Abstract
Recent studies have intensively investigated the possibility of kombucha application as non-conventional starter culture in manufacture of various fermented dairy products. Furthermore, natural extracts from medicinal and aromatic plants contain different biologically active components which often have antioxidant properties. Based on the stated above, the aim of this research was to investigate the possibility of kombucha inoculum application as a new starter culture in fresh cheese technology, as well as to investigate effects of sage (Salvia officinalis) herbal dust (by-product from filter tea factory), its essential oil and supercritical fluid extract on antioxidative activity and sensory characteristics of produced fresh kombucha cheese during 10 days of storage. In all samples, higher ABTS than DPPH radical scavenging activity was determined. Freshly prepared and 10 days stored kombucha cheeses fortified with different types of sage preparations had significantly higher FRAP values than the control sample. All analysed samples had satisfied sensory characteristics and same scores of sensory evaluation after the production. Kombucha fresh cheese with addition of different types of sage preparations can be an innovative and valuable dairy product.
Keywords: Fresh cheese, Kombucha, Sage, Antioxidative activity, By-products
Introduction
Produced from the enzymatic coagulation of milk with rennet and starter cultures, fresh cheeses have soft texture, slightly acidic flavour, high moisture and low price. Due to these characteristics, fresh cheeses are widely consumed dairy products (Barukcic et al. 2020).
Use of non-conventional starter cultures have a great potential in manufacture of various fermented dairy products with desirable technological and nutritional characteristics (Hati et al. 2014). Kombucha is a consortium of yeasts (Saccharomyces, Zygosaccharomyces, Pichia, Schizosaccharomyces, Saccharomycodes, Brettanomyces, Torulaspora and Candida), acetic acid bacteria (Acetobacter and Gluconobacter) and lactic acid bacteria (LAB) (Morales 2020; Marsh et al. 2014). Kombucha beverages has traditionally been prepared by fermentation different sweetened teas. Its specific microbiological composition led to the generation of various chemical compounds: organic acids, ethanol, proteins, sugars, polyphenols (depending on the utilized raw material), vitamins (B1, B6, B12, C), minerals (Cu, Fe, Mn, Ni, Zn), anions (F−, Cl−, Br−, I−, NO3−, HPO4−, SO4−) and interesting bioactive compounds (d-saccharic acid-1,4-lactone (DSL) (Morales 2020). Recently, the possibility of kombucha application (as new starter culture) in production of fermented dairy products has been studied (Vukic et al. 2014; Kanuric et al. 2018). It was found that fermented dairy beverages produced with kombucha starter culture had satisfactory overall physico-chemical, nutritive and sensory characteristics (Popovic et al. 2016; Kanuric et al. 2018; Vukic et al. 2018). In addition to formation specific sensory characteristics, milk fermentation is an important way of formation of bioactive compounds; therefore, the usage of new starter cultures and prebiotics set a wide possibility to obtain new functional foods (Hrnjez et al. 2014; Skrzypczak et al. 2017; Vukic et al. 2018; Shariati et al.2020; Haji Ghafarloo et al. 2020).
In recent years, much attention has been focused on reuse of by-products and reduction of waste from food industry. The possibility of using agro-industrial wastes as natural antioxidants and preservatives in the food industry could be very important for environmental protections as well as for food production with additional functional value (Lorenzo et al. 2014). Sage (Salvia officinalis L.), which belongs to Lamiaceae family, has been widely used in pharmaceutical and food industry due to its chemical composition and high antioxidant potential (Pavlic et al. 2016; Zekovic et al. 2017). The most abundant compounds in sage essential oil are monoterpenes and oxygenated monterpenes (Pavlic et al. 2018).
The aim of this research was to investigate the possibility of kombucha inoculum application as a new starter culture in fresh cheese technology, as well as to investigate effects of sage (Salvia officinalis) herbal dust (by-product from filter tea factory), its essential oil and supercritical fluid extract on antioxidative capacity and sensory characteristics of produced fresh kombucha cheese during 10 days of storage.
Materials and methods
Plant material
Sage (Salvia officinalis L.) originated from Montenegro was kindly donated by local filter tea factory, Fructus DOO (Bačka Palanka, Serbia). Herbal dust fraction had particle size lower than 0.315 mm, thus it is discarded as a by-product in filter tea processing. Discarded plant material was collected and used as raw material in this research.
Hydrodistillation
The essential oil (EO) of sage herbal dust was obtained using the official hydrodistillation procedure (Ph. Eur. 2014). The yield of EO was 1.80%, while limonene (23.22 mg/100 g), eucalyptol (186.12 mg/100 g), camphor (319.32 mg/100 g), α-thujone (305.28 mg/100 g) were major terpenoids obtained from sage herbal dust using hydrodistillation technique (Pavlic et al. 2018).
Supercritical fluid extraction
The supercritical fluid extraction (SFE) experiments were performed on laboratory scale high pressure extraction plant (HPEP, NOVA, Swiss, Effretikon, Switzerland) described in detail by Pekić et al. 1995. The main plant parts and properties, by manufacturer specifications were: gas cylinder with CO2, the diaphragm type compressor with pressure range up to 1000 bar, extractor with heating jacket for heating medium with internal volume 200 mL, maximum operating pressure of 700 bar, separator with heating jacket for heating medium (with internal volume 200 mL, maximum operating pressure of 250 bar), pressure control. Extraction was performed according to optimized conditions (298 bar, 44 °C and 0.4 kg CO2/h) for simultaneously maximized yield and antioxidant activity previously determined by Pavlic et al. (2018).
Chemical profile of SFE extract
GC–MS analysis was used for identification of volatile compounds in lipid extract obtained from sage herbal dust according to a method previously published by Pavlic et al. 2018. Agilent GC890N system coupled to mass spectrometer model Agilent MS 5759, equipped with HP-5MS column (30 m length, 0.25 mm inner diameter and 0.25 μm film thickness). Helium flow rate was 2 mL/min. Obtained extracts were dissolved in methylene chloride (about 1 mg/mL) and injected volume of solution was 5 μL with split ratio 30:1. Temperature conditions were as follows: injector temperature 250 C, detector temperature 300 °C; initial 60 °C with linear increase of 4 °C/min to 150 °C. Compounds were identified using the NIST 05 and Wiley 7n data base. GC-FID analysis was used for quantitative determination of dominant compounds in sage essential oil (γ-terpinene, ( +)-limonene, linalool, camphor, geraniol, eucalyptol, α-terpineol, methyl chavicol, carvacrol, α-pinene, β-pinene, eugenol and α-thujone). Standard compounds dissolved in methylene chloride at different concentrations (1–500 μg/mL) were used to obtain retention equations which are describing dependence of peak area on different concentration (R2 > 0.99). Qualitative results were expressed relative percentage, while content of major terpenoids was expressed as milligrams per gram of extract (mg/g).
Preparation of fresh cheese
Fresh cheeses were manufactured in laboratory conditions, from pasteurized milk (75 °C for 30 s) with 2.8% fat (Dairy»Mlekoprodukt« AD, Zrenjanin, Serbia). Kombucha inoculum used for cheese production was prepared by procedure described by Kanurić et al. (2011) Kombucha inoculum in concentration of 10% was applied for cheese production at 35 °C. Kombucha inoculum in concentration of 10% was applied due to previous results obtained for production of fermented dairy products, where it has been proven as the optimal concentration. Enzyme for coagulation (CHY-MAX®Powder Extra NB, Chr. Hansen A/S, Denmark) was added into milk in concentration of 0.005% at 35 °C after addition of starter culture. Fermentation was continued until pH = 4.5–4.6 were reached. After coagulation, the coagulum was cut, pasteurized by gently stirring at 60 °C for 5 min, quickly cooled to 25 °C and drained. Different form of sage herbal dust preparations were used: sage essential oil (EO), sage supercritical fluid extract (SFE) and sage ground (G). All sage preparations were added separately after production, at concentrations of 8 μL/100 g for SFE and EO and 0.417 g/100 g for ground sage.
Finally, depending on which sage herbal dust preparations were used, four samples were produced and labelled as follows: 1. Kombucha fresh cheese control sample (KC), 2. Kombucha fresh cheese with added ground (KG), 3. Kombucha fresh cheese with added essential oil (KEO), 4. Kombucha fresh cheese with added supercritical fluid extract (KSFE).
All samples were produced in triplets. All cheese samples were homogenized and packaged in cups with a lid and stored in the refrigerator at 4 °C. Samples were analysed at distinct periods of storage after 0 and 10 days.
Physico-chemical properties
Chemical quality of fresh cheeses after production and 10 days of storage period were analysed using the following methods: dry matter (ISO Standard No. 5534: 2004); milk fat according to Van Gulik method (ISO Standard No. 3343: 2008); total proteins (TP) (ISO Standard No. 8968-1: 2014); ash (A) (ISO Standard No. 5545: 2008); aw values were determined using LabSwift-aw device (Novasina AG, Switzerland).
pH value
pH values were determined at room temperature ( 25 °C) using a pH-meter (pH Spear, Eutech Instruments Oakton, England); pH-meter was previously calibrated using buffers with pH value of 4 and 7 (Khodashenas and Jouki 2020).
In vitro antioxidant activity
Sample preparation
Fresh cheese was prepared for the spectrophotometric analysis of total phenols content and antioxidant capacity (DPPH, FRAP and ABTS assays) according to the following procedure: Approximately 2 g of fresh cheese was weighted in centrifugal tube and 8 mL of extraction solvent was added while 70% ethanol was used as moderately polar extraction solvent in order to extract bioactives from cheese with sage preparations (KG, KEO and KSFE) added in cheese. Extraction was carried out by shaking at the vortex for 1 min, which was followed by sonication at ultrasonic bath (EUP540A, Euinstruments, France) for 1 min, 30 °C and 42 W/L ultrasonic power. The same procedure was repeated in try cycles and extracts were further centrifuged for 10 min at 3000 rpm (1100 g) and the supernatant was used removed, separately collected in glass sealed containers and used for further analysis.
Determination of total phenols content
The determination of the total phenols content in cheese samples was done by the Folin-Ciocalteu spectrophotometric method (Milani et al. 2020). Absorbance was measured at 750 nm using a 6300 Spectrophotometer, Jenway, UK and gallic acid was used as standard for preparation of calibration curve. Results are expressed as mg of gallic acid equivalents (GAE) per g of cheese sample (mg GAE/g).
DPPH test
The cheese’s ability to neutralize 2,2-diphenyl-1-picrylhydrazyl free radicals (DPPH) was measured using a modified method (Alipoorfard et al. 2020). Diluted cheese samples (0.1 mL) and methanolic soulution of DPPH reagent (2.9 mL) were added to a glass cuvette and incubated at room temperature for 60 min. Absorbance determinations were performed at 517 nm using a UV–VIS spectrophotometer (6300 Spectrophotometer, Jenway, UK). All measurements were performed in triplicate and results are expressed as μM of Trolox equivalent per g of cheese sample (μM TE/g).
FRAP test
The samples’ ability to reduce Fe3+ was determined by the FRAP test (Benzie and Strain 1996). FRAP reagent was freshly prepared from 300 mM acetate buffer (pH = 3.6), 10 mM 2,4,6-tris (2-pyridyl) -s triazine (TPZT), 40 mM HCl and 20 mM FeCl3 aqueous solution. Solutions were mixed in a ratio of 10: 1: 1 (v/v/v). Properly diluted cheese samples and FRAP reagent were mixed (0.1 mL + 2.9 mL) and incubated in the dark at 37 °C for 10 min. Absorbance was measured at 593 nm with UV–VIS spectrophotometer (6300 Spectrophotometer, Jenway, UK). Calibration was performed using freshly prepared Fe2+ (Fe2SO4) aqueous solutions (0–0.23 mM, R2 = 0.999). All experiments were performed in triplicate and results were expressed as μM of Fe2+ equivalents per g of cheese sample (μM Fe2+/g).
ABTS test
The ability to scavenging ABTS free radicals was determined using a modified spectrophotometric assay reported by Re et al. (1999). ABTS stock solution was freshly prepared from mixture (1:1, v/v) of 2.45 mM potassium persulfate aqueous solution and 7 mM ABTS (2,2'-azino-bis-(-3-ethylbenzothiazoline-6-sulfonic acid)-diammonium salt aqueous solution and left in the dark at room temperature for 16 h. A stock solution was diluted using 300 mM acetate buffer (pH = 3.6) to an absorbance of 0.70 (± 0.02). Properly diluted cheese samples and ABTS reagent were mixed (0.1 + 2.9 mL) and incubated at room temperature for 5 h in the dark. Absorbance was measured at 734 nm with UV–VIS spectrophotometer (6300 Spectrophotometer, Jenway, UK). Freshly prepared Trolox aqueous solutions (0–0.8 mM, R2 = 0.987) were used to obtain the calibration curve. Results were expressed as μM of Trolox equivalents per gram of cheese sample (μM TE/g).
Sensory analysis
Sensory analysis of the samples was performed by a panel of 10 consumers between 26 and 60 years old, selected from University staff members. All samples were stored at 4 °C until the moment of sensory evaluation. Prior to sensory evaluation, samples were opened, encoded, divided into 10 equal portions and presented simultaneously to each of the ten appraisers (Milani et al. 2020). Each sample was evaluated for flavour, colour, consistency appearance and taste. Sensory evaluation was performed with the five points system (1–5, from dislike extremely (1) to like extremely (5)). The sensory analysis was performed immediately after production (0) and after 10 days of refrigerated storage.
Colour measurement
The colour of fresh cheese is determined by photoelectric tristimulus colorimeter CHROMAMETER CR-400, Konica Minolta CRA33 extension. The colour differences of products surfaces (whiteness (L-), red/greenness (a-), and yellow/blueness (b-)) were measured. Prior to each measurement the tristimulus colorimeter was calibrated with a black and white ceramic plate (Milani et al. 2020). The software SpectroMagic NXPROQCver 2.0 was used for the results processing.
The evaluation of colour changing were analysed though the total colour difference ΔE, which was calculated between measurements of fresh cheese after production and cheese samples after 10 days of storage according to Equation [1]:
| 1 |
where – value of fresh cheese colour components after production. L*,a*,b*–value of cheese sample colour components after 10 days of storage.
Statistics
All analyses were performed in triplicate for all produced samples and values were expressed as average value. Univariate treatment of the data was performed by analysis of variance (ANOVA), using a”Statistica 13″ software. Duncan's multiple range test was performed to evaluate significant differences among the analysed parameters. Differences among analysed parameters of produced samples with addition of different sage preparations were considered statistically significant when P < 0.05.
Results and discussion
Physico-chemical characteristics
The acidification time of milk during the fresh cheese manufacturing by kombucha starter culture was 12 h and 45 min. pH value of sample was measured during the manufacturing process and stopped when the pH value reached 4.5. Kombucha starter has characteristic parabolic flow of acidification which is different from previously determined sigmoidal shape for kombucha fermented dairy products (Kanurić et al., 2011). The pH value of kombucha cheese declined more slowly in the beginning of the fermentation process and then dropped rapidly after 10 h. Usually, the pH value of fresh cheese is between 4.6 and 5.2. Slightly lower pH values obtained in studied samples could be related to activity of bioactive compounds originated from sage preparations, which is in accordance with previous research (Ribas et al. 2019). During the 10 day storage all samples had slight increase of pH values, but still within the expected limits.
Regardless which sage preparations were used, the kombucha fresh cheeses were not significantly different in dry matter and fat content, neither at the first nor at the last day of the storage period. These results were expected because sage was added after the cheese production. According to the performed statistical analysis, there were no statistically significant differences (P ≥ 0.05) in ash contents among different samples at the first day of the storage period (Table 1). Slight increase of ash was noticed in sample with added ground sage (KG), which could be consequence of the ash present in sage ground. Decrease of ash as main indicator of mineral content in milk products was observed during the storage in all samples expect the KG. Considering changes within the same sample during the storage, slight increase in protein content was observed for all samples. The aw value remain almost the same during the 10 days of storage in all samples. (Table 1 here).
Table 1.
Physico-chemical characteristics of kombucha fresh cheese’s
| Sample | KC | KEO | KSFE | KG | ||||
|---|---|---|---|---|---|---|---|---|
| Day of storage | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 |
| Dry matter % | 49.83 ± 0.2a | 52.57 ± 0.5a | 51.19 ± 0.2a | 49.57 ± 0.25a | 50.68 ± 0.15a | 50.99 ± 0.2a | 51.39 ± 0.09a | 50.23 ± 0.10a |
| Fat % | 26.75 ± 0.25a | 26.50 ± 0.00a | 26.75 ± 0.10a | 26.70 ± 0.15a | 26.65 ± 0.00a | 26.50 ± 0.02a | 26.70 ± 0.10a | 26.50 ± 0.10a |
| Ash % | 1.48 ± 0.08ab | 1.42 ± 0.04ab | 1.52 ± 0.06ab | 1.27 ± 0.02a | 1.58 ± 0.08ab | 1.55 ± 0.07ab | 1.53 ± 0.07ab | 1.71 ± 0.03b |
| Total protein % | 21.33 ± 0.91a | 22.54 ± 0.02a | 20.82 ± 0.04a | 22.49 ± 0.17a | 21.06 ± 0.08a | 22.58 ± 0.09a | 21.31 ± 0.21a | 22.54 ± 0.25a |
| pH | 4.93 ± 0.01a | 5.05 ± 0.00a | 4.92 ± 0.02a | 5.12 ± 0.00a | 4.88 ± 0.00a | 4.99 ± 0.00a | 4.89 ± 0.03a | 5.26 ± 0.00a |
| AW | 0.967 ± 0.002a | 0.965 ± 0.001a | 0.964 ± 0.00a | 0.964 ± 0.00a | 0.965 ± 0.00a | 0.964 ± 0.00a | 0.968 ± 0.00a | 0.962 ± 0.00a |
Different letters (a, b, c, d) in the same row represent statistically significant difference at significance level P < 0.05
Bioactives content and in vitro antioxidant activity
Different sage preparations were added as potential source of bioactive compounds in cheese samples. According to Pavlic et al. (2017), sage herbal dust (G) represents a good source of polyphenols, thus it was expected that its addition will release polyphenols in the cheese. On the other hand, sage essential oil (EO) and supercritical fluid extract (SFE) were used as source of lipophilic bioactives such as terpenoids. Chemical profile of EO was published elsewhere (Pavlic et al. 2018) and major terpenoids were present in following content: α-thujone (169.6 mg/g), camphor (177.4 mg/g), eucalyptol (103.4 mg/g), limonene (12.9 mg/g), α-pinene (16.6 mg/g), methyl-chavicol (2.7 mg/g) and carvacrol (2.4 mg/g). Chemical profile of terpenoids determined in sage extract obtained by SFE is given in Table 2. It could be observed that relative percentage of detected terpenoids was very similar with compounds detected in sage EO (Pavlic et al. 2018). Quantitative content of major terpenoids: α-thujone, camphor, eucalyptol, limonene, α-pinene, methyl-chavicol and carvacrol was 47.0, 56.6, 24.2, 2.2, < 1, < 1 and < 1 mg/g, respectively. It could be observed that content of major monoterpenes was lower in supercritical extract comparing to EO. However, previous study suggested that other lipids, particularly diterpenes, co-extracted by SFE could improve in vitro antioxidant activity (Pavlic et al. 2018). Furthermore, the same study suggested that extracts obtained by SFE could be produced in substantially higher yield comparing to EO obtained by hydrodistillation which could be particularly important from the techno-economical aspect of cheese production. (Tabale 2 here).
Table 2.
Relative percentage of detected terpenoids in sage extract obtained by SFE
| Compound | Retention time [min] | Relative percentage [%] |
|---|---|---|
| β-Myrcene | 4.460 | 0.07 |
| α-Terpinene | 5.077 | 0.09 |
| p-Cymene | 5.283 | 0.30 |
| Limonene | 5.341 | 0.51 |
| Eucalyptol | 5.439 | 6.04 |
| γ-Terpinene | 6.106 | 0.07 |
| cis-Linalool oxide | 6.566 | 0.05 |
| Dehydro-p-Cymene | 7.007 | 0.09 |
| α-Tujone | 7.497 | 9.23 |
| β-Tujone | 7.781 | 2.83 |
| Camphor | 8.653 | 13.66 |
| Borneol | 9.466 | 4.01 |
| Menthol | 9.631 | 0.08 |
| 4-Terpineol | 9.682 | 0.18 |
| n.i.* | 10.763 | 0.44 |
| Carvotanacetone | 11.483 | 0.05 |
| Trans-Geraniol | 12.208 | 0.06 |
| Bornyl acetate | 12.861 | 1.40 |
| Camphene | 13.111 | 0.11 |
| Thymol | 14.067 | 0.40 |
| Carvacrol | 14.355 | 0.14 |
| n.i | 15.329 | 2.02 |
| n.i | 15.445 | 2.30 |
| γ-Caryophyllene | 16.517 | 0.09 |
| n.i | 16.682 | 0.12 |
| trans-Caryophyllene | 16.914 | 1.70 |
| Aromadendrene | 17.503 | 0.13 |
| n.i | 17.791 | 0.78 |
| α-Humulene | 17.983 | 3.03 |
| n.i | 18.236 | 1.20 |
| Ledene | 19.222 | 0.41 |
| Caryophyllene oxide | 21.914 | 0.63 |
| Viridiflorol | 22.386 | 16.18 |
| Ledole | 22.586 | 0.13 |
| Humulene oxide | 22.718 | 0.38 |
| n.i | 23.542 | 3.49 |
| iso-Geraniol | 24.244 | 1.56 |
| Caryophyllene | 24.69 | 0.70 |
| n.i | 28.452 | 0.33 |
| n.i | 30.178 | 0.77 |
| n.i | 30.402 | 0.36 |
| n.i | 32.447 | 0.82 |
| Epirosmanol | 34.136 | 20.47 |
| n.i | 34.814 | 1.49 |
| Phytol | 35.306 | 0.24 |
| Ferruginol | 40.253 | 0.91 |
| Total | 100 |
*Not identified
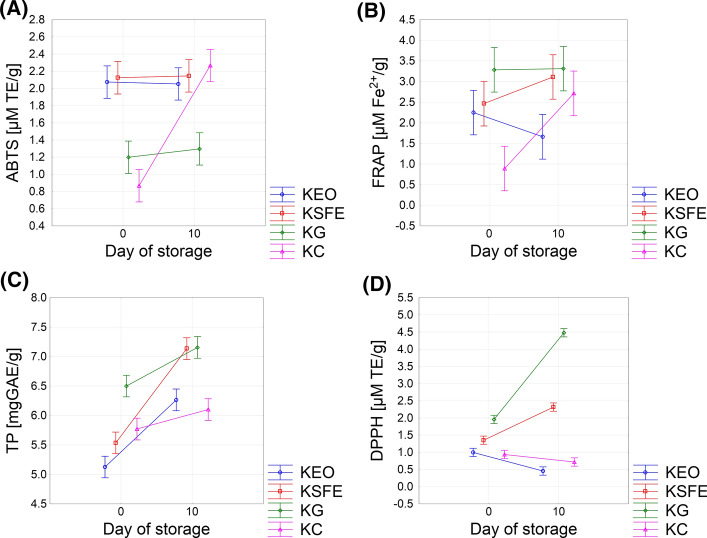
In the present investigation, the commonly accepted in vitro assays: DPPH, FRAP and ABTS were used for the evaluation of antioxidant activity of produced kombucha fresh cheeses. The total phenolic content was also determined since it was assumed that polyphenols could be released from the sage herbal dust. The results of these analyses are presented in the Fig. 1.
Fig. 1.
The changes of antoxidative activity during the storage period in: a ABTS b FRAP c TP; d DPPH
During the fermentation, there is an increase in polyphenols in comparison to raw milk and kombucha inoculum used for cheese production (total phenols content of milk and kombucha were 1.20 ± 0.01 and 0.125 ± 0.09 mg GAE/g, respectively). These results are in accordance with the observations of Jakubczyk et al. (2020), who found an increase in polyphenols during kombucha fermentation. Our results are also in accordance with previous research that determined up to three times increase of total phenol content during milk fermentation by kombucha inoculum (Ozyurt, 2020). Owing to the presence of total phenols in all samples, cheeses supplemented with ground and SFE extract did not show higher contents of total phenols than control kombucha fresh cheese (KC) after the production. This result is in accordance with the study performed by Deolindo et al. (2019) who also did not find increase of total polyphenols by addition of grape seed extracts in Petite Suisse cheese. With the progress of storage period, total phenols content increased in all prepared cheeses. The fresh cheeses with addition of different type of sage extracts show significant (P < 0.05) variations in content of total phenols. Sample with essential oil showed significantly (P < 0.05) lower value compared to other samples and it was not significantly different from the control sample (Fig. 1c).
The antioxidative activity towards DPPH radicals of kombucha fresh cheese with addition of sage SFE was higher compared to the sample fortified with EO obtained by hydrodistillation (Fig. 1d). This could be explained by higher antioxidative capacity of SFE than EO as well as higher content of diterpene polyphenols in sage extracts obtained by SFE (Pavlic et al. 2018). Therefore, KEO sample showed lower DPPH radical scavenging activity compared to KSFE (0.4663 and 0.987 μM TE/g of extract, respectively) and it was not significantly different from the control sample (KC), confirming that phenolic compounds are likely to contribute to the DPPH radical scavenging activity. The maximum DPPH value was shown in cheese with addition of ground sage which is in correlation with their total phenolic content.
All analysed samples showed significant scavenging ability against ABTS radical (Fig. 1a). Although KG sample showed the highest effect on DPPH free radical scavenging activity, they had the lowest values of ABTS activity (1.1197 ± 0.2038 and 1.286 ± 0.0657 μM TE/g, KG0 and KG10 respectively). It could be estimated that the phenolic compounds present in the extracts are not only antioxidative active compound. There were no significant differences in activity towards ABTS between KC and KG samples, as well as between KSFE and KEO samples, after production. The antioxidant activity of the kombucha control cheese (KC) can be attributed to the presence of bioactive peptides as well as effects of kombucha inoculum (Morales 2020). The production of bioactive peptides with antioxidative activity during milk fermentation should be further investigated to investigate effects of kombucha starter culture on antioxidative capacity of fermented milk beverages. The TEAC values of the analysed cheeses ranged from 1.22 to 2.20 mg TEAC/g depending on the sample. According to the obtained results, it is evident that the TEAC values of all samples, except the KG, obtained by ABTS assay were higher than those obtained by DPPH assay. It was observed that the phenolic content in the extracts showed much higher correlation with DPPH radical scavenging activity than with the reducing power measured by ABTS assay (r = 0.6986 and 0.179 DPPH and ABTS, respectively).
The difference between antioxidative effect determined by DPPH and ABTS assays could be partially due to the previously reported disadvantage of DPPH assay if used for hydrophilic antioxidants, such as protein hydrolysates (Tang et al. 2010). This is mostly due to the fact that the DPPH can be dissolved only in organic media (especially in alcoholic media), which can lead to precipitation of proteins with antioxidative activity, which could not be measured. Contrary, ABTS can be solubilised in aqueous and organic media; therefore radical scavenging activity of both hydrophilic and lipophilic compounds can be measured (Tang et al. 2010). Our results also confirmed this finding.
Freshly prepared and 10 days stored kombucha cheeses supplemented with different types of sage preparations presented significantly higher FRAP values than the control sample (KC). (Fig. 1c). The maximum value of FRAP (3.3302 μM Fe2+/g) was recorded in KG sample.
Antioxidative effect of different cheeses could be potentially explained by different mechanisms as well as different synergistic effects of terpenoids and other polyphenols present in sage preparations with milk peptides realised by protein hydrolisation. Yildirim-Elikoglu et al. 2021 pointed out the remarkable importance of the interactions between milk coagulation enzymes and phenols from green tea extracts (GTE) for functional properties of dairy products. It was found the negative effect of GTE addition on milk coagulation properties. On the other hand, starter culture through its metabolic activity, could also contribute to the antioxidative activity of produced samples. The capability of kombucha fermented milk beverages to scavenge different free radicals such as hydroxyl, superoxide anions and reducing power were anteriorly attributed to the phenolic, flavonoids, vitamin C and vitamins B presented in the kombucha inoculums was reported in previous researches (Morales 2020). Notwithstanding, the contribution of some other compounds, primarily bioactive peptides, should be further investigatedin more detail.
The sensory analysis
All samples of fresh cheeses fortified with sage preparations had typical mild milky odour with specific pleasant refreshing taste and conspicuous flavour. All analysed samples had the same score of sensory evaluation after production. During the storage period overall sensory characteristics were reduced in all samples (Table 3). The most intensive changes were observed for consistency and taste. A decrease score of consistency was described by comments such as untypical, irregular, grainy while taste changes were describe as too sour.
Table 3.
Sensory characteristics of fresh cheese samples
| Characteristics | KK | KEO | KSCF | KG | ||||
|---|---|---|---|---|---|---|---|---|
| 0 day | 10 day | 0 day | 10 day | 0 day | 10 day | 0 day | 10 day | |
| Appearance | 4a | 3.5b | 4a | 3.5b | 4a | 3.5b | 4a | 3.5b |
| Colour | 4a | 3.5b | 4a | 3.5b | 4a | 3.5b | 4a | 3.5b |
| Consistency | 4a | 3.5b | 4a | 3.5b | 4a | 3.5b | 4a | 3.5b |
| Flavour | 4a | 3.5b | 4a | 3.5b | 4a | 3.5b | 4a | 3.5b |
| Taste | 4a | 3.5b | 4a | 3.5b | 4a | 3.5b | 4a | 3.5b |
| Total | 20a | 17.5b | 20a | 17.5b | 20a | 17.5b | 20a | 17.5b |
Different letters (a, b, c, d) in the same row represent statistically significant difference at significance level P < 0.05
Nevertheless, this results indicated that kombucha inoculum with addition of sage dust extracts could ensure manufacturing of fresh cheese with satisfactory sensory characteristics.
At the end of the storage period all samples had the same sensory scores. Therefore, the final acceptance of the kombucha fresh cheese with addition of sage preparations will depend on consumers’ requirements.
Colour assay
The cheeses colours were measured by means of the CIE Lab colour system. Photoelectric tristimulus colorimeter quantifies the colour in terms of tristimulus values (L, a, b), which are calculated in a CIE L, a, and b colour spaces.
The whiteness values (L) of KC and KG samples kombucha cheese were not significantly changed after 10 days of storage but it significantly decreased in cheese samples with addition of SFE and EO. The samples KSFE and KEO also had the significant higher L value after production compared with KC and KG samples (Table 4).
Table 4.
Effect of sage addition on the colour of fresh cheese during storage assayed by CIE LAB system
| Sample | L | a | b | ∆E | |||
|---|---|---|---|---|---|---|---|
| 1 day | 10 day | 1 day | 10 day | 1 day | 10 day | 1 day | |
| KC | 85.55a | 84.993a | – 1.183a | – 1.095a | 18.078A | 19.810a | 1.8167a |
| KEO | 87.672B | 84.597b | – 0.952b | – 0.911b | 17.733B | 20.418b | 4.0825b |
| KSFE | 88.037C | 86.123c | – 0.955C | – 1.007c | 18.242C | 20.802c | 3.1968c |
| KG | 82.247d | 81.583d | – 1.088d | – 1.073d | 16.925D | 19.508d | 2.6670d |
Different letters (a, b, c, d) in the same column represent statistically significant difference (P < 0.05)
Different letter case (A,a; B,b; C,c; D,d) in the same row represent statistically significant difference (P < 0.05) between the parameters values during the storage
Furthermore, samples KC and KG had the lowest value of red/greenness coefficient a. Storage time did not have significant effect on the a values of the fresh cheeses. The value of coefficient b significantly increased during the storage period. The samples KSFE and KEO had higher value of b coefficient after 10 days. In addition, these samples also had the highest changes of ΔE (4.0825 and 3.1968 respectively) while the least change of ΔE was calculated in control kombucha fresh cheese (KC—1.8167). (Table 4 here).
Conclusion
Considering obtained results, the addition of different preparations of sage in cheeses contributed with higher content of phenolic compounds and antioxidant activity of samples improving their functional potential. Significant ABTS, FRAP and DPPH antioxidative activity was determined in all kombucha fresh cheeses, which increased during the storage. The differences in all measured colour ‘s parameters among the four groups of fresh cheeses were observed. The control and all fortified cheeses had overall high sensory quality. Hence, as a final conclusion of this research, the successful production of kombucha fresh cheese with non-conventional starter culture and its enrichment with sage dust fraction generated as by-product in filter tea processing, represent a valorisation of this by-product through convenient new dairy product which satisfies consumer`s interest in functional food.
Acknowledgements
This work was supported by Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant Number 451-03-68/2020-14/ 200134).
Authors' contributions
DV Conceptualization, Formal analysis, Writing—Original Draft. MI Project administration, Supervision, Writing—Review and Editing. VV Methodology, Validation, Writing—Review and Editing. BP Methodology, Formal analysis, Writing—review and editing, KK Investigation Resources, Writing – Review and Editing. MB Investigation, Formal analysis. ZZ Supervision, Writing—Review and Editing.
Declarations
Conflicts of interest
All authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dajana Vukić, Email: dajanavukic@uns.ac.rs.
Branimir Pavlić, Email: bpavlic@uns.ac.rs.
Vladimir Vukić, Email: vukicv@uns.ac.rs.
Mirela Iličić, Email: panim@uns.ac.rs.
Katarina Kanurić, Email: stay@uns.ac.rs.
Maja Bjekić, Email: maja.bjekic@yahoo.com.
Zoran Zeković, Email: zzekovic@tf.uns.ac.rs.
References
- Alipoorfard F, Jouki M, Tavakolipour H. Application of sodium chloride and quince seed gum pretreatments to prevent enzymatic browning, loss of texture and antioxidant activity of freeze dried pear slices. J Food Sci Technol. 2020;57(9):3165–3175. doi: 10.1007/s13197-020-04265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barukcic I, Scetar M, Marasovic I, Jakopovic KL, Galic K, Bozanic R. Evaluation of quality parameters and shelf life of fresh cheese packed under modified atmosphere. J Food Sci Tech MYS. 2020;57:2722–2731. doi: 10.1007/s13197-020-04308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Deolindo CTP, Monteiro PI, Santos JS, Cruz AG, da Silva MC, Granato D (2019) Phenolic-rich Petit Suisse cheese manufactured with organic Bordeaux grape juice, skin, and seed extract: Technological, sensory, and functional properties. Lwt-Food Science and Technology 115.
- Haji Ghafarloo M, Jouki M, Tabari M. Production and characterization of synbiotic Doogh, a yogurt-based Iranian drink by gum arabic, ginger extract and B. bifidum. J Food Sci Technol. 2020;57(3):1158–1166. doi: 10.1007/s13197-019-04151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hati S, Vij S, Mandal S, Malik RK, Kumari V, Khetra Y. α-Galactosidase activity and oligosaccharides utilization by lactobacilli during fermentation of soy milk. J Food Process Preserv. 2014;38:1065–1071. doi: 10.1111/jfpp.12063. [DOI] [Google Scholar]
- Hrnjez D, Vukić V, Milanović S, Iličić M, Kanurić K, Torbica A, Tomić J. Nutritive aspects of fermented dairy products obtained by kombucha application. Agro Food Ind Hi Tech. 2014;25:70–73. [Google Scholar]
- International Organization for Standardization (2004) Cheese and processed cheese - determination of the total solid content (ISO Standard No. 5534: 2004). https://www.iso.org/standard/35249.html
- International Organization for Standardization (2008) Milk - Determination of fat content - Van Gulik method (ISO Standard No. 3343: 2008). https://www.iso.org/standard/46336.html
- International Organization for Standardization (2014) Milk and Milk products - Determination of nitrogen content - Part 1: Kjedahl principle and crude protein calculation (ISO Standard No. 8968-1: 2014)
- International Organization for Standardization (2008) Rennet caseins and caseinates - Determnation of ash (Reference method) (ISO Standard No. 5545: 2008). https://www.iso.org/standard/46333.html
- Jakubczyk K, Kaldunska J, Kochman J, Janda K (2020) Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 9. [DOI] [PMC free article] [PubMed]
- Kanuric K, Milanovic S, Ikonic B, Loncar E, Ilicic M, Vukic V, Vukic D. Kinetics of lactose fermentation in milk with kombucha starter. J Food Drug Anal. 2018;26:1229–1234. doi: 10.1016/j.jfda.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanurić KG, Hrnjez DV, Ranogajec MI, Milanović SD, Iliĉić MD, Vukić VR, Milanović ML (2011) The effect of fermentation temperature on the functional dairy product quality. Acta Period. Technol.
- Khodashenas M, Jouki M. Optimization of stabilized probiotic Doogh formulation by edible gums and response surface methodology: assessment of stability, viability and organoleptic attributes. J Food Sci Technol. 2020;57:3201–3210. doi: 10.1007/s13197-020-04351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorshidian N, Yousefi M, Khanniri E, Mortazavian AM. Potential application of essential oils as antimicrobial preservatives in cheese. Innov Food Sci Emerg Technol. 2018;45:62–72. doi: 10.1016/j.ifset.2017.09.020. [DOI] [Google Scholar]
- Lorenzo JM, Sineiro J, Amado IR, Franco D. Influence of natural extracts on the shelf life of modified atmosphere-packaged pork patties. Meat Sci. 2014;96:526–534. doi: 10.1016/j.meatsci.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Marsh AJ, O'Sullivan O, Hill C, Ross RP, Cotter PD. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014;38:171–178. doi: 10.1016/j.fm.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Milani A, Jouki M, Rabbani M. Production and characterization of freeze-dried banana slices pretreated with ascorbic acid and quince seed mucilage: physical and functional properties. Food Sci Nutr. 2020;8:3768–3776. doi: 10.1002/fsn3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales D. Biological activities of kombucha beverages: the need of clinical evidence. Trends Food Sci Technol. 2020;105:323–333. doi: 10.1016/j.tifs.2020.09.025. [DOI] [Google Scholar]
- Ozyurt H. Changes in the content of total polyphenols and the antioxidant activity of different beverages obtained by Kombucha ‘tea fungus’. Int J Agric Environ Food Sci. 2020;4(3):255–261. [Google Scholar]
- Pavlic B, Vidovic S, Vladic J, Radosavljevic R, Cindric M, Zekovic Z. Subcritical water extraction of sage (Salvia officinalis L.) by-products-process optimization by response surface methodology. J Supercritical Fluids. 2016;116:36–45. doi: 10.1016/j.supflu.2016.04.005. [DOI] [Google Scholar]
- Pavlic B, Teslic N, Vidakovic A, Vidovic S, Velicanski A, Versari A, Radosavljevic R, Zs Z. Sage processing from by-product to high quality powder: I. Bioactive Potential Ind Crops Prod. 2017;107:81–89. doi: 10.1016/j.indcrop.2017.05.031. [DOI] [Google Scholar]
- Pavlic B, Bera O, Teslic N, Vidovic S, Parpinello G, Zekovic Z. Chemical profile and antioxidant activity of sage herbal dust extracts obtained by supercritical fluid extraction. Ind Crops Prod. 2018;120:305–312. doi: 10.1016/j.indcrop.2018.04.044. [DOI] [Google Scholar]
- Pekić B, Zeković Z, Petrović L, Tolić A. Behavior of (–)-α-bisabolol and (–)-α-bisabololoxides A and B in camomile flower extraction with supercritical carbon dioxide. J Sep Sci. 1995;30:3567–3576. doi: 10.1080/01496399508015137. [DOI] [Google Scholar]
- Popovic R, Milanovic S, Ilicic M, Ranogajec M, Kanuric K, Vukic V, Hrnjez D. Nutritive characteristics and market prospects of kombucha fermented milk beverages. Agro Food Ind Hi Tech. 2016;27:48–51. [Google Scholar]
- Ribas JCR, Matumoto-Pintro PT, Vital ACP, Saraiva BR, Anjo FA, Alves RLB, Santos NW, Machado E, Agustinho BC, Zeoula LMs, Influence of basil (Ocimum basilicum Lamiaceae) addition on functional, technological and sensorial characteristics of fresh cheeses made with organic buffalo milk. J Food Sci Tech MYS. 2019;56:5214–5224. doi: 10.1007/s13197-019-03990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariati Z, Jouki M, Rafiei F. Flavored functional drinking yogurt (Doogh) formulated with Lactobacillus plantarum LS5, cress seed gum, and coriander leaves extract. Food Sci Nutr. 2020;8:894–902. doi: 10.1002/fsn3.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypczak K, Gustaw W, Szwajgier D, Fornal E, Wasko A. kappa-Casein as a source of short-chain bioactive peptides generated by Lactobacillus helveticus. J Food Sci Tech MYS. 2017;54:3679–3688. doi: 10.1007/s13197-017-2830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Xueyan, He Zhiyong, Dai Yanfeng, Xiong Youling L., Xie Mingyong, Chen Jie. Peptide Fractionation and Free Radical Scavenging Activity of Zein Hydrolysate. Journal of Agricultural and Food Chemistry. 2010;58(1):587–593. doi: 10.1021/jf9028656. [DOI] [PubMed] [Google Scholar]
- Vukic VR, Hrnjez DV, Kanuric KG, Milanovic SD, Iličic MD, Torbica AM, Tomic JM. The effect of kombucha starter culture on the gelation process, microstructure and rheological properties during milk fermentation. J Texture Stud. 2014;45:261–273. doi: 10.1111/jtxs.12071. [DOI] [Google Scholar]
- Vukic DV, Vukic VR, Milanovic SD, Ilicic MD, Kanuric KG. Modeling of rheological characteristics of the fermented dairy products obtained by novel and traditional starter cultures. J Food Sci Tech MYS. 2018;55:2180–2188. doi: 10.1007/s13197-018-3135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim-Elikoglu S, Vural H, Erdem YK (2021) Effect of phenolic compounds on the activity of proteolytic enzymes during rennet induced coagulation of milk and ripening of miniature cheese. LWT - Food Sci Technol 136.
- Zekovic Z, Pintac D, Majkic T, Vidovic S, Mimica-Dukic N, Teslic N, Versari A, Pavlic B. Utilization of sage by-products as raw material for antioxidants recovery ultrasound versus microwave-assisted extraction. Ind Crops Prod. 2017;99:49–59. doi: 10.1016/j.indcrop.2017.01.028. [DOI] [Google Scholar]