Abstract
Patient-reported outcome measures (PROMs) are questionnaires that assess health outcomes meaningful to the patient. PROMs have multiple applications, such as supporting clinicians’ decision-making for patient care, understanding the impact of disease on patient functioning, and evaluating the efficacy of therapeutics. Though PROMs were developed for various eye conditions, no PROM was tailored to pediatric patients with inherited retinal disease (IRD). Hence, a literature search was conducted using MEDLINE and Embase to identify PROMs potentially relevant to this patient population. This review evaluated selected pediatric PROMs against the US Food and Drug Administration (FDA) guidelines and found restricted use in the context of IRD. As there is a need for PROMs tailored to pediatric patients with IRD, we provide a perspective on applying the International Society for Pharmacoeconomics and Outcomes Research and FDA standards on the development of PROMs specific to IRD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-022-00514-x.
Keywords: Patient-reported outcome, Retinal diseases, Ophthalmology, Pediatrics, Clinical trial, Genetic therapy, Vision impairment
Plain Language Summary
Inherited retinal diseases refer to a group of genetic conditions that affect the eye’s light-sensing cells and lead to vision loss. When a patient undergoes an eye assessment, the measures used are technical (e.g., visual acuity, visual field) and do not routinely address the patient’s experience. It is increasingly evident that the technical tools used do not really reflect how patients’ vision affects their daily lives. Questionnaires designed to assess how a condition impacts a daily activity are referred to as patient-reported outcome measures. The perspective of the impact of a condition on daily activities differs between adults and children. These tools are being created to evaluate health outcomes important to the patient on the basis of their condition and age. This is especially important when determining the value of therapies from the patient perspective. To date, no such questionnaire has been designed for pediatric patients with inherited retinal disease, an important cause of blindness. We explored the literature to evaluate existing pediatric vision tools and found that those could not be used to fill this gap. Given that we found a need to develop questionnaires tailored to pediatric patients with IRD, we also provide insight into how such a tool can be created for this population.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-022-00514-x.
Key Summary Points
| There is an increasing need to assess meaningful health outcomes in pediatric patients with inherited retinal disease (IRD), especially with the development of gene therapies for these conditions. |
| There are no published patient-reported outcome measures (PROMs) that have been tailored to patients with IRD. |
| Evaluation of “general pediatric vision” PROMs against the US Food and Drug Administration (FDA) guidelines has shown low applicability for pediatric patients with IRD. |
| A need to develop PROMs tailored to pediatric patients with IRD has been identified. |
Introduction
Inherited retinal diseases (IRDs) are a group of hereditary diseases of the retina that can result in progressive photoreceptor degeneration, subsequent severe vision loss, and blindness in children and adults. IRDs underlie an expressive economic and psychosocial burden. In 2019 alone, IRDs contributed approximately US $14,685.1–37,003.8 million in economic and well-being costs in the USA and Canada [1]. After deciphering the genetic basis of many IRDs, gene-specific therapies are being developed to restore some sight and slow or halt disease progression. A recent ocular gene replacement therapy, voretigene neparvovec-rzyl, was the first to be approved by the US Food and Drug Administration (FDA), European Medicines Agency, and Health Canada for patients with RPE65-Leber congenital amaurosis (LCA), an early and severe form of IRD. Since then, numerous clinical trials for other IRDs therapies have been underway [2]. In principle, novel interventions should aim to minimize the impact of disability on daily living, which can only be measured by patient-reported outcome measures (PROMs). This could not be thoroughly assessed in patients undergoing these trials as IRD-specific tools were not available.
Patient-reported outcome measures are self-reported questionnaires measuring health outcomes from the patient perspective. The FDA has defined specific features of PROM development [3, 4]. A validated PROM should be created by incorporating an in-depth qualitative analysis of patient input (i.e., interviews or focus groups) and thorough quantitative psychometric analysis to establish the measure’s validity, interpretability, reliability, and ability to detect a change in the target population. When coupled with other clinical tests, well-designed PROMs can holistically measure a therapeutic impact on a patient’s daily life [4–6].
To date, many clinical trials for voretigene neparvovec-rzyl and other ophthalmic therapies have used the National Eye Institute-Visual Function Questionnaire-25 (VFQ-25) as a secondary outcome measure, among others. The VFQ-25 is a widely used PROM, stated to assess vision function [7]; however, it is not IRD-specific. That said, until recently IRD-specific PROMs did not exist. The Michigan Retinal Degeneration Questionnaire (MRDQ) and Michigan Vision-related Anxiety Questionnaire (MVAQ) were recently developed adult IRD-tailored PROMs that assess vision ability and associated psychosocial health, respectively [8, 9]. The MRDQ generates an ability score from adult patient-reported visual function in domains representative of physiological visual function pathways and thereby can record a patient’s perspective on emerging therapeutics for these domains by using theta scores (item response theory) derived using a graded response model. The MRDQ was originally validated in adults, limiting its current use in the pediatric population as infants, children, and adolescents are at vastly different developmental stages than adults [10]. A patient-centric PROM requires consideration of the characteristics and perspectives of the age group. PROMs for pediatric patients should reflect their language level and the relevancy of experiences encapsulated by the questions to their age group as well as their condition.
There is an increasing need to assess the effect of therapies for pediatric patients with IRDs using PROMs to better understand changes observed from the patient perspective. This present review aims to evaluate if existing PROMs for pediatric patients with vision loss can be used for the IRD population and provide a perspective for developing PROMs for pediatric patients.
Methods
Search Methods for Identifying Studies
Patient-reported outcome measures (PROMs) tailored to pediatric ophthalmic conditions were identified in two ways. First, this review included the ophthalmic PROMs described in Tadic et al.’s [11] systematic review. Second, additional PROMs were identified from MEDLINE and Embase databases by adapting Tadic et al.’s [11] search strategy to cover PROMs developed after 2013 that could be potentially used for inherited retinal disease (IRD) and its substituent conditions. The strategy was verified by consultation with an information scientist (Table S1, Supplementary Material). The search was restricted till 2013 to avoid overlap with Tadic and colleagues’ search [11]. The citations were exported to the Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia; http://www.covidence.org) to de-duplicate and complete multilevel screening [12].
Study Selection
Studies were selected after two levels of screening (Fig. 1). First, title/abstract screening was conducted to remove papers if they were (a) focused on non-ophthalmic diseases, on a non-IRD ophthalmic disease, or not focused on visual impairment as a whole; (b) were gray literature, including conference abstracts, non-peer-reviewed text, or records only on the preliminary phases of PROM development; (c) PROM development or validation was not the primary objective or non-primary research, including reviews; (d) PROMs were not validated or made in English (transcultural adaptions into English were kept); and (e) participants were over 18 years of age. Then full-text screening was done with the title/abstract eligibility criteria, additionally filtering out studies included in Tadic et al.’s [11] systematic review.
Fig. 1.
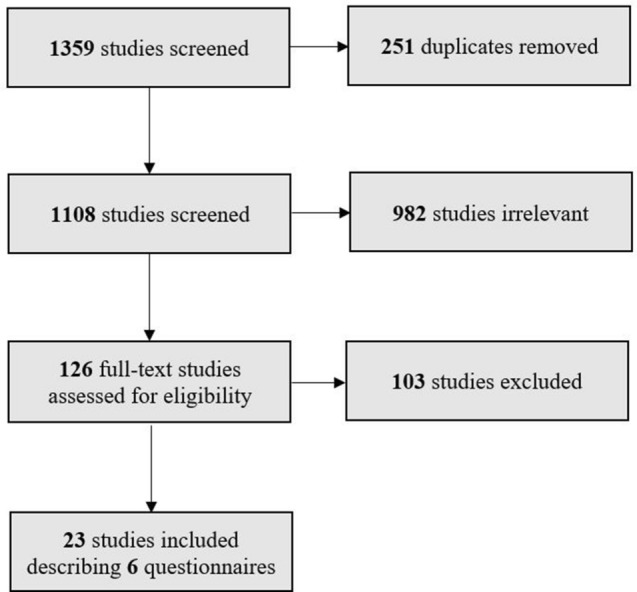
Flow diagram of literature screening
Data Extraction and Descriptive Analysis
Data extracted from each study included study description (i.e., age of participants, country, year, eligibility criteria, number of patients with IRD for each phase of PROM development), version of PROM (i.e., original or revised), and PROM description (i.e., number of items, questionnaire respondent, purpose, and subscale(s)/domains). The study characteristics are summarized in Table 1. Criteria set by the US FDA were used to evaluate each PROM’s applicability to pediatric patients with IRD (summarized in Table 2). A validated PROM has undergone an in-depth qualitative analysis of patient input (i.e., interviews or focus groups) and thorough quantitative psychometric analysis to establish the measure’s validity, interpretability, reliability, and ability to detect a change in the target population [4, 13]. As such, a representative sample is required for PROM development [3]. The study characteristics were qualitatively explored as part of the synopsis of each paper to identify the merits and limitations of the PROM’s applicability in pediatric patients with IRD.
Table 1.
Characteristics of included ophthalmic patient-reported outcome measures for pediatric patients with vision impairment
| PROMs | Country and year of publication(s) | Original or reviseda | Number of items (age range, respondent) | Number (%) of patients with IRD in sample | Purpose/construct | Subscale(s)/domain(s) |
|---|---|---|---|---|---|---|
| Children’s Visual Function Questionnaire (CVFQ) [14, 15] | USA, 2004–2007 | Original | 35 (0–3 years, self-report); 40 (3–7 years, self-report) | 29*/403 (7%) for pilot testing [14]; 58*/397 (15%) in < 3 years and 80*/376 (21%) in ≥ 3 years for validation [14]; 0*/194 were IRD for further validation [15] | To measure vision-specific QoL in children with VI | General health; general vision; competence; personality; family impact; treatment |
| Cardiff Visual Ability Questionnaire for Children (CVAQC) [16] | UK, 2010 | Original | 25 (5–18 years, self-report) | Not given | To assess VA in children and young people VI | Education; near vision; distance vision; getting around; social interaction; entertainment; sports |
| Impact of Visual Impairment for Children (IVI_C) [17, 18] | Australia, 2008–2011 | Original | 23 (8–18 years, self-report) | Not given | To measure the effect of impaired vision on QoL in children with VI | No subscale(s) reported |
| Pediatric Eye Questionnaires (PedEyeQ) [20–24] | USA, 2017–2021 | Original | 40 (5–11 years, self-report); 39 (12–17 years, self-report); 29 (0–4 years, parent-proxy); 39 (5–11 years, parent-proxy); 42 (12–17 years, parent-proxy); 35 (parent self-report) | 12**/124 (9.6%) in 0–4 years (parent-proxy), 12**/117 (10.3%) in 5–11 years (self-report and parent-proxy), 13**/87 (14.9%) in 12–17 years (self-report and parent-proxy) for content development [20]; 55*/444 (12.4%) in 0–17 years (self-report and parent-proxy) for pilot testing [21]; 5/45 (11.1%) in 0–4 years (parent-proxy), 5/40 (12.5%) in 5–11 years (self-report and parent-proxy), 3/22 (13.4%) in 12–17 years (self-report and parent-proxy) for validation [22] | To assess eye-related quality of life in VI children | (5–11 years and 12–17 years, self-report): functional vision; bothered by eyes/vision; social; frustration/worry (0–4 years, parent-proxy): functional vision; bothered by eyes/vision; social (5–11 years and 12–17 years, parent-proxy): functional vision; bothered by eyes/vision; social; frustration/worry; eye care (parent self-report): impact on parent/family; worry regarding child’s eye condition; worry regarding child’s self-perception and interactions; worry regarding child’s visual function |
| Vision-related Quality of Life of Children (VQoL_C) and Vision-related Quality of Life of Children (VQoL_YP) [29] | UK, 2011–2021 | Revised | 20 (8–12 years, self-report); 22 (13–17 years, self-report) | 7*/12 (75%) in 7–9 years, 12*/17 (70.6%) in 16–19 years for content development [29]; 8*/12 (66.7%) in 7–10 years, 9*/16 (56.3%) in 13–18 years for pretesting [29]; 15*/26 (57.7%) in 8–12 years, 18*/23 (78.3%) in 13–17 years for pilot testing [29]; 56*/87 (64.4%) in 7–13 years, 50*/73 (68.5%) in 13–18 years for validation [29] | To measure vision-specific QoL in children with VI | No subscale(s) reported |
| Functional Vision Questionnaire for Children (FVQ_C) and Functional Vision Questionnaire for Young People (FYQ_YP) [32] | UK, 2011–2021 | Revised | 28 (8–12 years, self-report); 38 (13–18 years, self-report) | 9*/12 (75%) in 6–9 years, 12*/17 (70.6%) in 16–19 years for content development [32]; 8*/12 (66.7%) in 7–10 years, 9*/16 (56.3%) in 13–18 years for pretesting [32]; 71*/113 (62.8%) in 7–13 years, 68*/96 (70.8%) in 13–18 years for pilot testing and validation [32] | To assess the functional impact of VI on activities of daily living in children and young people | No subscale(s) reported |
PROM patient-reported outcome measure, QoL quality of life, VI vision impairment
* = IRD + retinal disease; ** = IRD + retinal disease + optic neuropathy
aRevised = New edition of PROM released since initial validation
Table 2.
Summary of criteria to evaluate applicability of vision impairment PROMs based on FDA guidelines
| Criteria | Definition |
|---|---|
| Validity |
The capacity of the PROM to describe the trait it intends to measure e.g., content-related, criterion-related, predictive |
| Reliability |
The consistency of the measurements taken by the PROM “How the person functions” in regards to their vision e.g., test–retest, internal consistency, inter-interviewer reproducibility |
| Sensitivity |
The instruments’ ability to detect a change in the population e.g., effect size, standard error |
| Representative populationa |
A PROM should be developed with the target population for the target population In the case of IRD, there are many subtypes and so a representative sample for PROM development consisting of IRD’s three electroretinogram subtypes (rod-cone dystrophy, cone/cone-rod dystrophy, and macular dystrophy) |
PROM patient-reported outcome measure
aAdditional criteria set out by the US Food and Drug Administration (FDA)
This article is based on previously published studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Study Selection
Many PROMs are available for pediatric patients with different ophthalmic conditions [11]. There were no IRD-specific PROMs identified for pediatric patients; however, the PROMs described is this section have been developed for general visual impairment and may have included some patients with IRD in the making. That said, in the absence of IRD-specific PROMs, we focused on available tested and validated PROMs tailored to visual impairment (VI) in pediatric patients (Table 1).
Children’s Visual Function Questionnaire (CVFQ) [14, 15]
First developed by Felius, Birch, and colleagues in 2004, the Children’s Visual Function Questionnaire (CVFQ) covers all concerns young children (at most 7 years of age) have related to their vision impairment [14, 15]. The initial PROM combined several behavioral and visual function questionnaires alongside clinician input [14]. After pilot testing in patients, it was divided into two measures for two age groups (less than 3 years and 3–7 years) based on response frequency; these drafts were tested in a second set of patients. Factor analysis showed the presence of multiple subscales, which were defined as Competence, Personality, Family Impact, and Treatment Difficulty. The CVFQ was further validated in pediatric patients by assessing its sensitivity to different patient situations: unilateral vs. bilateral disease (i.e., cataracts), different severity of visual impairment (i.e., retinopathy of prematurity patients grouped by acuity), the difficulty of treatment regimen (i.e., optical, surgery, surgery + optical, and surgery + optical + occlusion), and alternative treatments for the same condition (i.e., intraocular lens, and aphakic contact lenses) [15]. Meaningful differences were found between the predefined groups, solidifying CVFQ’s importance in these types of clinical trials. Both studies were informative, but the measure’s development lacked visual impairment patient input when developing items. Additionally, 7–21% of the samples in the item development phases had IRD or other unspecified forms of retinal disease, while no patients had IRD in the validation phase—a critical yet missing piece to conclude on the sensibility to a PROM’s target group. Outside of patients with IRD, this tool still has significant validity for use in patients with a cataract.
Cardiff Visual Ability Questionnaire for Children (CVAQC) [16]
There was a need to assess pediatric patients’ (aged 5–18 years) difficulty in performing activities of daily living and track changes in difficulty performing these activities after rehabilitation. Khadka and colleagues [16] developed the Cardiff Visual Ability Questionnaire for Children (CVAQC) to meet this need and found no differential item functioning by age. Several subscales are defined in the measure: Education, Near vision, Distance Vision, Getting Around, Social Interaction, Entertainment, and Sports. The measure showed high measurement precision (person separation 2.28 and reliability 0.84; item separation 6.20 and reliability 0.97), and test–retest reliability (interclass correlation of 0.89 for persons (95% CI 0.80–0.94) and 0.94 for six items (95% CI 0.87–0.97)). However, the study is limited as it does not go into much depth on how the items were derived, severely VI patients were excluded from the psychometric validation phase, and patient breakdown by VI conditions is not given, so the IRD sample is undeterminable. Many PROMs following identified CVAQC as a precedent for measuring a change in patient experience following an ophthalmic intervention and is informative for PROM development.
Impact of Vision Impairment on Children (IVI_C) [17, 18]
Cochrane and colleagues (2008) aimed to assess the impact of VI on visually impaired patients (aged 8–18 years) and their caregivers, especially given that most measures developed then were solely based on caregiver or clinician-researcher input and did not include the patient perspective [17, 18]. An adult version of the Impact of Vision Impairment on Children (IVI_C [19]) PROM existed; however, it was not adapted to this age group; instead, the IVI_C was developed de novo. Focus groups were conducted to gain input from patients and other relevant stakeholders (i.e., teachers, caregivers, or specialists) [17]. Items developed from the qualitative portion were validated by Rasch analysis, yet no subscales were defined [18]. However, the inclusion criteria excluded patients with low VI (0.3 logMAR or less), and patient breakdown by VI condition is not given, so the IRD sample is undeterminable. Outside of patients with IRD, the measure showed particular use in other patients with low vision and near blindness.
Pediatric Eye Questionnaires (PedEyeQ) [20–24]
Holmes, Hatt, and colleagues (2021) [23] wanted to assess pediatric patients’ health and well-being across the entire spectrum of eye disease. They conducted concept elicitation interviews based on methods from their previous interview-based studies with different VI patient groups [25–28]. Through a thematic analysis, they identified common elements between the participants, which they used to create the first draft of the Pediatric Eye Questionnaires (PedEyeQ) PROM [20, 21]. There were four PROMs made for the VI patients, two sets for the groups of children aged 5–11 years and 12–17 years (each group having a patient self-report and a caregiver-report measure). The PROMs were then validated where the following domains were identified: child PedEyeQ domains are functional vision, bothered by eyes/vision, social, frustration/worry; and proxy PedEyeQ domains are functional vision, bothered by eyes/vision, social, frustration/worry, eye-care [22]. While there was a breakdown of IRD conditions given for different PROM development phases [20–22], only 9.6–14.9% of participants from the samples had IRD.
Vision-Related Quality of Life of Children (VQoL_C) and Vision-Related Quality of Life of Children (VQoL_YP) [29]
The Vision-related Quality of Life of Children (VQoL_C) and Vision-related Quality of Life of Children (VQoL_YP) recently developed by Rahi, Tadić, and colleagues (2021) are age-specific extensions of their 2011 VQoL_CYP [30, 31]. These tools were developed to assess all impacts of VI on visually impaired pediatric patients (aged 8–12 years, 13–17 years) on the basis of their perspective, otherwise known as vision-related quality of life (VRQoL). The strength of this PROM is its age-specific extensions. All editions were validated in the pediatric VI group. The studies had a large proportion of retinal patients (56.3–78.26% depending on the study phase) [32]; the number of patients with the inherited type was not specified. Additionally, they excluded participants with visual acuity in the better eye of logMAR ≤ 0.48 [31].
Functional Vision Questionnaire for Children (FVQ_C) and Functional Vision Questionnaire for Young People (FYQ_YP) [32]
Again by Rahi, Tadić, and colleagues (2021), the Functional Vision Questionnaire for Children (FVQ_C) and Functional Vision Questionnaire for Young People (FVQ_YP) are age-specific extensions of the FVQ_CYP from 2011 [30, 33]. The original and its extensions were developed to assess a visually impaired child’s perspective of their functional vision (aged 8–12 years, 13–18 years). All editions for this have been validated by Rasch analysis. The study had many patients with any retinal disease (56.3–75% depending on the study phase) [32]; the number of patients with the inherited type was not specified. As well, participants were excluded if the visual acuity in their better eye was logMAR ≤ 0.48 [33].
Discussion
Why Use PROMs Tailored to the Condition?
Patient-reported outcome measures can assess the impact of a condition on activities of daily living. The FDA [34] designates value in using an appropriate PROM in a therapeutic trial to highlight clinically meaningful differences in patients’ health and visual outcomes, where a general PROM may not have that capacity [35, 36]. General PROMs are multistate, grouping several conditions to assess health outcomes in general (e.g., general vision). This can result in floor or ceiling effects, where responses are pooled to extremes of a score distribution [37].
Current Status of PROMs in Inherited Retinal Disease Clinical Trials
Many past or ongoing gene therapeutic clinical trials included patients with IRD, specifically for retinitis pigmentosa, choroideremia, achromatopsia, LCA, Usher syndrome, X-linked retinoschisis, and Stargardt disease [38]. However, most of the peer-reviewed studies published did not include PROM results or any evaluation of the patient experience [5]. One study included the VFQ-25 [39], but as discussed earlier, the FDA states a validated PROM should be developed in the target population [4, 13], and so while VFQ-25 does have its strengths, it was not tailored to the pediatric IRD population.
New PROMs for Adult Patients with IRD
The Michigan Retinal Degeneration Questionnaire and Michigan Vision-related Anxiety Questionnaire are patient-reported outcome measures for adult patients with IRD to assess their vision ability and associated psychosocial health, respectively [8, 9]. These IRD-specific measures will begin to be incorporated into clinical trials studying voretigene neparvovec-rzyl as part of their outcomes and other observational studies. The use of MRDQ and MVAQ in a pediatric sample lacks validation. Studies are in progress to fulfill this need to holistically evaluate the efficacy of an intervention in children with IRD.
Perspectives on Pediatric PROM Development
Creating PROMs is iterative [3], and the product is held to the same rigorous standards as other outcome measures in clinical trials [13, 40]. The FDA and the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) have created their own methods for designing a PROM [3, 10, 13, 40]. Both groups emphasize the importance of the PROM showing adequate validity and reliability. Thus far, no PROMs have been made for pediatric patients with IRDs, so the ISPOR guidelines are summarized here in the context of pediatric patients.
Determine the Construct
The construct or patient-reported outcome (PRO) is the concept or outcome of interest as defined by the patient [13]. This can be related to the disease, such as assessing condition-specific quality of life, functional ability, or an intervention for the patient group. Unlike adults, the pediatric group carries vast developmental differences [10]. The selection of age groups is relatively arbitrary, which is why this varies between studies. One solution toward more uniformity could be to refer to ISPOR’s guidelines (less than 5 years, 5–7 years, 8–11 years, and 12–18 years) [10].
Concept Elicitation and Analysis
Once a construct is defined, the researchers need to create items for the first draft of the PROM. First, an interview or focus-group guide is developed to lead interviews [13]. The guide itself will help elicit ideas, experiences, and concerns the patients have about their condition, which will be used to create the PROM. The design of the guide can be based on the literature or focus groups/interviews with stakeholders for the PROM (such as patients and relevant clinicians) [3, 13]. However, it is highly recommended to include the patient input into the guides as they are the intended group for the measure in development and to ensure construct validity [41]. In the case of infants and young children (less than 6 years), the majority of the concepts will come from the caregiver [10]. Between 6 and 12 years, the reliability and validity of the child’s responses can be variable, a problem not exclusive to PROMs, so incorporating both the child’s and caregiver’s responses is recommended. The focus group and interview transcripts should be thematically analyzed (i.e., looking for commonalities that can be used to create the questions in the interview/focus group guides) [13]. Once these guides are created, they can be administered to larger groups of patients to develop that repertoire of experiences needed to make the first draft of the PROM. Again, these experiences need to be thematically analyzed.
Pilot the Draft Instrument
A draft instrument should be ready by this stage. As with the previous stages, depending on the age group, the draft should be designed with the respondent in mind [42]. For caregiver-proxy PROMs, where the caregiver responds on behalf of the pediatric patient, the vocabulary can be more complex than patient self-report PROMs, but children’s PROMs should always have age-appropriate language. Following is one last stage of qualitative improvement before psychometric validation. The instrument can be administered in a small group of patients, where each participant should consider each question for relevance and comprehensibility [13]. This can be done through cognitive interviews, an evidence-based, qualitative method of improving and validating questionnaires, which uses the respondent’s cognition to identify incorrect or difficult items [42]. Once pilot-tested, the final draft of the PROM is ready for quantitative validation.
Psychometric Validation
The final draft of the PROM should be tested in a large sample of patients dependent on the appropriate sample size calculations and characteristics of the population. As such, in the case of orphan diseases like IRDs, the sample size calculations may be forgone, given the uncommonness of the condition in the general population [37]. Many different techniques exist to quantitatively assess the validity and reliability of the PROM [40]. This depends on the complexity of questions and so is at the researcher’s discretion. An important marker to identify in patient populations such as IRDs is the presence of floor or ceiling effects. The distribution of responses should have some level of correlation to the physiological impairment the patient faces [5]. Additionally, test–retest reliability, defined as the stability of scores over time when no change is expected in the concept of interest test–retest reliability, should be established for the questionnaire [43].
Conclusion
At the time of writing this review, there are no tailored patient-reported outcome measures available to assess the impact of IRD on pediatric patients; most pediatric tools are designed to assess the impact of general visual impairment. Outcome measures used for a specific condition should be rigorously developed with mixed methods input from its target condition and age cohort as advised by FDA and ISPOR. As such, the use of general visual impairment PROMs is not recommended for garnering significant insight for the purposes of clinical decision-making and evaluating the efficacy of therapies. This review establishes the need to develop PROMs for pediatric patients with IRDs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Elena Springall, an information scientist at the Gerstein Science Information Centre (University of Toronto Libraries, Toronto, Ontario, Canada), for their support in designing the search strategy.
Funding
Funding for this review was provided by The Henry Brent Chair in Innovative Pediatric Ophthalmology Research (The Hospital for Sick Children, Toronto, Ontario, Canada; held by EH), the Vision Science Research Program (University Health Network and University of Toronto, Toronto, Ontario, Canada; held by KS), and Foundation Fighting Blindness (USA; Grant CD-CL-0617-0727-HSC held by AV). The journal’s Rapid Service Fee was also covered by The Henry Brent Chair in Innovative Pediatric Ophthalmology Research grant.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
KS, MFA, AV, and EH conceived the review. KS designed the search strategy and prepared the manuscript. KS and MFA selected the studies. KS completed data extraction and data analysis with support from GDL. KS and MFA contributed to the first draft. All authors revised the manuscript critically for important intellectual content and gave final approval of the manuscript. EH had full access to all the data in the study and final responsibility for the decision to submit for publication.
Disclosures
Elise Héon is consultant for Novartis and on a DSMB (for Sanofi Atsena). Ajoy Vincent is a consultant for Novartis and Adverum Biotechnologies Inc. Kavin Selvan, Maria Fernanda Abalem, and Gabrielle D. Lacy declare no conflicts or competing financial interests related to this publication.
Compliance with Ethics Guidelines
This article is based on previously published studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Contributor Information
Kavin Selvan, Email: kavin.selvan@mail.utoronto.ca.
Maria F. Abalem, Email: mabalemd@med.umich.edu
Gabrielle D. Lacy, Email: gabdlacy@med.umich.edu
Ajoy Vincent, Email: ajoy.vincent@sickkids.ca.
Elise Héon, Email: elise.heon@sickkids.ca.
References
- 1.Gong J, Cheung S, Fasso-Opie A, et al. The impact of inherited retinal diseases in the United States of America (US) and Canada from a cost-of-illness perspective. Clin Ophthalmol. 2021;15:2855–2866. doi: 10.2147/OPTH.S313719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heon E, Koenekoop R. Treatments for inherited retinal degenerations are coming to Canada: brief update on a new standard of care for inherited retinal degenerations. Can J Ophthalmol. 2021;56:e34–e35. doi: 10.1016/j.jcjo.2020.10.019. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services FDA Center for Drug Evaluation and Research, US Department of Health and Human Services FDA Center for Biologics Evaluation and Research, US Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:79. 10.1186/1477-7525-4-79. [DOI] [PMC free article] [PubMed]
- 4.Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10:S125–S137. doi: 10.1111/j.1524-4733.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 5.Lacy GD, Abalem MF, Musch DC, et al. Patient-reported outcome measures in inherited retinal degeneration gene therapy trials. Ophthalmic Genet. 2020;41:1–6. doi: 10.1080/13816810.2020.1731836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayasundera KT, Abuzaitoun RO, Lacy GD, et al. Challenges of cost-effectiveness analyses of novel therapeutics for inherited retinal diseases. Am J Ophthalmol. 2022;235:90–97. doi: 10.1016/j.ajo.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-list-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 8.Lacy GD, Abalem MF, Andrews CA, et al. The Michigan Retinal Degeneration Questionnaire: a patient-reported outcome instrument for inherited retinal degenerations. Am J Ophthalmol. 2021;222:60–68. doi: 10.1016/j.ajo.2020.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacy GD, Abalem MF, Andrews CA, et al. The Michigan Vision-related Anxiety Questionnaire: a psychosocial outcomes measure for inherited retinal degenerations. Am J Ophthalmol. 2020 doi: 10.1016/j.ajo.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matza LS, Patrick DL, Riley AW, et al. Pediatric patient-reported outcome instruments for research to support medical product labeling: report of the ISPOR PRO good research practices for the assessment of children and adolescents task force. Value Health. 2013;16:461–479. doi: 10.1016/j.jval.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Tadic V, Hogan A, Sobti N, et al. Patient-reported outcome measures (PROMs) in paediatric ophthalmology: a systematic review. Br J Ophthalmol. 2013;97:1369–1381. doi: 10.1136/bjophthalmol-2013-303350. [DOI] [PubMed] [Google Scholar]
- 12.McKeown S, Mir ZM. Considerations for conducting systematic reviews: evaluating the performance of different methods for de-duplicating references. Syst Rev. 2021;10:38. doi: 10.1186/s13643-021-01583-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 1—eliciting concepts for a new PRO instrument. Value Health. 2011;14:967–977. doi: 10.1016/j.jval.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Felius J, Stager DR, Berry PM, et al. Development of an instrument to assess vision-related quality of life in young children. Am J Ophthalmol. 2004;138:362–372. doi: 10.1016/j.ajo.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Birch EE, Cheng CS, Felius J. Validity and reliability of the Children’s Visual Function Questionnaire (CVFQ) J AAPOS. 2007;11:473–479. doi: 10.1016/j.jaapos.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khadka J, Ryan B, Margrain TH, et al. Development of the 25-item Cardiff Visual Ability Questionnaire for Children (CVAQC) Br J Ophthalmol. 2010;94:730–735. doi: 10.1136/bjo.2009.171181. [DOI] [PubMed] [Google Scholar]
- 17.Cochrane G, Lamoureux E, Keeffe J. Defining the content for a new quality of life questionnaire for students with low vision (the Impact of Vision Impairment on Children: IVI_C) Ophthalmic Epidemiol. 2008;15:114–120. doi: 10.1080/09286580701772029. [DOI] [PubMed] [Google Scholar]
- 18.Cochrane GM, Marella M, Keeffe JE, et al. The Impact of Vision Impairment for Children (IVI_C): validation of a vision-specific pediatric quality-of-life questionnaire using Rasch analysis. Investig Ophthalmol Vis Sci. 2011;52:1632–1640. doi: 10.1167/iovs.10-6079. [DOI] [PubMed] [Google Scholar]
- 19.Weih LM, Hassell JB, Keeffe J. Assessment of the impact of vision impairment. Investig Ophthalmol Vis Sci. 2002;43:927–935. [PubMed] [Google Scholar]
- 20.Hatt SR, Leske DA, Wernimont SM, et al. Patient-derived questionnaire items for patient-reported outcome measures in pediatric eye conditions. J AAPOS. 2018;22:445. doi: 10.1016/j.jaapos.2018.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatt SR, Leske DA, Castañeda YS, et al. Development of pediatric eye questionnaires for children with eye conditions. Am J Ophthalmol. 2019;200:201–217. doi: 10.1016/j.ajo.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leske DA, Hatt SR, Castañeda YS, et al. Validation of the Pediatric Eye Questionnaire (PedEyeQ) in children with visual impairment. Am J Ophthalmol. 2019;208:124–132. doi: 10.1016/j.ajo.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leske DA, Hatt SR, Wernimont SM, et al. Quality of life and functional vision across pediatric eye conditions assessed using the PedEyeQ. J AAPOS. 2021 doi: 10.1016/j.jaapos.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leske DA, Hatt SR, Wernimont SM, et al. Association of visual acuity with eye-related quality of life and functional vision across childhood eye conditions. Am J Ophthalmol. 2021;223:220–228. doi: 10.1016/j.ajo.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castaneda YS, Birch EE, Leske DA, et al. Quality of life concerns in parents of children with esotropia. Investig Ophthalmol Vis Sci. 2015;56:5204. [Google Scholar]
- 26.Liebermann L, Leske DA, Castañeda YS, et al. Childhood esotropia: child and parent concerns. J AAPOS. 2016;20:295–300.e1. doi: 10.1016/j.jaapos.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liebermann L, Leske DA, Hatt SR, et al. Bilateral childhood visual impairment: child and parent concerns. J AAPOS. 2017;21:183.e1–183.e7. doi: 10.1016/j.jaapos.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatt SR, Leske DA, Wernimont SM, et al. Comparison of rating scales in the development of patient-reported outcome measures for children with eye disorders. Strabismus. 2017;25:33–38. doi: 10.1080/09273972.2016.1276941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tadic V, Robertson AO, Cortina-Borja M, et al. An age- and stage-appropriate patient-reported outcome measure of vision-related quality of life of children and young people with visual impairment. Ophthalmology. 2020;127:249–260. doi: 10.1016/j.ophtha.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Rahi JS, Tadić V, Keeley S, et al. Capturing children and young people’s perspectives to identify the content for a novel vision-related quality of life instrument. Ophthalmology. 2011;118:819–824. doi: 10.1016/j.ophtha.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Tadic V, Robertson A, Rahi JS, et al. Measuring vision-related quality of life (VQoL) of children and young people with visual impairment. Qual Life Res. 2016;25:90. doi: 10.1007/s11136-016-1390-7. [DOI] [Google Scholar]
- 32.Robertson AO, Tadić V, Cortina-Borja M, et al. A patient-reported outcome measure of functional vision for children and young people aged 8 to 18 years with visual impairment. Am J Ophthalmol. 2020;219:141–153. doi: 10.1016/j.ajo.2020.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Tadić V, Cooper A, Cumberland P, et al. Development of the functional vision questionnaire for children and young people with visual impairment: the FVQ_CYP. Ophthalmology. 2013;120:2725–2732. doi: 10.1016/j.ophtha.2013.07.055. [DOI] [PubMed] [Google Scholar]
- 34.US Food and Drug Administration. Value and use of Patient-Reported Outcomes (PROs) in assessing effects of medical devices. 2016. https://www.fda.gov/media/109626/download.
- 35.Sedaghat AR. Understanding the minimal clinically important difference (MCID) of patient-reported outcome measures. Otolaryngol Head Neck Surg. 2019;161:551–560. doi: 10.1177/0194599819852604. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. How to use the ICF: A practical manual for using the International Classification of Functioning, Disability and Health (ICF). Exposure draft for comment Geneva: WHO. 2013;13. https://www.who.int/publications/m/item/how-to-use-the-icf---a-practical-manual-for-using-the-international-classification-of-functioning-disability-and-health.
- 37.Slade A, Isa F, Kyte D, et al. Patient reported outcome measures in rare diseases: a narrative review. Orphanet J Rare Dis. 2018;13:61. doi: 10.1186/s13023-018-0810-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nuzbrokh Y, Ragi SD, Tsang SH. Gene therapy for inherited retinal diseases. Ann Transl Med. 2021;9:1278. doi: 10.21037/atm-20-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2—assessing respondent understanding. Value Health. 2011;14:978–988. doi: 10.1016/j.jval.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Wiering B, de Boer D, Delnoij D. Patient involvement in the development of patient-reported outcome measures: a scoping review. Health Expect. 2017;20:11–23. doi: 10.1111/hex.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright J, Moghaddam N, Dawson DL. Cognitive interviewing in patient-reported outcome measures: a systematic review of methodological processes. Qual Psychol. 2021;8:2–29. doi: 10.1037/qup0000145. [DOI] [Google Scholar]
- 43.Qin S, Nelson L, McLeod L, et al. Assessing test–retest reliability of patient-reported outcome measures using intraclass correlation coefficients: recommendations for selecting and documenting the analytical formula. Qual Life Res. 2019;28:1029–1033. doi: 10.1007/s11136-018-2076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary information files.


