Abstract
Objective
To assess the antibody response to disease-modifying antirheumatic drug (DMARD) therapy after the first and second dose of the ChAdOx1nCov-19 (AstraZeneca (AZ)) and BNT162b (Pfizer) vaccines in patients with immune-mediated inflammatory disease (IMID) compared with controls and if withholding therapy following the first vaccination dose has any effect on seroconversion and SARS-CoV-2 antibody (Ab) levels.
Methods
A multicentre three-arm randomised controlled trial compared the immunogenicity of the Pfizer and AZ vaccines in adult patients on conventional synthetic (csDMARD), biologic (bDMARD) or targeted synthetic (tsDMARD) therapy for IMID (n=181) with a control group (n=59). Patients were randomised to continue or withhold DMARD therapy for 1–2 weeks post first dose vaccination only. Serum SARS-CoV-2 IgG detection (IgG ≥1.0 U/mL) and titres against the S1/S2 proteins were measured at baseline, 3–4 weeks post first vaccination and 4 weeks post second vaccination.
Results
AZ vaccination was given to 47.5%, 41.5% and 52.5% in the continue, withhold and control groups, respectively while Pfizer vaccination was given to 52.5%, 58.5% and 47.5% among the continue, withhold and control groups, respectively. Seroconversion rates following the first dose in the AZ and Pfizer groups were only 27.3% vs 79.2% (p=0.000) and 64.58% vs 100% (p=0.000), respectively in the IMID groups who continued therapy compared with the AZ and Pfizer controls, respectively. Withholding DMARD therapy following the first vaccination dose resulted in higher seroconversion to 67.7% and 84.1% in the AZ and Pfizer groups, respectively. Following the second AZ and Pfizer vaccinations when all DMARDs were continued, despite a slightly lower seroconversion rate (83.7% vs 100%, p=0.000 and 95.9% vs 100%, p=0.413), respectively, the mean SARS-CoV2 IgG Ab titres were not significantly different in the csDMARD and bDMARD groups compared with the controls regardless of hold while it was significantly lower in patients taking tsDMARD (12.88 vs 79.49 U/mL, p=0.000).
Conclusions
Following the first vaccination dose, antibody responses were lower in IMID on DMARD therapy, however the final responses were excellent regardless of hold with the exception of the tsDMARD group where withholding therapy is recommended. At least 2 vaccinations are therefore recommended preferably with an messenger RNA vaccine.
Trial registration number
ANZCTR: 12621000661875.
Keywords: COVID-19, antirheumatic agents, vaccination
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT?
It is known that the immunogenicity of the Pfizer and AstraZeneca COVID-19 vaccines are reduced in patients with immune-mediated inflammatory disease (IMID) who take disease-modifying antirheumatic drug (DMARD) therapy.
It is therefore vital that vaccination strategies are developed for these patients.
WHAT DOES THIS STUDY ADD?
The antibody response in patients with IMID treated with DMARD therapies are impaired following the first vaccination compared with the controls however after the second dose of the vaccine, the antibody responses were not significantly different to the controls with the exception for those on targeted synthetic DMARD (tsDMARD) therapy.
The antibody response was also influenced by vaccine type.
HOW MIGHT THIS IMPACT ON CLINICAL PRACTICE OR FURTHER DEVELOPMENTS?
Full vaccination with at least two doses preferably with a messenger RNA vaccine is recommended in those with IMID.
Withholding tsDMARD therapy specifically after SARS-CoV-2 vaccination is a recommended strategy to improve antibody response.
Introduction
Around the globe, COVID-19 has spread uncontrollably with an estimated 476 million cases and over 6.1 million cumulative deaths as of March 2022.1 Quickly developed vaccines have demonstrated protective immunity in the general population as characterised by the detection of SARS-CoV-2-specific antibodies.2–4 Patients with immune-mediated inflammatory disease (IMID) have not been included in efficacy studies of SARS-CoV-2 vaccines and it has become apparent that the vast majority of patients with IMID on disease-modifying antirheumatic drugs (DMARDs) still respond to SARS-CoV2 vaccination, however the antibody responses maybe delayed and reduced, especially on regimens including mycophenolate, abatacept or rituximab.5–7 In January and February 2021, the BNT162b (Pfizer/BioNTech) COVID-19 messenger RNA (mRNA) and the ChAdOx1nCov-19 (AstraZeneca (AZ)/Oxford) vaccines, respectively, were provisionally approved for use in Australia by the Therapeutics Goods Administration. Both these COVID-19 vaccines target the spike protein of SARS-CoV-2 leading to the inhibition of binding to the ACE-2 receptor and hence viral entry into the host cell. They both have been shown to be safe and effective in the normal population.2 3 8 9
The COVID-19 Global Rheumatology Alliance physician registry has shown that age, male sex, chronic lung disease, cardiovascular disease combined with hypertension and high disease activity are factors associated with an increased risk for COVID-19-related death while methotrexate (MTX) or biological monotherapy are not associated with adverse COVID-19 outcomes.10 Patients with rheumatoid arthritis (RA) who were treated with rituximab or a Janus kinase inhibitor (JAKi) had poorer COVID-19 outcomes compared with tumour necrosis factor inhibitors (TNFi) as characterised by higher hospitalisation and death rates.11 12 In addition, glucocorticoids (>10 mg/day), rituximab, sulfasalazine and immunosuppressant therapy (azathioprine, cyclophosphamide, ciclosporin, mycophenolate or tacrolimus) were also associated with COVID-19-related death.10 Pausing MTX for at least 10 days after second vaccination has been shown to improve immunogenicity of COVID-19 vaccination in patients ≥60 years with rheumatic disease.13
RESCUE1 is an investigator-led three-arm randomised controlled trial aiming to investigate the effect of DMARD therapies in patients with IMID compared with a control group following the first and second doses of the AZ and Pfizer vaccinations and to evaluate whether withholding therapy after the first dose improves immunogenicity.
Methods
Study design and patient population
Consecutive participants at their routine clinic visit were recruited at a private Perth-based rheumatology practice at St John of God Hospital and at the IBD unit at St Vincent’s Hospital Melbourne between the 1 May 2021 and 30 September 2021. The eligibility criteria were age 18 years and over, have a diagnosis of an IMID and deemed to be in clinical remission on DMARD therapy with no disease flares for >4 weeks prior to enrolment. The inclusion criteria for an IMID were: RA/American College of Rheumatology (ACR)/EULAR 2010 classification criteria; psoriatic arthritis (PsA)/classification criteria for PsA; axial spondyloarthritis/Assessment of SpondyloArthritis International Society classification criteria; systemic lupus erythematosus/1997 ACR criteria; Crohn’s disease and ulcerative colitis: European Crohn’s and Colitis Organisation criteria. The exclusion criteria were prior vaccination to COVID-19, history of COVID-19 infection, prednisolone use within 4 weeks of COVID-19 vaccination, inability to have the COVID-19 vaccine or previous thromboembolism, myocarditis or pericarditis. Volunteers who did not have a diagnosis of IIMD and who were not DMARDs were also recruited as the controls. These participants consisted of patients with non-inflammatory rheumatic disease and/or their partners, health professionals, friends and family.
Procedures
Demographic data which included age, sex, ethnicity, height and weight, smoking status, DMARD therapy, concomitant medications and disease activity were collected at the baseline visit. At study commencement, the Australian Technical Advisory Group on Immunisation (ATAGI) on COVID-19 vaccines recommended for those aged 16 to under 60 years to have the Pfizer as the preferred vaccine due to a higher risk of thrombosis and thrombocytopenia syndrome related to the AZ vaccine.14 15 From 16 September 2021, eligibility for the Pfizer vaccine was expanded to people over age 60 years. The Pfizer and AZ vaccines were given 3 and 12 weeks apart, respectively and were administered by the participant’s general practitioner or government run vaccination hub.
The DMARDs were grouped into conventional (csDMARD), biological (bDMARD) and targeted synthetic (tsDMARD) therapies (table 1). Subjects on combination cs/bDMARDs were grouped according to the csDMARD as the influence of COVID-19-related death from MTX and sulfasalazine are known to the exceed that of bDMARDs (eg, TNFi).10 Patients on combination cs/tsDMARDs were considered solely in the tsDMARD group. Approximately 50% of participants were randomised using a random allocation table allocated to each DMARD group uploaded into the REDCap database hosted at the University of Western Australia to withhold their current immunosuppressive therapy. Participants on csDMARDs withheld therapy for 2 weeks after the first vaccine dose. MTX was administered weekly and the vaccination was attempted to be timed on the day the dose was due however the MTX dose was paused on the day of vaccination for two cycles. If however the first dose of the vaccine was <1 week following the last dose of MTX, then the dose prior to vaccination was withheld for two cycles. Participants on daily DMARDs withheld therapy for 1 week starting on the day of first vaccination. Participants on bDMARDs delayed their therapy by 1 week following their usual injection or infusion cycle. For example, for a bDMARD administered fortnightly, the vaccination was timed at the end of the 2 weeks and then restarted 1 week later leaving an interval of 3 weeks. All participants withheld therapy following the first vaccination dose only. For the subjects randomised to withhold their usual DMARDs, the withhold dates were calculated and adherence was confirmed and checked by the dates recorded by each participant.
Table 1.
Disease-modifying antirheumatic drug (DMARD) therapy
| AZ | |||||||||||
| csDMARD | bDMARD | tsDMARD | |||||||||
| Withhold (n) | Continue (n) | Withhold (n) | Continue (n) | Withhold (n) | Continue (n) | ||||||
| MTX | 7 | MTX | 9 | ADA | 3 | ADA | 2 | BARI | 3 | BARI | 1 |
| MTX+HCQ | 2 | MTX+HCQ | 3 | ETA | 2 | ETA | 1 | TOF | 4 | TOF | 3 |
| MTX+SSZ | 2 | SSZ | 1 | GOL | 1 | GOL | 2 | TOF+MTX | 3 | TOF+MTX | 3 |
| MTX+IXE | 0 | MTX+IXE | 1 | IXE | 2 | IXE | 3 | UPA | 5 | UPA | 8 |
| MTX+SEC | 0 | MTX+SEC | 1 | SEC | 2 | SEC | 3 | ||||
| MTX+TOC | 1 | MTX+TOC | 1 | TOC | 1 | TOC | 4 | ||||
| UST | 0 | UST | 2 | ||||||||
| Total | 12 | 16 | 11 | 17 | 15 | 15 | |||||
| Pfizer | |||||||||||
| csDMARD | bDMARD | tsDMARD | |||||||||
| Withhold (n) | Continue (n) | Withhold (n) | Continue (n) | Withhold (n) | Continue (n) | ||||||
| LEF | 1 | LEF | 1 | ADA | 4 | ADA | 5 | BARI | 4 | BARI | 4 |
| MTX | 7 | MTX | 8 | CTZ | CTZ | 2 | BARI+MTX | 1 | BARI+MTX | 1 | |
| MTX+HCQ | 4 | MTX+HCQ | 3 | ETA | 1 | ETA | 1 | TOF | 3 | TOF | 2 |
| MTX+SSZ | 1 | MTX+SSZ | 3 | GOL | 0 | GOL | 1 | TOF+MTX | 0 | TOF+MTX | 0 |
| MTX+ETA | 0 | MTX+ETA | 1 | GUS | 1 | GUS | 0 | UPA | 7 | UPA | 7 |
| MTX+SEC | 1 | MTX+SEC | 0 | INF | 1 | INF | 1 | UPA+MTX | 0 | UPA+MTX | 1 |
| MTX+TOC | 0 | MTX+TOC | 0 | IXE | 4 | IXE | 5 | ||||
| SEC | 2 | SEC | 1 | ||||||||
| TOC | 2 | TOC | 2 | ||||||||
| UST | 0 | UST | 1 | ||||||||
| Total | 14 | 16 | 15 | 20 | 15 | 15 | |||||
MTX, methotrexate; HCQ, hydroxychloroquine; SSZ, sulfasalazine; LEF, leflunamide; ADA, adalimumab; CTZ, certolizumab; ETA, etanercept; GOL, golimumab; GUS, guselkumab; INF, infliximab; IXE, ixekizumab; SEC, secukinumab, TOC, tocilizumab, UST, ustekinumab; BARI, baricitinib; TOF, tofacitinib; UPA, upadacitinib.
ADA, adalimumab; BARI, baricitinib; bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; CTZ, certolizumab; ETA, etanercept; GOL, golimumab; GUS, guselkumab; HCQ, hydroxychloroquine; INF, infliximab; IXE, ixekizumab; LEF, leflunamide; MTX, methotrexate; SEC, secukinumab; SSZ, sulfasalazine; TOC, tocilizumab; TOF, tofacitinib; tsDMARD, targeted synthetic DMARD; UPA, upadacitinib; UST, ustekinumab.
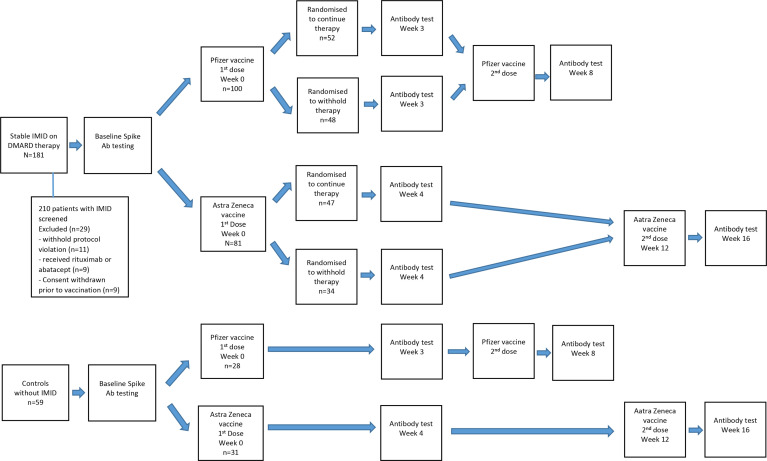
Any disease flares or adverse reactions to the COVID-19 vaccines were recorded by the participant and the outcomes were followed up via a phone consultation or scheduled clinic visit. If a participant flared then their current DMARD therapy which was withheld was immediately reinstituted. Blood samples were collected within 1 week prior to the first vaccine dose, 3–4 weeks after the first dose (just before the second dose in those receiving the Pfizer vaccine) and 4 weeks after the second dose. The baseline test prior to the vaccination was to ensure the participants had not had prior infection with SARS-CoV-2 (figure 1).
Figure 1.
Study flow chart. DMARD, disease-modifying antirheumatic drug; IMID, immune-mediated inflammatory disease.
Laboratory methods
SARS-CoV2 IgG antibody was measured using the Siemens ADVIA Centaur sCOVG assay which is a two-step sandwich immunoassay using indirect chemiluminescent technology. This assay detects antibodies against the S1-RBD antigen and can be used for qualitative and quantitative detection of SARS-CoV2 IgG. The results are given as U/mL with the cut-off for positivity defined as ≥1.0 U/mL.16
Sample size and statistical analysis
A prospective power calculation was performed according to equation 4 specified in the study by Whitley and Ball to determine the number of subjects per group required to detect a difference between proportions of 20% assuming a power of 80% and a two-sided significance level of 95%.17 Based on observations that neutralising activity against wild-type SARS-CoV-2 was significantly lower in patients receiving MTX and targeted immunosuppressive therapy (median 50% inhibitory dilution) than in controls,18 the calculated sample size required was 180 patients (81 AZ and 100 Pfizer) to detect a difference in the SARS-CoV-2 IgG levels in the study group.
For categorical variables, the Fisher’s exact parametric test was used to assess the seroconversion rates between the DMARD and control groups while the Wilcoxon-Mann-Whitney U non-parametric test was used to assess the continuous variable of antibody levels between the different groups. The data are given as a frequency (%) or mean with SD. Initial univariate logistic regression analysis was performed to assess for associations between patient characteristics and odds of achieving protective SARS-CoV2 IgG antibody titres. Multivariate analysis was carried out using multiple logistic regression in the variables which were found to be significant on univariate analysis. Statistical analyses were performed using STATA (StataCorp, USA) and SPSS (IBM, USA). A p value of <0.05 was considered statistically significant.
Outcome measures
The primary outcome was antibody seroconversion rates defined as the detection of SARS-CoV-2 antispike (S) protein receptor-binding antibodies (IgG titre ≥1.0 U/mL) between the controls and subgroups of patients with IMID 3 weeks after the first vaccination and three to 3–4 weeks after the second vaccination. The secondary outcomes were SARS-CoV2 antispike (S) protein receptor-binding antibody titres.
Results
Patient characteristics
The IMID cohort consisted of 73.2% females with a mean age of 54.2 years (±13.3, range 18–84) and 82.5% were Caucasian. Overall, 53.7% of patients had RA, 32.9% had PsA and 7.3% had ankylosing spondylitis. The controls were 59.6% females with a mean age of 54.4 years (±12.6, range 26–78) and 69.0% Caucasian. Eighty-one (44.5%) patients received the AZ vaccine while 100 (55.0%) received the Pfizer vaccine; 41.5% and 58.5% of the AZ and Pfizer IMID participants, respectively, withheld DMARD therapy. Eleven (4.5%) patients in the withhold group were excluded from the study for not following the hold protocol. Twenty-nine (12%) participants missed the baseline testing. Immunogenicity was evaluated at a mean duration of 32.6 and 29.8 days in the AZ group and 22.7 and 31.1 days in the Pfizer group following the first and second doses, respectively. The mean age of the IMID group taking MTX was 57.6 years (±14.6) with the mean dose being 16.93 mg weekly. The mean treatment duration was 9.04 years for the bDMARD, 3.39 years for the csDMARD and 3.82 years for the tsDMARD groups. Table 2 contains detailed participant characteristics.
Table 2.
Demographics and clinical characteristics of patients with IMID and controls
| Trial arm | Autoimmune disease group | Controls | Total | |
| Withhold therapy | Continue therapy | Controls | ||
| N | 82 | 99 | 59 | 240 |
| Vaccine | ||||
| CHadOx1nCov-19 (AstraZeneca) | 34 (41.5) | 47 (47.5) | 31 (52.5) | 112 (46.7) |
| BNT162b2 (Pfizer) | 48 (58.5) | 52 (52.5) | 28 (47.5) | 128 (53.3) |
| Sex | ||||
| Female | 60 (73.2) | 65 (66.3) | 33 (59.6) | 158 (66.4) |
| Male | 22 (26.8) | 33 (33.7) | 25 (43.1) | 80 (33.6) |
| BMI | 29.2 (7.1) | 28.8 (6.1) | 26.2 (5.0) | 28.3 (6.3) |
| Ethnicity | ||||
| African-American or black | 1 (1) | 1 (1) | 1 (1.7) | 3 (1.3) |
| Arabic | 0 (0) | 1 (1) | 0 (0) | 1 (0.4) |
| Asian | 8 (10) | 8 (8) | 14 (24.1) | 30 (12.7) |
| Indian | 2 (2.5) | 3 (3) | 1 (1.7) | 6 (2.5) |
| Indigenous | 2 (2.5 | 3 (3) | 0 (0) | 5 (2.1) |
| White | 66 (82.5) | 83 (84) | 40 (69) | 189 (79.8) |
| Other | 1 (0) | 0 (0) | 2 (3.4) | 3 (1.3) |
| Age | 55.4 (12.6) | 53.3 (14.4) | 54.4 (12.6) | 54.2 (13.3) |
| Smoking status | ||||
| Non-smoker | 55 (67.1) | 72 (73.5) | 48 (84.2) | 175 (73.8) |
| Current smoker | 9 (11.0) | 7 (7.1) | 5 (8.8) | 21 (8.9) |
| Ex-smoker | 18 (22.0) | 19 (19.4) | 4 (7.0) | 41 (17.3) |
| Medication class | ||||
| csDMARD | 26 (31.7) | 32 (32.3) | n/a | 58 (32.0) |
| bDMARD | 26 (31.7) | 37 (37.4) | n/a | 63 (34.8) |
| tsDMARD | 30 (36.6) | 30 (30.3) | n/a | 60 (33.2) |
| Autoimmune disease | ||||
| Ankylosing spondylitis | 6 (7.3) | 13 (13.1) | n/a | 19 (10.5) |
| Crohn’s disease | 1 (1.2) | 1 (1.0) | n/a | 2 (1.1) |
| Psoriatic arthritis | 27 (32.9) | 26 (26.3) | n/a | 53 (29.3) |
| Rheumatoid arthritis | 44 (53.7) | 53 (53.5) | n/a | 97 (53.6) |
| SLE/Sjögren's/CTD | 3 (3.7) | 2 (2.0) | n/a | 5 (2.8) |
| Ulcerative colitis | 0 (0) | 2 (2.0) | n/a | 2 (1.1) |
| Other | 1 (1.2) | 2 (2.0) | n/a | 3 (1.7) |
All data are n (%) or mean (SD) unless otherwise indicated.
bDMARD, biologic DMARD; BMI, body mass index; csDMARD, conventional synthetic DMARD; CTD, connective tissue disease; DMARD, disease-modifying antirheumatic drug; IMID, immune-mediated inflammatory disease; n/a, not available; SLE, systemic lupus erythematosus; tsDMARD, targeted synthetic DMARD.
Four patients on tocilizumab were changed to an alternative bDMARD or tsDMARD during the study due to an Australia-wide critical shortage of the drug however in all cases this occurred after both doses of the AZ or Pfizer vaccines had been administered and hence did not affect the post second vaccination SARS-CoV-2 IgG antibody (Ab) seroconversion and IgG Ab level analysis.
SARS-CoV-2 vaccination responses in AZ and Pfizer compared with controls
A total of 207 patients were included in the analysis of the response following the first dose vaccine and 210 patients following the second dose vaccine due to missing serology tests. In the AZ vaccine group following the first vaccine dose, the seroconversion rate was significantly higher in the withhold group (67.7% vs 27.3%, p=0.002) while there was no statistically significant difference between the withhold group and controls (67.7% vs 79.2%, p=0.380). Continuing therapy resulted in a significantly lower seroconversion rate compared with the controls (27.3% vs 79.2%, p=0.000). Following the second vaccine dose, the continue group had a significantly lower rate of seroconversion compared with the group who initially withheld following the first vaccine dose (83.7% vs 100%, p=0.038) and this was also significantly lower than the control group (83.7% vs 100%, p=0.000) (table 3). The mean SARS-CoV2 IgG Ab titre following the first vaccine dose were significantly lower in the continue group compared with both withhold and control groups (1.25 vs 2.17 and 6.50 U/mL, p=0.014 and p=0.000, respectively). Following the second vaccine dose, there was no significant difference in the mean SARS-CoV2 antibody titres regardless of hold (26.55 vs 14.70 U/mL, p=0.709 and 26.55 vs 13.10 U/mL, p=0.119 for withhold and continue groups, respectively) (table 3).
Table 3.
Immunological response in subjects who received AstraZeneca
| Withhold therapy | Continue therapy | Controls | Total | |
| Detectable SARS-CoV2 Ab* | ||||
| Post first vaccine dose | 21/31 (67.7) | 9/33 (27.3) | 19/24 (79.2) | 49/88 (55.68) |
| Withhold versus continue (p=0.002) | ||||
| Withhold versus controls (p=0.380) | ||||
| Continue versus controls (p=0.000) | ||||
| Post second vaccine dose | 27/27 (100) | 36/43 (83.7) | 22/22 (100) | 85/92 (92.39) |
| Withhold versus continue (p=0.038) | ||||
| Withhold versus controls | ||||
| Continue versus controls (p=0.000) | ||||
| Mean SARS-CoV2 Ab titre† | ||||
| Post first vaccine dose | 2.17 (2.32) | 1.25 (1.58) | 6.50 (11.47) | 3.00 (6.51) |
| Withhold versus continue (p=0.014) | 1.4 (2.7) | 0.5 (0.6) | 2.1 (5.05) | 0.95 (2.25) |
| Withhold versus controls (p=0.105) | ||||
| Continue versus controls (p=0.000) | ||||
| Post second vaccine dose | 26.55 (33.00) | 14.70 (22.73) | 13.10 (11.53) | 17.79 (24.76) |
| Withhold versus continue (p=0.086) | 7.7 (46.5) | 4.9 (18.1) | 10.6 (13.2) | 6.5 (19.9) |
| Withhold versus controls (p=0.709) | ||||
| Continue versus controls (p=0.119) |
Data are presented as proportions (%) or mean (SD) and median (IQR).
*Using Fisher’s exact test with significance cut-off 5%.
†Using Wilcoxon-Mann-Whitney U test with significance cut-off 5%.
Ab, antibody.
In the Pfizer vaccination group following the first vaccine dose, the seroconversion rate was significantly lower in the continue group compared with the control (64.58% vs 100%, p=0.000). Following the second vaccine dose, there was no significant difference in the seroconversion rate compared with the controls regardless of hold (p=0.413). Following the first and second vaccine doses, the mean SARS-CoV2 Ab titre however were significantly lower in the continue therapy compared with the control group (4.94 vs 11.05 U/mL, p=0.0000 and 76.18 vs 133.26 U/mL, p=0.033). There was no significant difference in the SARS-CoV2 Ab titre levels in the group that withheld therapy during the first dose and followed through to the second dose (136.02 vs 133.58, p=0.980) (table 4 and figure 2).
Table 4.
Immunological response in subjects who received Pfizer
| Withhold therapy | Continue therapy | Controls | Total | |
| Detectable SARS-CoV2 antibodies* | ||||
| Post first vaccine dose | 37/44 (84.1) | 31/48 (64.58) | 27/27 (100) | 95/119 (79.83) |
| Withhold versus continue (p=0.056) | ||||
| Withhold versus controls (p=0.039) | ||||
| Continue versus controls (p=0.000) | ||||
| Post second vaccine dose | 42/42 (100) | 47/49 (95.9) | 27/27 (100) | 116/118 (98.31) |
| Withhold versus continue (p=0.497) | ||||
| Withhold versus controls | ||||
| Continue versus controls (p=0.413) | ||||
| Mean SARS-CoV2 Ab titre† | ||||
| Post first vaccine dose | 7.36 (9.79) | 4.94 (7.19) | 11.05 (11.82) | 7.24 (9.59) |
| Withhold versus continue (p=0.019) | 3.4 (8.7) | 1.85 (7.1) | 5.1 (10.2) | 3.4 (8.8) |
| Withhold versus controls (p=0.052) | ||||
| Continue versus controls (p=0.000) | ||||
| Post second vaccine dose | 136.02 (169.68) | 76.18 (77.63) | 133.58 (166.26) | 110.40 (139.53) |
| Withhold versus continue (p=0.032) | 73.7 (111.3) | 50.8 (92) | 66.8 (78.3) | 66.8 (97.2) |
| Withhold versus controls (p=0.980) | ||||
| Continue versus controls (p=0.033) |
Data are presented as proportions (%) or mean (SD) and median (IQR).
*Using Fisher’s exact test with significance cut-off 5%.
†Using Wilcoxon-Mann-Whitney U test with significance cut-off 5%.
Ab, antibody.
Figure 2.
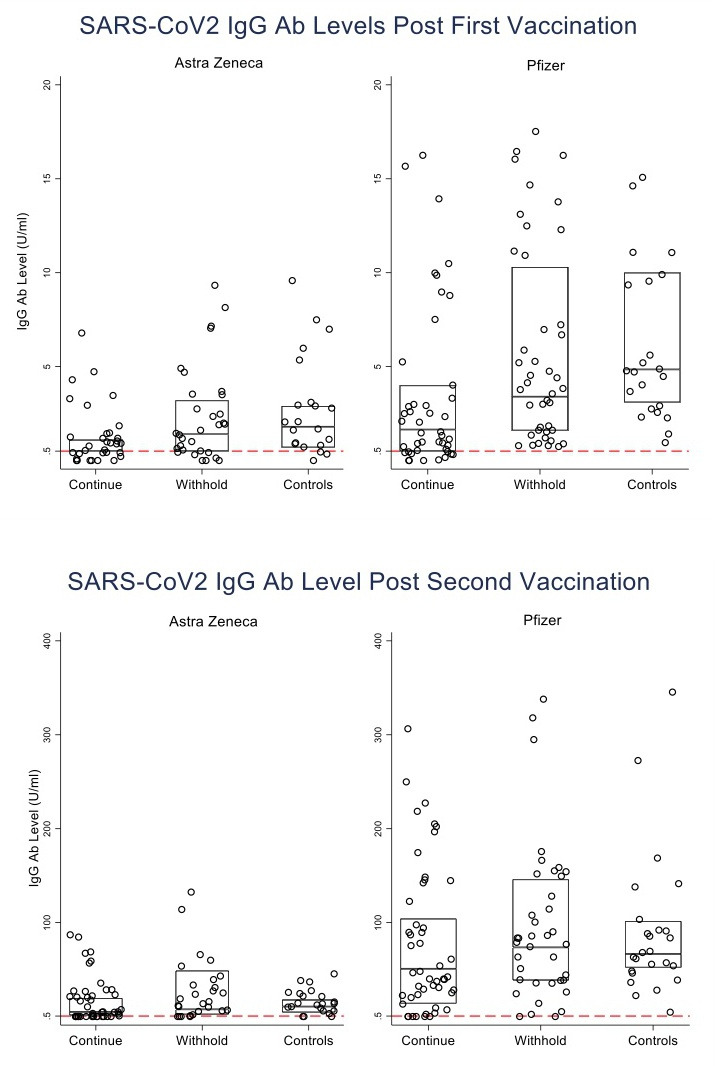
Distribution of anti-SARS-CoV-2 IgG antibody levels after the first and second AstraZeneca and Pfizer vaccine doses in the different treatment groups.
Comparison of SARS-CoV-2 vaccination responses within each of the DMARD groups
When stratifying to each of the DMARD groups, in the participants who continued with therapy in the perivaccination period, there was a statistically significant lower rate of seroconversion following the first dose only and not the second dose with the tsDMARD group showing the lowest seroconversion rate among the different DMARDs (p=0.000) (table 5). In the continue DMARD group, when analysing the mean SARS-CoV2 IgG Ab titres there was a significant difference following the first and second vaccinations among each of the DMARD groups (p=0.000 and p=0.009, respectively) (table 5).
Table 5.
Impact of DMARD class on vaccine response in the IMID and control groups
| csDMARD | bDMARD | tsDMARD | Controls | |
| Continue group | ||||
| Detectable SARS-CoV2 IgG Abs* | ||||
| Post first vaccine dose (p=0.000) | 9/22 (40.91) | 25/32 (78.13) | 5/26 (19.23) | 46/51 (90.20) |
| P=0.000 | P=0.116 | P=0.000 | ||
| Post second vaccine dose (p=0.748) | 28/30 (93.33) | 30/34 (88.24) | 23/26 (88.46) | 49/49 (100) |
| P=0.141 | P=0.025 | P=0.039 | ||
| Mean SARS-CoV2 IgG Ab titre† | ||||
| Post first vaccine dose (p=0.000) | 2.40 (4.90) | 5.96 (7.56) | 1.24 (2.5) | 8.91 (11.77) |
| 0.5 (1.9) | 3.3 (7.2) | 0.5 (0.75) | 4.2 (8.4) | |
| P=0.000 | P=0.232 | P=0.000 | ||
| Post second vaccine dose (p=0.009) | 62.11 (80.90) | 59.19 (67.47) | 12.88 (18.23) | 79.49 (136.74) |
| 22.95 (77.9) | 41.95 (80.1) | 4.9 (20.2) | 48.4 (67.2) | |
| P=0.490 | P=0.551 | P=0.000 | ||
| Withhold group | ||||
| Detectable SARS-CoV2 IgG Ab* | ||||
| Post first vaccine dose (p=0.070) | 16/20 (76.19) | 22/24 (91.67) | 18/28 (64.3) | 46/51 (90.20) |
| P=0.143 | P=0.603 | P=0.007 | ||
| Post second vaccine dose | 20/20 (100) | 20/20 (100) | 27/27 (100) | 49/49 (100) |
| Mean SARS-CoV2 IgG Ab titre† | ||||
| Post first vaccine dose (p=0.110) | 6.05 (12.31) | 4.85 (3.98) | 3.4 (4.66) | 8.91 (11.77) |
| 2.85 (3.9) | 3.7 (6.5) | 1.4 (2.75) | 4.2 (8.4) | |
| P=0.076 | P=0.431 | P=0.002 | ||
| Post second vaccine dose (p=0.617) | 70.57 (107.13) | 74.05 (71.60) | 86.27 (150.6) | 79.49 (136.74) |
| 43.45 (96.4) | 64.65 (74) | 40.65 (91.9) | 48.4 (67.2) | |
| P=0.642 | P=0.319 | P=0.831 |
Data are presented as proportions (%) or mean (SD) and median (IQR).
Comparison of DMARD categories with controls using Fisher’s exact test or Wilcoxon-Mann-Whitney U test as appropriate with significance cut-off 5%.
*Using Fisher’s exact test with significance cut-off 5%.
†Using Kruskal-Wallis test among the immunosuppression groups only with significance cut-off 5%.
Ab, antibody; bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease-modifying antirheumatic drug; IMID, immune-mediated inflammatory disease; tsDMARD, targeted synthetic DMARD.
In the withhold group, there was no significant difference in the seroconversion rate between each of the DMARD groups following the first and second vaccine doses (p=0.070). In addition, there were also no significant differences in the mean SARS-CoV2 IgG Ab titres following the first and second vaccine doses among the DMARD groups (p=0.110 and p=0.617, respectively) (table 5).
When comparing intervention within each of the DMARD classes, withholding tsDMARD resulted in a significantly higher mean SARS-CoV2 IgG Ab titre following the first and second vaccinations (p=0.000 and p=0.001), respectively while significance was only reached in the csDMARD group following the first vaccination (p=0.018). There was no difference in vaccine response observed between the groups who withheld or continued bDMARDs (table 6).
Table 6.
Results of significance testing (p values) comparing withholding therapy versus continuing therapy within DMARD classes
| csDMARD | bDMARD | tsDMARD | |
| Detectable SARS-CoV2 IgG Abs* | |||
| Post first vaccine dose | 0.031 | 0.274 | 0.001 |
| Post second vaccine dose | 0.510 | 0.285 | 0.111 |
| Mean SARS-CoV2 IgG Ab titre† | |||
| Post first vaccine dose | 0.018 | 0.706 | 0.000 |
| Post second vaccine dose | 0.620 | 0.231 | 0.001 |
*Using Fisher’s exact test with significance cut-off 5%.
†Using Kruskal-Wallis test among the immunosuppression groups only with significance cut-off 5%.
Ab, antibody; bDMARD, biologic DMARD; csDMARD, conventional synthetic DMARD; DMARD, disease-modifying antirheumatic drug; tsDMARD, targeted synthetic DMARD.
Comparison of SARS-CoV-2 vaccination responses in DMARD groups compared with controls
Compared with the controls, the continue therapy group SARS-CoV-2 IgG seroconversion rates were significantly lower in csDMARD and tsDMARD following the first dose (40.91% vs 90.20%, p=0.000 and 19.23% vs 90.20%, p=0.000), respectively while following the second dose the bDMARD and tsDMARD groups had slightly lower seroconversion rates (88.24% vs 100%, p=0.025 and 88.46% vs 100%, p=0.039, respectively). Of those who mounted a serological response in the withhold group, only the tsDMARD group had a significantly lower seroconversion rate following the first dose (64.3% vs 90.20%, p=0.007) along with a lower mean SARS-CoV-2 IgG Ab titre (3.4 vs 8.91 U/mL, p=0.002). All DMARD groups who withheld therapy after the first dose seroconverted following the second vaccine dose (table 5).
For both the csDMARD and bDMARD groups, withholding therapy during the first vaccine dose did not result in a significant difference in the seroconversion rate compared with the control group (76.19% vs 90.20%, p=0.143 and 91.67 vs 90.20%, p=0.603, respectively). Following the second vaccine dose, there was no significant difference in the mean SARS-CoV2 IgG Ab titre in all the DMARD groups compared with the controls (table 5).
Analysis of factors affecting vaccine response
We analysed factors that influenced the immunisation response and found that patients receiving the Pfizer vaccine were at substantially higher odds of mounting a protective response compared with patients receiving the AZ vaccine (OR=16.24, 95% CI: 8.18 to 32.25, p=0.000). In addition, withholding DMARD therapy was found to confer higher odds of seroconverting (OR=2.55, 95% CI: 1.33 to 4.90). In contrast, there was a slightly reduced odds of seroconversion in those with longer durations between vaccinations (OR=0.952, 95% CI: 0.941 to 0.964, p=0.000) (online supplemental table 1).
rmdopen-2022-002301supp001.pdf (200.1KB, pdf)
After controlling for the impact of age, sex, BMI, lymphocyte count, renal impairment, IMID and vaccine type on vaccine response, only vaccine type and withholding DMARD therapy remained significantly associated with protective IgG antibody levels. Patients receiving the Pfizer vaccine were at 489% higher odds of achieving a serum IgG antibody titre of above 25 U/mL following two doses of the vaccine (OR=5.89, 95% CI: 2.19 to 15.85, p=0.000) while in the withhold group there was 3.59 times greater odds of seroconverting post second vaccine dose when an IgG cut-off of 25 U/mL was used relative to patients who continued with DMARD therapy (OR=3.590, 95% CI: 1.441 to 8.943). After controlling for the effect of vaccine type, the interval between vaccine doses was no longer a predictor of seroconversion (OR=0.974, 95% CI: 0.944 to 1.004). Lastly, there was a reduced OR of seroconverting with the use of tsDMARD after controlling for DMARD category (OR 0.409, 95% CI: 0.146 to 1.144) (online supplemental table 2).
Side effects and flares following the SARS-CoV-2 vaccination
The tolerability of both the Pfizer and AZ vaccines were similar across the IMID and HC (Healthy Control) groups except for the higher incidence of rash in the group that continued with DMARD therapy (p=0.0478). The most common adverse event in all groups was injection site pain and fatigue. Ten versus six patients (8.3% vs 6.52%) in the withhold and continue therapy groups, respectively had an IMID flare however was not statistically significant (p=0.294) (table 7).
Table 7.
Vaccine side effects
| Side effect* | Autoimmune disease group | Healthy controls | Total | |
| Withhold therapy | Continue therapy | |||
| Fever (p=0.4253) | 6/83 (7.23) | 3/92 (3.26) | 2/55 (3.64) | 11/230 (4.78) |
| Fatigue (p=0.1186) | 19/83 (22.89) | 12/92 (13.04) | 14/55 (25.45) | 45/230 (19.57) |
| Headache (p=0.0786) | 5/83 (6.02 | 15/92 (16.3) | 9/55 (16.36) | 29/230 (12.61) |
| Chills (p=0.2200) | 10/83 (12.05) | 5/92 (5.43) | 7/55 (12.73) | 22/230 (9.57) |
| Nausea/Vomiting (p=0.9624) | 3/83 (3.61) | 4/92 (4.35) | 2/55 (3.64) | 9/230 (3.91) |
| Diarrhoea (p=0.5099) | 0/83 (0) | 1/92 (1.09) | 1/55 (1.82) | 2/230 (0.87) |
| Muscle pain (p=0.1842) | 24/83 (28.92) | 26/92 (28.26) | 23/55 (41.82) | 73/230 (31.74) |
| Joint pain (p=0.8629) | 6/83 (7.23) | 5/92 (5.43) | 3/55 (5.45) | 14/230 (6.09) |
| Antipyretic use (p=0.4629) | 1/83 (1.20) | 4/92 (4.35) | 2/55 (3.64) | 7/230 (3.04) |
| Disease flare (p=0.294) | 10/83 (12.05) | 6/92 (6.52) | n/a | 16/175 (9.14) |
| Rash (p=0.0478*) | 0/83 (0.00) | 4/92 (4.35) | 0/55 (0.00) | 4/230 (1.74) |
| Other (p=0.2217) | 0/83 (0.00) | 2/92 (2.17) | 0/55 (0.00) | 2/230 (0.87) |
| Nil (p=0.7581) | 29/83 (34.94) | 34/92 (36.96) | 17/55 (30.91) | 90/230 (34.78) |
Data are presented as proportions (%).
*Using Fisher’s exact test with significance cut-off 5%.
n/a, not available.
Discussion
Our study demonstrates that the antibody response following the first dose of the Pfizer and AZ vaccines in the IMID patients who continued with DMARD therapy were delayed and reduced compared with the patients that temporarily suspended DMARD therapy. Despite this, the mean SARS-CoV2 IgG levels in the AZ group were not significantly different between the patients and controls after the second vaccine dose, irrespective of DMARD hold. In the Pfizer group however, holding therapy resulted in higher SARS-CoV2 IgG antibody levels than the continue group, which was comparable to the controls. The seroconversion rates and SARS-CoV-2 IgG Ab levels were higher in the Pfizer than the AZ group across all study arms suggesting that the Pfizer vaccine is more immunogenic. Receiving a second vaccine dose appears to have an additive effect on cumulative immunogenicity sufficient to mitigate the effect of being on a csDMARD but not tsDMARD hence supporting the need for two full vaccinations for efficient vaccination responses.19
Our study also suggests that DMARD therapy has an immunomodulatory effect on SARS-CoV-2 IgG antibody production with the most important period following the initial vaccination when naïve T cells are being primed. In the group who continued DMARD therapy, the post first dose seroconversion rate was reduced but then increased significantly after the second vaccination suggesting a delayed antibody response. Given the recognised patterns of antibody production in COVID-19 infection, it is biologically plausible that interrupting DMARD therapy following the initial vaccination improves the antibody response and resultant trajectory of antibody production following the second vaccination dose. It is postulated that if logistically feasible, withholding DMARD therapy following the second vaccination may result in further increases in SARS-CoV-2 IgG antibody levels with the boosting of the already primed T cells, however this must be weighed with the potential increased risk for disease flare. Alternatively, offering an additional or third vaccine dose as part of an extended primary series may also help patients on DMARDs to achieve a sufficient protective immune response.
Drug elimination is dependent on a number of pharmacokinetic parameters, which include age, distribution, renal and hepatic function, genetic variation, smoking, route of administration and half-life.20 Withholding each drug dependent on these factors would be complicated and the duration required would be different for each subject and medication. Hence for practical considerations, the DMARDs were withheld for 1 week after the first vaccine dose with the exception of MTX, which was withheld for two doses based on data from influenza vaccines.21–23 The risk for potential flares were also considered and hence therapy was not withheld for longer than 2 weeks nor following the second vaccination.24 In addition, given the complexity of withholding therapy around each of the two vaccine doses for the Pfizer vaccine which are spaced 3 weeks apart, the DMARDs were withheld following the first dose only.
The withhold regimen was an effective strategy for all DMARD groups, however most so in the group taking tsDMARDs. This could be due to the relatively short half-lives of these medications being 3.2, 12.5 and 9–14 hours for tofacitinib, baricitinib and upadacitinib, respectively.25–27 JAKs mediate signal transduction for numerous cytokines including those involved in T-cell activation and proliferation. The JAK-signal transducer and activator of transcription (STAT) pathway is important for both innate and adaptive immunity.28 STAT2 deficiency has been described to increase the susceptibility to viral infections as it is required for type 1 interferon signalling and studies with tofacitinib and baricitinib have been shown to diminish the responsiveness to the pneumococcal vaccine.29–31 When tofacitinib therapy was interrupted for 2 weeks before the pneumococcal vaccine, there was no significant difference however a higher dose of tofacitinib at 10 mg twice daily was used in that study suggesting that the most important time to withhold therapy is immediately after vaccination.29 Our study was not sufficiently powered to test whether there were any differences in SARS-Cov-2 IgG antibody responses with JAK inhibitor selectivity. In contrast to another study, our results support the withholding of JAKi in relation to the COVID-19 vaccination as recommended by the ACR COVID-19 vaccination guidelines.32 33
JAKi with baricitinib and corticosteroids in patients with moderate-to-severe SARS-CoV-2 pneumonia have been associated with greater improvement in pulmonary function compared with corticosteroids alone.34 A systematic review has also found the all-cause mortality rate at day 28 was lower among patients receiving JAKi compared with the control group.35 The discordance in findings that JAKi reduces SARS-CoV-2 IgG antibody responses with the improved clinical outcomes of patients with COVID-19 could come down to timing. Patients with severe COVID-19 present with an exaggerated immune response characterised by the increased production of interleukin (IL)-6, IL-2, IL-7, IL-10, granulocyte-colony-stimulating factor, interferon-gamma, macrophage inflammatory protein 1α and tumour necrosis factor-α. JAK1 and JAK2 inhibitors may inhibit the signalling of type I interferon, IL-6, interferon-gamma and IL-2 dampening the effects of the immune dysregulation and cytokine storm, however when given too early may conversely impair the immune response to SARS-CoV-2.36
Despite the heterogeneous cohort of immunosuppressive medications used, they can be grouped by similar mechanisms of action enabling the interrogation of drug-specific classes on the effects of vaccine immunogenicity. Our study showed that seroconversion is lower in patients receiving the AZ vaccine compared with the Pfizer vaccine in both the control and IMID cohorts, however the average age of the AZ group was older given the ATAGI age preference for vaccine eligibility being >60 years old at the time of study enrolment.15 Immunosenescence especially in those aged over 70 years have been shown to result in lower total IgG against the RBD spike protein and neutralising antibody titres than younger subjects.9
The safety of both the AZ and Pfizer vaccines were reassuring for a good safety profile with most adverse events being mild and temporary consistent with other studies and registry data.32 37 38 Withholding the DMARD contemporaneous to the first vaccination did not drive disease flare ups significantly.
The importance of both cellular and humoral immunity for the protection against SARS-CoV-2 infection remains yet to be fully elucidated. Our study assessed humoral immunity only using the Siemens electrochemiluminescence immunoassay to measure antibody concentrations instead of using a neutralisation assay. Despite this, the quantitative values of antibodies against the RBD of the S-Protein of SARS-Cov-2 have been shown to be a good correlation with virus neutralisation titres (r=0.843; p<0.0001) and an overall qualitative agreement of 98.5%.39 When compared head to head with other SARS-CoV-2 assays, only the Siemens and Roche assays achieved a sensitivity of at least 98.1% and specificity of at least 98% without further optimisation.40
The Siemens ADVIA Centaur sCOVG assay reports a range of quantification of 0.5–750.0 U/mL, 99.4% specificity and 90.5% sensitivity while the S1-RBD antibody levels show a good correlation with virus neutralisation titres (r=0.843; p<0.0001).39 It is still unclear what level of SARS-CoV-2 IgG antibody and consequently neutralising antibody levels are required for protection against severe SARS-CoV-2 infection. A predictive model of immune protection has shown that a 50% protective neutralisation level has been estimated to be approximately 20% of the average convalescent level while for a 50% level of protection from severe infection, only approximately 3% of the average convalescent level is required.41 In rhesus macaques, the neutralising antibody titre thresholds for full protection against SARS-CoV-2 was approximately 500 while a titre of approximately 50 provided partial protection.42 Since these titres are readily achievable by vaccination in humans, a SARS-CoV-2 IgG titre cut-off of 7 and 25 U/mL were used as a reference during the logistic regression analysis for our study as this equated to a neutralising antibody titre of 50 and 500, respectively based on the strong correlation to viral neutralisation testing as per the graph on page 14 of the Siemens Advia Centaur kit insert.16 Hence, even with relatively low SARS-CoV-2 levels in the IMID group which correlate with low neutralising antibody titres, there could still be adequate protection against SARS-CoV-2 suggesting that T-cell immune responses are also contributory to protection.42
Our study had several limitations, one of which included not withholding therapy following the second vaccination. This was mainly due to the Pfizer vaccination which was given 3 weeks apart and withholding therapies given fortnightly or greater would not have been possible. In addition, there were concerns for disease flare if therapy was withheld consecutively in a short timeframe which was an ethical consideration. Despite this, it has been shown that seroconversion rates and respective antibody titres after the second vaccination are not significantly affected by DMARD monotherapy,19 with the exception of tsDMARDs which was highlighted in our study. Combinations with csDMARD were possible in the tsDMARD group, however the majority of patients were on monotherapy (51/60, 85%). Numerically, all the DMARD groups still showed higher responder rates and antibody titres after the second vaccine when withholding, however was only statistically significant for tsDMARDs. The small sample size and hence a type 2 error cannot be excluded. Although the patients were instructed to withhold therapy following the first vaccine dose only, the possibility remains that in a minority of patients that these instructions were not adhered to and therapy was also withheld following the second vaccination. The intention-to-treat principle was applied to the final analysis.
In Australia, there had been a recommendation to vaccinate people aged 60 years and over with the AZ and 16–59 years old people with the Pfizer vaccine.15 Given the age disparity, the median age of the AZ vaccine group was higher compared with the Pfizer group and hence the two groups could not be age-matched. Hence in addition to age, other factors including disease activity or type were not considered in the main analysis (tables 3 and 4). The majority of patients in this study were also able to afford private healthcare. Medicare-dependent patients may produce different results dependent on socioeconomic and compliance factors.
The blood sampling regimen of our study was quite intensive with three episodes required. The participants who missed the baseline SARS-CoV-2 IgG test however they were all from Western Australia and were unlikely to affect the vaccine responses as there were no cases of community transmission during the study period. The antinuceleocapsid Ab could be tested in such patients for confirmation.
In summary, the seroconversion rate with both the AZ and Pfizer vaccines were impaired following the first vaccination however was mitigated following DMARD hold. Despite this, the mean SARS-CoV2 antibody levels were not affected following the second vaccination regardless of hold with the exception of the tsDMARD group. This places emphasis on withholding tsDMARD therapy following the first vaccination dose and the IMID group having at least two vaccinations preferably with an mRNA vaccine to ensure adequate immune responses.
Acknowledgments
We thank the patients and controls who participated in this study. This research was supported by an Australian Government Research Training Programme (RTP) Scholarship. We thank Dr Francis Cheng, Dr Priya Chowalloor, Ms Joanne CZ Ding, and Dr Ivan Lee for assistance with patient recruitment.
Contributors: All authors contributed to the intellectual content of the submission and preparation of the manuscript and all have approved the final version for submission. Dr A.P Tran is responsible for the overall content as the guarantor of the study.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Antibody RESponse to Covid-19 ChAdOx1nCov-19 and BNT162b vaccines after temporary suspension of DMARD therapy in immUne-mediated inflammatory diseasE
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by both the St John of God and St Vincent’s Hospital Melbourne Human Research Ethics Committee (HREC approval 1809 and 099/21). All participants provided written informed consent prior to commencement.
References
- 1.WHO . Who coronavirus (COVID-19) Dashboard. Available: https://covid19.who.int/table [Accessed 26 Mar 2022].
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–15. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. The Lancet 2021;397:99–111. 10.1016/S0140-6736(20)32661-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahase E. Covid-19: UK approves Moderna vaccine to be given as two doses 28 days apart. BMJ 2021;372:n74. 10.1136/bmj.n74 [DOI] [PubMed] [Google Scholar]
- 5.Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021:1098–9. 10.1136/annrheumdis-2021-220289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon D, Tascilar K, Fagni F, et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis 2021;80:1312–6. 10.1136/annrheumdis-2021-220461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun-Moscovici Y, Kaplan M, Braun M, et al. Disease activity and humoral response in patients with inflammatory rheumatic diseases after two doses of the pfizer mRNA vaccine against SARS-CoV-2. Ann Rheum Dis 2021;80:1317–21. 10.1136/annrheumdis-2021-220503 [DOI] [PubMed] [Google Scholar]
- 8.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020;383:2427–38. 10.1056/NEJMoa2028436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. The Lancet 2020;396:1979–93. 10.1016/S0140-6736(20)32466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis 2021;80:930–42. 10.1136/annrheumdis-2020-219498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann Rheum Dis 2021;80:1137–46. 10.1136/annrheumdis-2021-220418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avouac J, Drumez E, Hachulla E, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol 2021;3:e419–26. 10.1016/S2665-9913(21)00059-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arumahandi de Silva AN, Frommert LM, Albach FN, et al. Pausing methotrexate improves immunogenicity of COVID-19 vaccination in elderly patients with rheumatic diseases. Ann Rheum Dis 2022. 10.1136/annrheumdis-2021-221876. [Epub ahead of print: 14 Mar 2022]. 10.1136/annrheumdis-2021-221876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon SF, Clothier HJ, Morgan H, et al. Immune thrombocytopenia following immunisation with Vaxzevria ChadOx1-S (AstraZeneca) vaccine, Victoria, Australia. Vaccine 2021;39:7052–7. 10.1016/j.vaccine.2021.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ATAGI statement on revised recommendations on the use of COVID-19 vaccine AstraZeneca, 17 June 2021. A statement from the Australian technical Advisory group on immunisation (ATAGI) on the AstraZeneca COVID-19 vaccine in response to new vaccine safety concerns, 2021. Available: https://www.health.gov.au/news/atagi-statement-on-revised-recommendations-on-the-use-of-covid-19-vaccine-astrazeneca-17-june-2021
- 16.Siemens ADVIA Centaur SARS-CoV-2 IgG assay, in Centaur SARS-CoV-2 IgG kit insert 2020;14. [Google Scholar]
- 17.Whitley E, Ball J. Statistics review 4: sample size calculations. Crit Care 2002;6:335–41. 10.1186/cc1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol 2021;3:e627–37. 10.1016/S2665-9913(21)00212-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simader E, Tobudic S, Mandl P, et al. Importance of the second SARS-CoV-2 vaccination dose for achieving serological response in patients with rheumatoid arthritis and seronegative spondyloarthritis. Ann Rheum Dis 2022;81:416–21. 10.1136/annrheumdis-2021-221347 [DOI] [PubMed] [Google Scholar]
- 20.Garza AZ PS, Kocz R. Drug elimination, 2021. Available: https://www.ncbi.nlm.nih.gov/books/NBK547662/ [PubMed]
- 21.Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2017;76:1559–65. 10.1136/annrheumdis-2017-211128 [DOI] [PubMed] [Google Scholar]
- 22.Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018;77:898–904. 10.1136/annrheumdis-2018-213222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JK, Kim MJ, Choi Y, et al. Effect of short-term methotrexate discontinuation on rheumatoid arthritis disease activity: post-hoc analysis of two randomized trials. Clin Rheumatol 2020;39:375–9. 10.1007/s10067-019-04857-y [DOI] [PubMed] [Google Scholar]
- 24.Araujo CSR, Medeiros-Ribeiro AC, Saad CGS, et al. Two-Week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS-CoV-2 vaccine: a randomised clinical trial. Ann Rheum Dis 2022. 10.1136/annrheumdis-2021-221916. [Epub ahead of print: 22 Feb 2022]. [DOI] [PubMed] [Google Scholar]
- 25.Dowty ME, Lin J, Ryder TF, et al. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a Janus kinase inhibitor, in humans. Drug Metab Dispos 2014;42:759–73. 10.1124/dmd.113.054940 [DOI] [PubMed] [Google Scholar]
- 26.Australian Prescriber . Baricitinib for rheumatoid arthritis, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Australian Prescriber . Upadacitinib for rheumatoid arthritis, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Shea JJ, Holland SM, Staudt LM. Jaks and STATs in immunity, immunodeficiency, and cancer. N Engl J Med 2013;368:161–70. 10.1056/NEJMra1202117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winthrop KL, Silverfield J, Racewicz A, et al. The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis 2016;75:687–95. 10.1136/annrheumdis-2014-207191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winthrop KL, Bingham CO, Komocsar WJ, et al. Evaluation of pneumococcal and tetanus vaccine responses in patients with rheumatoid arthritis receiving baricitinib: results from a long-term extension trial substudy. Arthritis Res Ther 2019;21:102. 10.1186/s13075-019-1883-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med 2015;66:311–28. 10.1146/annurev-med-051113-024537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. 10.1136/annrheumdis-2021-220647 [DOI] [PubMed] [Google Scholar]
- 33.Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients With Rheumatic and Musculoskeletal Diseases: Version 2. Arthritis Rheumatol 2021;73:e30–45. 10.1002/art.41877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Garcia JL, Sanchez-Nievas G, Arevalo-Serrano J, et al. Baricitinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: an observational cohort study. Rheumatology 2021;60:399–407. 10.1093/rheumatology/keaa587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C-Y, Chen W-C, Hsu C-K, et al. Clinical efficacy and safety of Janus kinase inhibitors for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Int Immunopharmacol 2021;99:108027. 10.1016/j.intimp.2021.108027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med Overseas Ed 2020;383:2255–73. 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021;80:1306–11. 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machado PM, Lawson-Tovey S, Strangfeld A, et al. Safety of vaccination against SARS-CoV-2 in people with rheumatic and musculoskeletal diseases: results from the EULAR coronavirus vaccine (COVAX) physician-reported registry. Ann Rheum Dis 2022;81:695-709. 10.1136/annrheumdis-2021-221490 [DOI] [PubMed] [Google Scholar]
- 39.Irsara C, Egger AE, Prokop W, et al. Clinical validation of the Siemens quantitative SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. Clin Chem Lab Med 2021;59:1453–62. 10.1515/cclm-2021-0214 [DOI] [PubMed] [Google Scholar]
- 40.National SARS-CoV-2 Serology Assay Evaluation Group . Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis 2020;20:1390–400. 10.1016/S1473-3099(20)30634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 42.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2021;590:630–4. 10.1038/s41586-020-03041-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002301supp001.pdf (200.1KB, pdf)
Data Availability Statement
Data are available on reasonable request.