Abstract
Abstract
Purpose
Anti-cancer and anti-migration effects of lupeol as a biological pentacyclic triterpenoid were investigated individually and in combination with Doxorubicin (DOX) on MCF-7 and MDA-MB-231 breast cancer cells and human foreskin fibroblasts.
Methods
To uncover the anticancer effect of lupeol and the impact of its combination with DOX, cell viability and scratch assays and dual acridine-orange apoptotic staining were performed. Moreover, the expression of proapoptotic caspase-3 and metastasis-related MMP-9 at the mRNA and protein levels was analyzed using qPCR and western blot techniques.
Results
Lupeol synergistically increased the anti-proliferative effect of DOX with IC50 values of 42.55, 62.24 and 65.9 μM on MCF-7, MDA-MB-231 and HFF cells, respectively. Lupeol reduced the cell migration and lowered the DOX-induced cell migration, significantly (p < 0.05). The number of apoptotic cells elevated significantly (p < 0.05) when cancer cells were treated with the combination of lupeol and DOX. Lupeol individually and in combination with DOX up-regulated the expression of caspase-3. The proposed combination therapy synergized (3–4 fold) the down-regulation of MMP-9 expression in MCF-7 and MDA-MB-231 cells.
Conclusion
Our results indicate that lupeol could be considered as an anticancer agent and anticancer adjuvant in breast cancer-therapy. The anticancer properties of lupeol attribute to its antiproliferative, antimigrative and apoptotic effects.
Graphical abstract
Keywords: Apoptosis, Breast Cancer, Cell proliferation, Cell migration, Combination therapy, Gene expression
Introduction
Cancer is one of the deadliest diseases in the world and has the second place after cardiovascular diseases in case of mortality. The incidence of cancer among other diseases is very high as on 2018 it has been estimated a total number of 18.1 million new cases and 9.5 million deaths from cancer worldwide. Distribution of cancer type in females showed that 24.2% of new cases belongs to breast cancer with 15% mortality [1]. Breast cancer is known as a heterogeneous disease based on the existing of different receptors including: estrogen, progesterone and human epidermal growth factor receptor-2 (ERBB2; formerly Her-2). There are classically 3 major subtypes of breast cancer: luminal type: ER and/or PR positive and Her-2 negative (70% of patients), Her-2 positive (15%–20%), and triple-negative (lack of all 3 known molecular markers in tumors; 15%) [2].
Doxorubicin (DOX) as a chemotherapeutic agent is considered one of the most active chemical substance against breast cancer. DOX acts at cellular level by intercalation between two nitric bases of double DNA helix, interfering in the mitochondria enzymes function, production of free radicals, cell membrane lipid peroxidation and apoptosis induction [3]. Based on the clinical investigations it has been proven that in spite of potential anticancer capacity, DOX produces severe side effects including irreversible cardiomyopathy and liver toxicity [4]. To reduce the toxicity and chemoresistance of DOX, the combination therapy model could be an effective approach. Previous studies for instance showed that the combination of diltiazem and DOX increased the DOX cytotoxicity against MCF-7 cells and also reduced the multi drug resistance [5].
Lupeol as a dietary triterpene is found in a variety of fruits and in several medicinal plants. Lupeol has been used for the treatment of some disorders all around the world [6–8]. It’s relatively low toxicity and valuable pharmacological effects such as anti-hepatic toxicity, anti-renal toxicity, anti-heart diseases, anti-diabetes, anti-inflammation and anti-arthritis made this molecule much important than ever [9–11]. Recently, extensive studies performed to show the lupeol’s cancer chemo-preventive potential against various cancers like prostate cancer, pancreatic cancer [12], skin cancer [13], hepatocellular carcinoma [14], epidermoid carcinoma [15] and melanoma [16]. The most documented molecular mechanisms of lupeol as an anticancer agent include: decrease of cell proliferation, inhibition of cell migration and induction of apoptosis. Moreover, other mechanisms such as androgen receptors inhibition and chemosensetization of tumor cells by lupeol have been also reported [17]. During the last decades co-administration of herbal medicines with chemical anticancer agents was to reduce the cancer symptoms and also to control the adverse effects of anticancer agents. This combination therapy would be medically useful when the combination outcome resulted in a remarkable synergistic effect against cancer. Synergistic approach in the cancer therapy can reduce the dose of anticancer chemicals and consequently their toxic effects. Previous studies reported the synergistic effects of quercetin with vincristine in the treatment of breast cancer, quercetin with tamoxifen in the treatment of prostate cancer and quercetin with doxorubicin in the treatment of leukemia in mice. The synergistic effects of quercetin with anticancer drugs were due to its capacity to inhibit the cell proliferation, regulate the angiogenesis and/or increase the antioxidant power of cancerous cases [18]. The synergistic antitumor effects of lupeol with S14161 (a PI3K inhibitor) on the inhibition of HCC tumor growth in in vivo model and potentiation of γ-radiation induced cell apoptosis in the HCC cell line SMMC-7721 have recently been demonstrated [19, 20].
Therefore, the aim of this study was to highlight the anti-proliferation, anti-migration and apoptotic effects of lupeol on two distinctive types of breast cancer cells (MCF-7 as receptor positive and MDA-MB-231 as triple negative cells) and also to highlight whether the combination of lupeol with DOX could be able to reduce the concentration of DOX and enhance its cytotoxicity against breast cancer cells. In addition of antiproliferative capacity of mono- and combination-therapy, the effects of proposed compounds in combination form on cancer cell migration and apoptosis induction were also examined.
Materials and methods
Chemicals
Lupeol (LPL, L5632) and doxorubicin (DOX, D1515) were purchased from Sigma-Aldrich (Germany). Both compounds were dissolved in DMSO and a 10 mg/ml primary stock solution was prepared and remained at −20 °C. MTT [3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide], PBS (Phosphate Buffered Saline) buffer, were purchased from Sigma-Aldrich and all the other chemicals and solutions were obtained from Merck (Germany). TRIzol reagent was purchased from Invitrogen, life technologies (Nieuwerkerk, The Netherlands).
Cell culture
Human breast cancer cell lines (MCF-7 and MDA-MB-231) and Human foreskin fibroblasts (HFF), were obtained from Iranian Biological Resource Center (Tehran, Iran). The cells were cultured in DMEM medium (Biowest, France), supplemented with 10% fetal bovine serum (Biowest, France) and 1% penicillin - streptomycin (Biowest, France). Cells were grown at 37 °C and humid incubator with 5% CO2 atmosphere. The reason for including two different types of breast cancer cells in this study is to highlight the possible influence of their different receptor content (MCF-7 cells as positive for estrogen, progesterone and androgen receptors and MDA-MB-231 cells are known as triple negative breast cancer cells, which are lacking the estrogen and progesterone receptors) on susceptibility and/or resistance of them against the test compounds.
Cell viability assessment
The cells with 80–90% confluency were sub-cultured and seeded in 96-well plates (1× 104 cells/well). After 24 h, the old medium was discarded and the cells were treated with fresh medium containing various concentrations of Lupeol (LPL: 25, 50 and 100 μM), Doxorubicin (DOX: 0.1, 0.5 and 1 μM), and the combination of LPL (50 μM) and DOX (0.25 μM) for 24 and 48 h. The control cells received the same concentration of DMSO as treated cells. The given concentrations were selected according to previous reports and corresponding to drug level in biological fluids after in vivo administration [21, 22]. Following the treatment times, cell viability was assessed by MTT assay at 570 nm using a microplate reader (Bio Tek Instruments, EPOCH2TC, Inc. Highland Park, Winooski, VT, USA). Cell viability was expressed as percentage of non-treated controls as follows:
Scratch assay
The cells (MCF-7, MD-MBA-230 and HFF) were seeded in 6-well plate (1 × 106 cells/well) and after 24 h with acceptable confluency indicating a monolayer (~ 80 to 90% confluency), scratches were made down on each well using a sterile sampler tip as described previously. Thereafter, the detached cells were removed with PBS and immediately the cells were treated with the effective concentrations of Lupeol (100 μM), DOX (0.5 μM), combination of LPL and DOX (50 μM + 0.25 μM) and/or control (medium containing the equal amount of test compounds solvent). Photographs of the scratches were taken at marked positions after 0 and 24 h. The scratch distances were quantified using ImageJ software [23].
Dual Acridin orange/ Ethidium bromide staining
MCF-7 and MD-MBA-230 cells were treated with different concentrations of LPL and DOX alone or in combination as stated in previous section. After exposure time (24 h), the old medium was removed and the cells were washed with PBS. Dual AO/EB staining was performed to demonstrate the induction of apoptosis in treated and non-treated cells. This method of staining is used to identify any changes of cell membranes during the process of apoptosis by using a fluorescent microscope (Nikon, TC-C-TC, 510563, Japan) [24]. Live cells will appear completely green, while apoptotic cells will incorporate ethidium bromide and therefore stain orange. Intensity of green and orange colors was quantified by using Image J/Ver:4, IHC plugin/IHC Toolbox.
Real-time PCR
The breast cancer cells were treated as mentioned in previous sections for 24 h and thereafter, total RNA was isolated using the TRIZOL method [25]. RNA concentrations were determined spectrophotometrically, and RNA purity was measured by NanoDrop 2000 (Thermo Scientific, Waltham, MA, USA) as A260/A280 ratio with expected values between 1.8 and 2. Subsequently, cDNA was synthesized in a 20 μl reaction mixture containing 1 μg RNA, oligo(dT) primer (1 μl), 5 × reaction buffer (4 μl), RNAse inhibitor (1 μl), 10 mM dNTP mix (2 μl) and M-MuLV Reverse Transcriptase (1 μl) according to the manufacturer’s protocol (Fermentas, GmbH, Germany). The cycling protocol was 5 min at 65 °C, followed by 60 min at 42 °C, and 5 min at 70 °C.
The produced cDNA was diluted with nuclease-free water (1:9) before qRT-PCR analysis. The qPCR reaction mixture, containing 10 μl of the diluted cDNA mixed with 12.5 μl SYBER Green PCR master mix (BioFACT Co., Ltd. Daejeon, Republic of Korea), forward and reverse primers (final concentration of 300 nM for each primer), and nuclease free water, was prepared according to manufacturer’s instructions (Cinagene Co. Tehran, Iran). qPCR was performed using the IQ5 multicolor real-time PCR detection system (Bio-Rad Laboratories). PCR cycle parameters were as follows: general denaturation at 95 °C for 3 min, 1 cycle, followed by 40 cycles of 95 °C for 20 s, annealing temperature (AT) for 30 s, and elongation at 72 °C for 30 s. Forward and reverse primers for Caspase-3, MMP-9 and β-actin (Table 1) were designed by using NCBI primer-BLAST and were manufactured commercially (Cinnagene Co. Tehran, Iran). Specificity and efficiency of primers were confirmed by qRT-PCR analysis and dilution series of pooled cDNA at a temperature gradient (55 °C to 65 °C) for primer-annealing and subsequent melting curve analysis. Glyceraldehydes 3-phosphate dehydrogenase (GAPDH) and ß-actin were tested as reference genes and the GeNorm software (version 3.5) was used to identify the most stable reference genes. The mRNA quantity was calculated relative to the expression of β-actin reference gene.
Table 1.
Nucleotide sequence and annealing temperature for used primers in qPCR
| Target Gene | Primer Sequence (5′-3′) | Accession number | AT (°C) |
|---|---|---|---|
| Cacpase-3 | FWD: AGAACTGGACTGTGGCATTGAG | NC_000004.12 | 59 |
| REV: GCTTGTCGGCATACTGTTTCAG | |||
| MMP-9 | FWD: GATGCGTGGAGAGTCGAAA | NC_000020.11 | 59 |
| REV: TAGGTGATGTTGTGGTGGTG | |||
| β-actin | FWD: CTTCTACAATGAGCTGCGTG | NC_000007.14 | 50 |
| REV: CATGAGGTAGTCAGTCAGG |
Western blotting for the caspase-3 and MMP-9 expression
MCF-7 and MD-MBA-230 cells were treated with various concentrations of test compounds (LPL and DOX) alone or in combination for 24 h. After 24 h treatment period, total protein concentrations were determined by a BCA protein assay kit (Thermo Fisher Scientific, MA, USA). Equal amounts of protein from boiled samples (20 μg) were separated by electrophoresis (Criterion TM Gel, 4–20% Tris-HCL, Bio-Rad Laboratories Inc. USA) and electro-transferred onto polyvinylidene difluoride membranes by using Turbo Trans-Blot Transfer Pack (Bio-Rad LaboratoriesInc. USA). The membrane then was blocked with Tris-buffered saline (TBS) containing 0.05% Tween-20 (TBST) supplemented with 5% bovine serum albumin (BSA) for 1 h. The membrane was incubated overnight at 4 °C with monoclonal antibodies against caspase-3 (1:1000), MMP-9 (1: 500) or β-actin (1:1000) (Santa Cruz, Dallas, Texas, USA). The membrane then was washed with TBST and incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000, Dako, Glostrup, Denmark) for 2 h at room temperature. Ultimately, the blot was washed in TBST and incubated with ECL prime western blotting detection reagent (Amersham Biosciences, Roosendaal, The Netherlands). Digital images were taken with the Chemi Doc TMMP imager (Bio-Rad Laboratories Inc. USA). Signal intensity was quantified using the Image J 1.52 software and the expression of different proteins was normalized with β-actin and expressed as mean fold change in relation to the control group.
Statistical analyses
For statistical analyses, mean and standard deviation of the measured parameters were calculated. The results were analyzed using Graph Pad Prism software (Version 8.02. Graph Pad Software Inc. San Diego, California, USA). The comparisons between groups were made by analysis of variance (ANOVA) followed by Bonferroni post-hoc test. A P value less than 0.05 was considered significant.
Results
Effects of LPL, DOX and LPL plus DOX on cell viability
All three cell lines including breast cancer cells and fibroblasts were treated with test compounds at various concentrations for 24 and 48 h and cell viability was assessed by MTT assay. Both compounds showed a time- and concentration-dependent antiproliferative effect on cancerous cells and normal fibroblasts. The results revealed that MCF-7 cells were significantly (p < 0.05) more sensitive than MDA-MB-231 cells to antiproliferative effects of LPL and DOX. Both compounds at all tested concentrations reduced the cell viability of fibroblasts in a concentration-dependent fashion. In both breast cancer cells, the antiproliferative effect of DOX was synergized by LPL when administered in combination form (Fig. 1A to C). Lupeol’s anti-proliferative effect was documented with calculated IC50 values of 42.55, 62.24 and 65.9 μM on MCF-7, MDA-MB-231 and HFF cells, respectively. While DOX after 48 h treatment period was not able to reach the IC50 in MDA-MB-231 cells, in MCF-7 and HFF cells however resulted in a remarkable cytotoxicity with IC50 values of 0.32 and 0.43 μM, respectively. Since, following 24 h treatment time, in both cancerous cell lines, the given concentrations resulted in a significant cytotoxicity, hence all further experiments were performed for 24 h and based on the closest values to the estimated IC50 values.
Fig. 1.
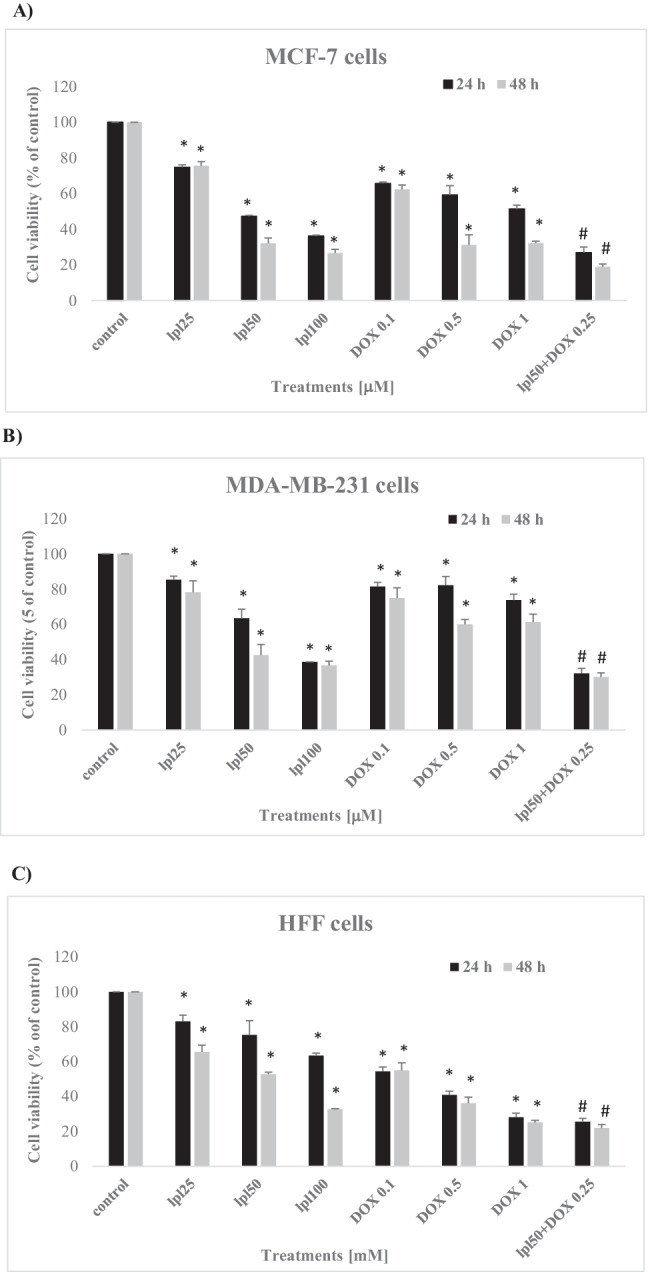
Effects of Lupeol, Doxorubicin and their combination on cell viability of MCF-7 cells (A), MDA-MB-231 cells (B) and HFF cells (C). Asterisks are representing significant difference (p < 0.05) between the corresponding control and treated cells and # is indicating a significant difference between the cells that received the combination of LPL and DOX and/or each single compound at all tested concentrations
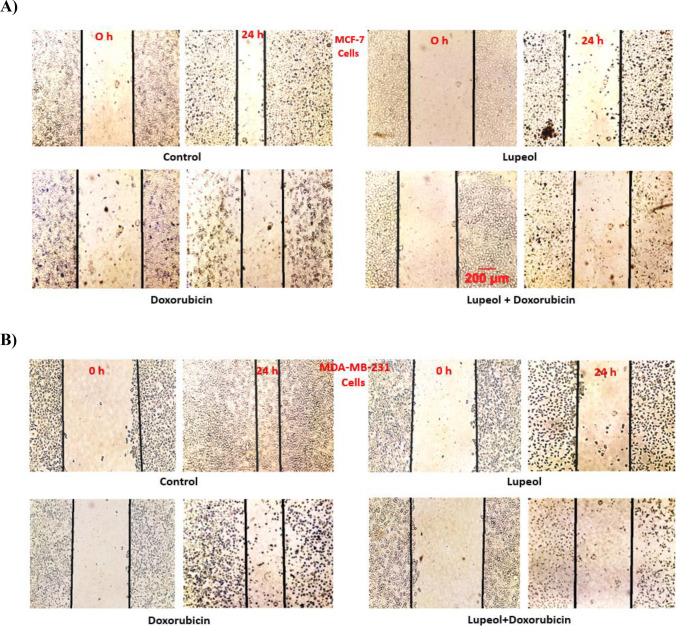
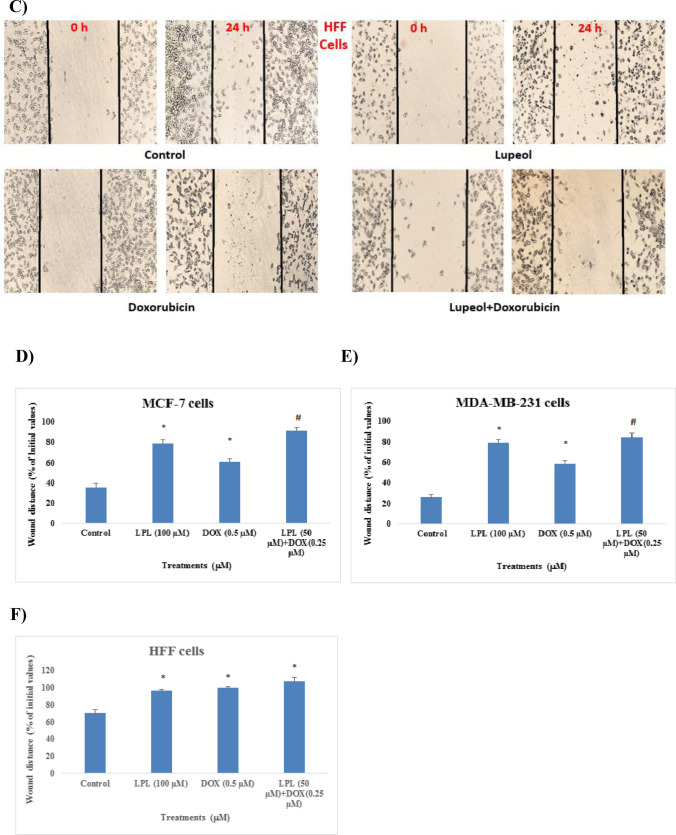
LPL plus DOX reduced the migration of breast cancer cells
Results of scratch assay on MCF-7, MDA-MB-231 and HFF cells showed that despite of the control group, which after 24 h scratch edges have been significantly closed to each other, in other test groups; firstly, the density of cells has been reduced remarkably and secondly the distance between two edges of scratch widened, when compared with the corresponding control groups. Those cells that received LPL alone or LPL in combination with DOX, showed the widest distance between edges of experimentally-induced scratch in all three tested cell lines after 24 h (Fig. 2A, B and C). LPL at 100 μM concentration in both cancerous cell lines reduced the cell migration stronger than DOX (0.5 μM), while in HFF cells we failed to find any statistical difference between LPL and DOX treated cells. The scratch distance in each group was expressed as % of initial values (Fig. 2D, E and F).
Fig. 2.
Lupeol in mono- and combination therapy reduced the migration of: A) MCF-7, B) MDA-MB-231 and C) HFF cells; Phase contrast images were taken immediately after scratch (0 h) and 24 h after treatment (24 h) and scratch distances were expressed as % of initial values (D, E and F). Asterisks are representing significant difference (p < 0.05) between the corresponding control and treated cells and # is indicating a significant difference between the cells that received the combination of LPL and DOX and/or each single compound. Scale bare represents 200 μm
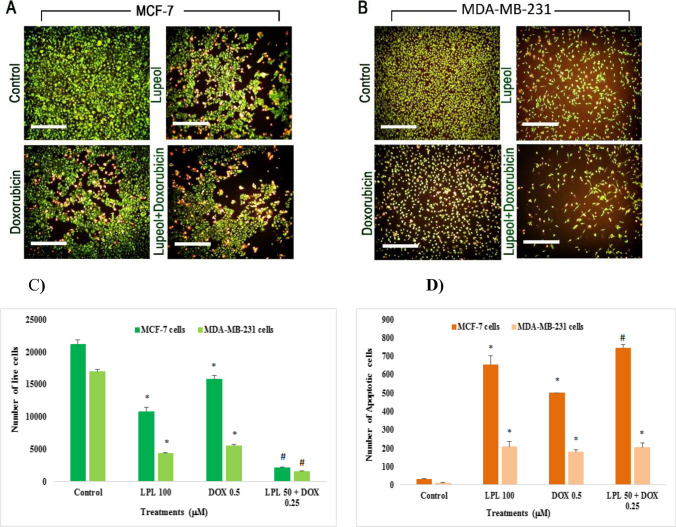
Lupeol increased the number of apoptotic cells
The mechanism of LPL-induced cytotoxicity was clarified by using a combined AO/EB staining method. The percentage of green and/or orange stained cells under fluorescent microscope reveals the rate of normal and apoptotic cells, respectively (Fig. 3A and B). The intensity of each color as indicator of cell condition was quantified by using a computer-based program of densitometry. The results showed that LPL and DOX in monotherapy regimen increased significantly (p < 0.05) the number of apoptotic cells in both cell lines when compared with the control cells. Interestingly, densitometric analyses revealed that LPL was able to potentiate significantly (p < 0.05) the apoptotic effects of DOX only in MCF-7 cells. (Fig. 3C and D).
Fig. 3.
Lupeol increased the number of Apoptotic cells in MCF-7 cells (A) and MDA-MB-231 cells (B); the cells were treated with test chemicals for 24 h and thereafter were stained with AO/EB. The test compounds-induced changes in the intensity of stained cells were measured using a computer-based program (Image J/Ver: 4, IHC plugin/IHC Toolbox) and illustrated as corresponding densitometry results (C and D). Asterisks are representing a significant difference between the control and treated cells and # is demonstrating a significant difference between mono-therapy and combination therapy (p < 0.05). Scale bare represents 300 μm
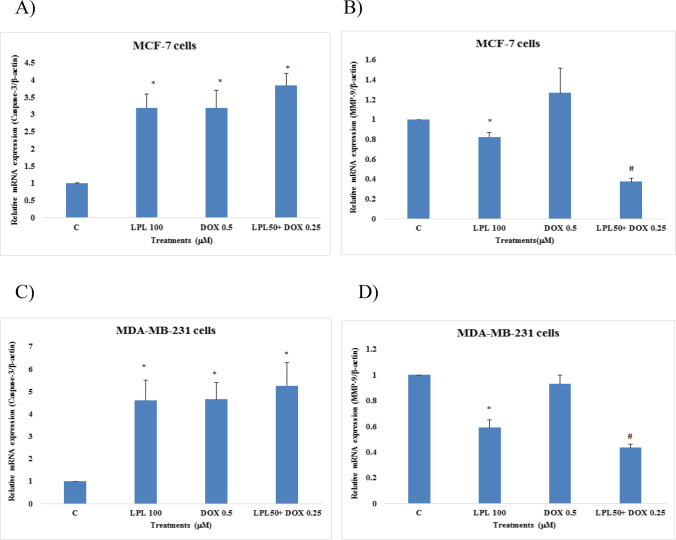
Lupeol sinergized the DOX-induced up-regulation of caspase-3 and downregulated the expression of MMP-9 in breast cancer cells
The mRNA expression of Caspase-3 and MMP-9 in the control and treated cells were analyzed using quantitative PCR. Results revealed that LPL, DOX and LPL plus DOX up-regulated 3–4 fold the expression of caspase-3 in MCF-7 and 4–5 fold in MDA-MB-231 cells. Although the combination of LPL plus DOX resulted in an upregulation of caspase-3 in both cell lines, there was however, no statistical differences between mon- and the combination therapy forms (Fig. 4A and C). The expression of MMP-9 in MCF-7 cells following the LPL treatment was downregulated, while DOX at 0.5 μM concentration showed a non-significant (p > 0.05) up-regulation of MMP-9 in MCF-7 cells. We found that the combination of LPL plus DOX resulted in a significant downregulation of MMP-9 in MCF-7 cells. The expression of MMP-9 in MDA-MB-231 cells showed that LPL and LPL plus DOX resulted in a significant downregulation of MMP-9 (p < 0.05), while DOX at the given concentration (0.5 μM) was not able to alter the expression of MMP-9 significantly (p > 0.05) at mRNA level (Fig. 4B and D).
Fig. 4.
Effects of LPL and DOX on the expression of Caspase-3 and MMP-9 at mRNA level in: A and B: MCF-7 and C and D: MDA-MB-231 cells; asterisks are representing a significant difference between the control and treated cells and # is demonstrating a significant difference between mono-therapy and combination therapy (p < 0.05)
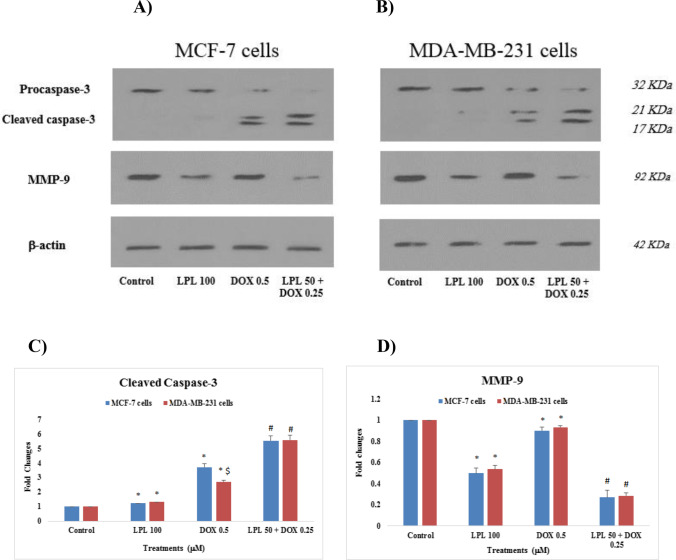
Effect of Lupeol on the expression of apoptotic and metastatic proteins (Caspase-3 and MMP-9)
The expression of caspase-3 and MMP-9 at protein level was analyzed using western blotting technique. The protein expression profile of the proposed proteins was clearly confirmed the mRNA expression (Fig. 5A and B). The highest up-regulation of cleaved caspase-3 protein was observed in the breast cancer cells, which were treated with the combination of LPL (50 μM) and DOX (0.25 μM). LPL individually increased the expression of cleaved caspase-3 slightly and significantly (p < 0.05). There was a significant difference between two studied cell lines in terms of the effect of DOX on cleaved caspase-3 protein expression and we found that DOX up-regulated the expression of cleaved caspase-3 in MCF-7 cells more than MDA-MB-231 cells (Fig. 5C). LPL and LPL plus DOX down-regulated the expression of MMP-9 protein in both breast cancer cells significantly (p < 0.05). DOX reduced the expression of MMP-9 in both cells very slightly in mono-therapy model (Fig. 5D).
Fig. 5.
Effects of LPL and DOX on the expression of Caspase-3 and MMP-9 at protein level in: A: MCF-7 and B: MDA-MB-231 cells; The densitometry results, which normalized based on the expression of β-actin are represented for cleaved caspase-3 (C) and for MMP-9 (D); Asterisks are representing a significant difference between the control and treated cells and # is demonstrating a significant difference between mono-therapy and combination therapy (p < 0.05)
Discussion
Breast cancer is metastatic, aggressive and resistance to chemotherapy with high mortality rate between women. Due to such an invasive behavior and much importantly its resistance to existing chemotherapeutic agents, discovering new anticancer agents or improving the existing protocols are required. Most of the studies to highlight the effectiveness and molecular mechanism(s) of new agents or currently used chemicals are taking place on cell culture models, which representing those cells with the same receptors in tumor form. The current study clarified the anticancer effects of LPL on receptor positive and negative models of breast cancer cells and confirmed the anticancer effects of DOX as currently used chemotherapeutic agent. Much importantly any beneficial interaction from combination therapy of LPL with DOX, was evaluated. Results of this study showed that LPL alone exerts cytotoxic effects on both breast cancer cells. The anti-migration and apoptotic effects of LPL were demonstrated by means of scratch assay and apoptotic staining method of Ao/EtB, respectively. Both aforementioned findings were confirmed with up-regulation of caspase-3 and down-regulation of MMP-9 proteins and encoding genes in breast cancer cells. Lupeol synergized remarkably the cytotoxicity, anti-migration and apoptotic effects of DOX and clearly showed that the combination of lower concentrations of DOX and LPL produce high cytotoxicity, powerful anti-migration property and potent apoptotic effects on breast cancer cells and in particular on MCF-7 cells as those are hormonal receptor positive. Of course the cytotoxicity of LPL and DOX on non-cancerous human foreskin fibroblasts also was examined. Both compounds with various IC50 values showed cytotoxicity on HFF cells, too.
DOX as an anthracycline is administered for the treatment of many tumors including breast cancers. It acts via various pathways including intercalation between bases of double DNA helix, inhibition of mitochondrial enzymes and cell respiration, lipid peroxidation in particular in cell membrane lipids, free radicals generation and DNA damage, impair of helicase activity and apoptosis induction [3]. Our findings also confirmed some of previously reported mechanisms as its cytotoxicity was demonstrated on breast cancer cells via interfering on mitochondrial enzymes, which reflected in MTT colorimetric assay. Comparing two cell lines viability after exposing against DOX showed that MCF-7 cells were more sensitive than MDA-MB- 231cells, which may related to different receptor content of two cell lines.
Further experiments demonstrated that DOX in monotherapy form and at 0.5 μM concentration slightly prevented the cell migration in all three tested cell lines. Cell migration in cancer cells is a complex event that is counted as primary step of metastases, which most of the chemotherapeutic agents supposed to prevent or reduce this capability of cancer cells. There are studies reporting that DOX at rather lower concentrations promoted the breast cancer cells migration and invasion [19]. Other recent studies also supporting our findings that DOX at nono-molar concentrations and in monotherapy regimen not only did not prevent from cell migration but also acted as a promoter of breast cancer cells migration [26]. Degradation of extracellular matrix (ECM) is one of essential steps in the migration of cancer cells and overall in the metastasis, which mediated by proteolytic enzymes and in particular by matrix metalloproteinases (MMPs). Among the others, oncogenes and tumor promoters are playing crucial role in the expression of MMPs. It has been documented that the expression of MMPs and their secretion is elevated in many type of cancers [27]. To explain the possible molecular pathway behind the migration of cancer cells in the current study, the molecular analyses were performed to highlight the expression changes in the mRNA and protein levels of MMP-9 in both cell lines. Our findings demonstrated that DOX was not able to change the expression of MMP-9 in the tested cancer cells, although it’s non-significant up-regulation on MMP-9 was recorded in MCF-7 cells. These findings confirmed our previous findings regarding the effect of DOX on cell migration as both cell lines showed markedly migration under DOX monotherapy.
Another important factor, which this study focused on was the cytotoxicity of test compounds in monotherapy and combination therapy forms on breast cancer cells. DOX showed that at all three used concentrations was able to reduce significantly the cell viability of tested cells. To find the molecular mechanism(s) of cell death due to exposure to test compounds, two apoptotic hallmarks were examined and our findings showed that in both cell lines DOX remarkably increased the number of apoptotic cells in special apoptosis staining and up-regulated significantly the expression of caspase-3 as one of the key factors in the regulation of apoptotic death in cells. It is noteworthy that there are increasing number of evidence demonstrating the expression of caspase-3 in both MCF-7 and MDA-MB-231 cells and its activation during apoptosis due to apoptotic signals [28, 29].
The major goal of this study was to investigate about the anticancer effect of LPL on two distinct model of breast cancer cells in terms of their receptor expression profile. We hypothesized if this phytosteroline (LPL) exerted an anticancer effect, what would be its effect on the combination therapy with DOX. Our results showed that LPL treatment resulted in a remarkable reduction of cell viability in all tested cell lines. Based on calculated IC50 values, MCF-7 cells were found to be the sensitive cells followed by MDA-MB-231 cells and ultimately HFF cells. To explain this finding, it is noteworthy that anti-androgenic effect of LPL has been previously documented (7). At the same time the androgen receptor-related proliferative effects via the mitogen-activated protein kinase in breast cancer cells also reported [30]. Therefore, cross-talk between AR and ER, where their transduction signaling can affect each other, will be resulted in cell proliferation and in turn inhibition of AR by LPL might explain the observed remarkable differences between the ER-positive (MCF-7) and triple negative (MDA-MB-231) breast cancer cells [31]. This finding is supported by previous reports where it has been demonstrated that AR inhibition resulted in a marked decline in estradiol-induced proliferation in multiple ER+/AR+ breast cancer cell lines [32].
Although LPL cell viability assay showed that it is not absolutely selective anticancer against the tested breast cancer cells, the higher IC50 value for HFF cells however is suggesting higher susceptibility of cancer cells to LPL. Our findings are in agreement with previous reports as it has been shown that LPL with IC50 value of 80 μM demonstrated an anticancer effect on MCF-7 cells [33]. There are several mechanisms of action for LPL cytotoxic effects including its capability in inhibition of topoisomerase II, inhibition of lyase activity of DNA polymerase β, inhibition of farnesyltransferase, inhibition of angiogenesis and eventually induction of apoptosis [15]. The mentioned cytotoxic effect of LPL was further confirmed with its anti-metastatic and apoptotic effects on breast cancer cells. Previously published reports are supporting our finding regarding anti-migration effect of LPL in breast cancer cells, but also in lung and colorectal cancer cells [34]. Wang (2016) reported that LPL was able to prevent from MDA-MB-231 cells migration through down-regulation of COX-II, MMP-2 and MMP-9 proteins [35]. Our gene and protein expression analyses confirmed the previous report as we demonstrated that in both MCF-7 and MDA-MB-231 cell lines, LPL down-regulated the expression of MMP-9 at mRNA and protein levels. Other mechanisms of anti-migration effect of LPL were described in human umbilical vein endothelial cells (HUVECs) and human lung cancer cells, which LPL by downregulation of TNF-α and subsequently suppression of VEGF signaling in HUVEC cells and suppression of Wnt-β-Catenin in colorectal cancer cells inhibited cell migration, respectively [36, 37].
Our apoptotic studies showed that LPL at 100 μM concentration increased the number of apoptotic cells in acridin orange and ethidium bromide staining and also markedly up regulated the expression of caspase-3 in both breast cancer cell lines. These findings confirmed our cell viability assessment and highlighted its molecular mechanism as caspase-3 dependent apoptosis behind LPL anticancer effect. The apoptosis induction by LPL in human epidermal carcinoma A431 cells was attributed to the up regulation of caspase 3, caspase 9, Bax and Apaf-1 and downregulation of Bcl-2 mediated through mitochondria [15]. The results of current study indicate that LPL in monotherapy form could be considered as a potential chemotherapeutic agent in breast cancer therapy.
Although as novelty of the current study, the anti-migration and apoptotic potential of LPL alone on breast cancer cells has been uncovered, nevertheless the most important findings could be the final outcome of LPL combination therapy with DOX as a currently used chemotherapeutic agent. LPL increased the antiproliferative effect of DOX on breast cancer cells as at half concentrations of LPL plus DOX, we observed a significantly higher antiproliferative effect. LPL also improved the other anticancer effects of DOX by increasing the number of apoptotic cells, upregulation of caspase-3 and downregulation of MMP-9 in breast cancer cells.
Combination chemotherapy is a useful strategy to decrease the side effects and increase the therapeutic effectiveness. This strategy decreases the side effects by reducing the dose and may also help to reduce the drug resistance. DOX despite having life threatening side effects such as cardiotoxicity, bone marrow suppression, immune suppression and nephrotoxicity is commonly used as one of the most effective anticancer agent [38]. The results of current study indicate that the combination of LPL and DOX resulted in both concentration reduction and higher effectiveness on breast cancer cells. Indeed the proposed combination therapy with low concentrations could result in rather less toxic effects of DOX on non-cancerous cells, too. There are increasing number of studies, reporting an improvement of DOX anti-cancer efficacy when it has been used in combination therapy regimens. A synergistic effect of: DOX and cisplatin against breast cancer cell lines, DOX and docetaxel on prostate cancer cell migration, DOX and curcumin against A549 cells, DOX and paclitaxel on breast and hepatocarcinoma cancers, DOX and quercetin against liver cancer and DOX and lovastatin on melanoma have been documented [39–42]. One of the important drawbacks for DOX in cancer therapy is its cell migration induction property, which our proposed combination therapy was able to reduce this property, as well. Previous reports indicated that furanodiene reduced the DOX-induced cell migration in MDA-MB-231 metastatic breast cancer cells [43]. The combination of hesperidin with DOX also resulted in a significant reduction of MCF-7HER-2 positive cell migration [44].
This study could be further completed as the receptors (estrogen, progesterone and androgen receptors) expression at mRNA and protein levels under monotherapy and combination therapy is studied. Moreover, pharmacokinetic and toxicokinetic studies on LPL and analyses of any interactions between LPL and DOX (drug –drug interactions) will make the LPL effectiveness much reliable as required.
Conclusions
This study in addition of highlighting the anticancer effects of LPL in monotherapy form on both breast cancer cell lines, which characterized by antiproliferative, antimigrative and apoptotic properties, revealed that LPL synergizes the anticancer potency of DOX. It would be practically important if the described combination therapy can be matter of further preclinical and clinical studies to reduce the DOX-induced side effects and drug resistance and increase the effectiveness of both tested compounds.
Acknowledgements
The authors wish to thank from Miss. Shima Zeinali-Moghaddam for her generous technical support.
Authors contributions
FM carried out the cell culture and qPCR experiments and wrote the first draft of the manuscript. FK designed the study and participated in data collection and analyses. MHKA participated in data analyses and also in the discussion of manuscript. HM carried out the statistical analyses, helped in the drafting of manuscript and discussed the results. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
All data generated or analyzed during this study are included in this published article.
Declarations
Ethical approval
This study was approved by Ethical committee of Urmia University of Medical Sciences (IR.UMSU.REC.1398.113). We are happy to announce that neither entire nor part of the current study has been or currently being considered for publication by other journals.
Consent to participate
All authors happily and pleasurably have participated in this study.
Consent to publish
All authors read the manuscript and agreed to have in the present form for publication in “DARU journal of Pharmaceutical Sciences”. .
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. IJC. 2019;144(8):1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Mariotto AB, Etzioni R, Hurlbert M, et al. Estimation of the number of women living with metastatic breast cancer in the United States. CEBP. 2017;26(6):809–815. doi: 10.1158/1055-9965.epi-16-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minotti G, Menna P, Salvatorelli E, et al. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 4.Greish K, Sawa T, Fang J, et al. SMA–doxorubicin, a new polymeric micellar drug for effective targeting to solid tumours. J of Cont Rel. 2004;97(2):219–230. doi: 10.1016/j.jconrel.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Al-malky HS, Damanhouri ZA, Al Aama JY, et al. Diltiazem potentiation of doxorubicin cytotoxicity and cellular uptake in human breast cancer cells. Breast Cancer Manag. 2019;8(4):BMT31. doi: 10.1186/s12935-019-0912-0. [DOI] [Google Scholar]
- 6.Iman S, Azhar I, Hasan M, et al. Two triterpenes lupanone and lupeol isolated and identified from Tamarindus indica L. Pak J Pharm Sci. 2007;20(2):125–127. [PubMed] [Google Scholar]
- 7.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285(2):109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You YJ, Nam NH, Kim Y, et al. Antiangiogenic activity of lupeol from Bombax ceiba. Phytother Res. 2003;17(4):341–344. doi: 10.1002/ptr.1140. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi PK, Bhui K, Shukla Y. Lupeol: connotations for chemoprevention. Cancer Lett. 2008;263(1):1–13. doi: 10.1016/j.canlet.2008.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10(3):475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi E, Shirakawa H, Hata K,Hiwatashi K, et al. Lupeol supplementation improves blood pressure and lipid metabolism parameters in stroke-prone spontaneously hypertensive rats. Biosci Biotechnol Biochem. 2012:1112062754-1112062754. 10.1016/j.canlet.2008.01.047. [DOI] [PubMed]
- 12.Murtaza I, Saleem M, Adhami VM, et al. Suppression of cFLIP by lupeol, a dietary triterpene, is sufficient to overcome resistance to TRAIL-mediated apoptosis in chemoresistant human pancreatic cancer cells. Cancer Res. 2009;69(3):1156–1165. doi: 10.1158/0008-5472.can-08-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleem M, Afaq F, Adhami VM, et al. Lupeol modulates NF-κ B and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23(30):5203–5214. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]
- 14.Liu F, He Y, Liang Y, Wen L, Zhu Y, Wu Y, et al. PI3-kinase inhibition synergistically promoted the anti-tumor effect of lupeol in hepatocellular carcinoma. Cancer cell intl. 2013;13(1):108. doi: 10.1186/1475-2867-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad S, Madan E, Nigam N, Roy P, George J, Shukla Y. Induction of apoptosis by lupeol in human epidermoid carcinoma A431 cells through regulation of mitochondrial, Akt/PKB and NF-kappaB signaling pathways. Cancer Biol Ther. 2009;8(17):1632–1639. doi: 10.4161/cbt.8.17.9204. [DOI] [PubMed] [Google Scholar]
- 16.Tarapore RS, Siddiqui IA, Saleem M, Adhami VM, Spiegelman VS, Mukhtar H. Specific targeting of Wnt/β-catenin signaling in human melanoma cells by a dietary triterpene lupeol. Carcinogenesis. 2010;31(10):1844–1853. doi: 10.1093/carcin/bgq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu K, Zhang X, Xie L, Deng M, Chen H, Song J, et al. Lupeol and its derivatives as anticancer and anti-inflammatory agents: molecular mechanisms and therapeutic efficacy. Pharmacol Res. 2021;1(164):105373. doi: 10.1016/j.phrs.2020.105373. [DOI] [PubMed] [Google Scholar]
- 18.Zou H, Ye H, Kamaraj R, Zhang T, Zhang J, Pavek P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine. 2021;1(92):153736. doi: 10.1016/j.phymed.2021.153736. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, He Y, Liang Y, et al. PI3-kinase inhibition synergistically promoted the anti-tumor effect of lupeol in hepatocellular carcinoma. Cancer Cell Int. 2013;13(1):1–7. doi: 10.1186/1475-2867-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin Y, Lyu Y, Tang X, Zhang Y, Chen J, et al. Lupeol enhances radiosensitivity of human hepatocellular carcinoma cell line SMMC-7721 in vitro and in vivo. Int J Radiat Biol. 2015;91(2):202–208. doi: 10.3109/09553002.2015.966209. [DOI] [PubMed] [Google Scholar]
- 21.Siddique HR, Mishra SK, Karnes RJ, Saleem M. Lupeol, a novel androgen receptor inhibitor: implications in prostate cancer therapy. Clin Cancer Res. 2011;17(16):5379–5391. doi: 10.1158/1078-0432.CCR-11-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinello PC, Panis C, Xavier Silva TN, Binato R, Abdelhay E, Rodrigues JA, et al. Metformin prevention of doxorubicin resistance in MCF-7 and MDA-MB-231 involves oxidative stress generation and modulation of cell adaptation genes. Sci Rep. 2019;9:5864. doi: 10.1038/s41598-019-42357-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang C-C, Park AY, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 24.Gherghi IC, Girousi ST, Voulgaropoulos A, Tzimou-Tsitouridou R. Study of interactions between DNA-ethidium bromide (EB) and DNA-acridine orange (AO), in solution, using hanging mercury drop electrode (HMDE) Talanta. 2003;61(2):103–112. doi: 10.1016/s0039-9140(03)00238-8. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction: twenty-something years on. Nat Protoc. 2006;1(2):581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 26.Bozzuto G, Ruggieri P, Molinari A. Molecular aspects of tumor cell migration and invasion. Ann dell'Istitut Super SanitÃ. 2010;46:66–80. doi: 10.4415/ann_10_01_09. [DOI] [PubMed] [Google Scholar]
- 27.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 28.Abd Razak N, Abu N, Ho WY, et al. Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci Rep. 2019;9(1):1–12. doi: 10.1038/s41598-018-37796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mondal A, Bennett LL. Resveratrol enhances the efficacy of sorafenib mediated apoptosis in human breast cancer MCF7 cells through ROS, cell cycle inhibition, caspase 3 and PARP cleavage. Biomed Pharmacother. 2016;84:1906–1914. doi: 10.1016/j.biopha.2016.10.096. [DOI] [PubMed] [Google Scholar]
- 30.Lange CA, Gioeli D, Hammes SR, Marker PC. Integration of rapid signaling events with steroid hormone receptor action in breast and prostate cancer. Annu Rev Physiol. 2007;69:171–199. doi: 10.1146/annurev.physiol.69.031905.160319. [DOI] [PubMed] [Google Scholar]
- 31.Venema CM, Bense RD, Steenbruggen TG, Nienhuis HH, Qiu SQ, van Kruchten M, et al. Consideration of breast cancer subtype in targeting the androgen receptor. Pharmacol Ther. 2019;200:135–147. doi: 10.1016/j.pharmthera.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 32.D'Amato NC, Gordon MA, Babbs B, Spoelstra NS, Butterfield KT, Torkko KC, et al. Cooperative dynamics of AR and ER activity in breast cancer. Mol Cancer Res. 2016;14(11):1054–1067. doi: 10.1158/1541-7786.MCR-16-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitchai D, Roy A, Ignatius C. In vitro evaluation of anticancer potentials of lupeol isolated from Elephantopus scaber L. on MCF-7 cell line. J Adv Pharm Technol 2014;5(4):179. 10.4103/2231-4040.143037. [DOI] [PMC free article] [PubMed]
- 34.Bhatt M, Patel M, Adnan M, et al. Anti-metastatic effects of Lupeol via the inhibition of MAPK/ERK pathway in lung Cancer. Curr Med Chem – Anti-Cancer Agents. 2021;21(2):201–206. doi: 10.2174/1871520620666200424131548. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Cui H, Sun C, Li G, Wang H, Xia C, et al. Effect of lupeol on migration and invasion of human breast cancer MDA-MB-231 cells and its mechanism. Yao xue xue bao= Acta Pharm Sin. 2016;51(4):558–562. [PubMed] [Google Scholar]
- 36.Kangsamaksin T, Chaithongyot S, Wootthichairangsan C, et al. Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α. PLoS One. 2017;12(12):e0189628. doi: 10.1371/journal.pone.0189628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Hong D, Qian Y, et al. Lupeol inhibits growth and migration in two human colorectal cancer cell lines by suppression of Wnt–β-catenin pathway. Onco Targets Ther. 2018;11:7987. doi: 10.2147/ott.s183925. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Beg T, Siddique YH, Ara G, et al. Genistein suppresses doxorubicin associated Genotoxicity in human lymphocytes. Lat Am J Pharm. 2011;0(7):1317–1324. [Google Scholar]
- 39.Gehl J, Boesgaard M, Paaske T, et al. Combined doxorubicin and paclitaxel in advanced breast cancer: effective and cardiotoxic. Ann Oncol. 1996;7(7):687–693. doi: 10.1093/oxfordjournals.annonc.a010717. [DOI] [PubMed] [Google Scholar]
- 40.Kim JH, Chae M, Kim WK, et al. Salinomycin sensitizes cancer cells to the effects of doxorubicin and etoposide treatment by increasing DNA damage and reducing p21 protein. Br J Pharmacol. 2011;162(3):773–784. doi: 10.1111/j.1476-5381.2010.01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park H-K, Lee J-E, Lim J, et al. Combination treatment with doxorubicin and gamitrinib synergistically augments anticancer activity through enhanced activation of Bim. BMC Cancer. 2014;14(1):1–9. doi: 10.1186/1471-2407-14-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsakalozou E, Eckman AM, Bae Y. Combination effects of docetaxel and doxorubicin in hormone-refractory prostate cancer cells. Biochem Res Int 2012;2012.10.1155/2012/832059 [DOI] [PMC free article] [PubMed]
- 43.Zhong Z-F, Tan W, Tian K, et al. Combined effects of furanodiene and doxorubicin on the migration and invasion of MDA-MB-231 breast cancer cells in vitro. Oncology Rep. 2017;37(4):2016–2024. doi: 10.3892/or.2017.5435. [DOI] [PubMed] [Google Scholar]
- 44.Nurhayati IP, Khumaira A, Ilmawati GPN, et al. Cytotoxic and antimetastatic activity of hesperetin and doxorubicin combination toward Her2 expressing breast cancer cells. APJCP. 2020;(5):21, 1259, 10.31557/apjcp.2020.21.5.1259. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.