Abstract
The potential of the biocontrol agent Trichoderma harzianum T-203 to trigger plant defense responses was investigated by inoculating roots of cucumber seedlings with Trichoderma in an aseptic, hydroponic system. Trichoderma-treated plants were more developed than nontreated plants throughout the experiment. Electron microscopy of ultrathin sections from Trichoderma-treated roots revealed penetration of Trichoderma into the roots, restricted mainly to the epidermis and outer cortex. Strengthening of the epidermal and cortical cell walls was observed, as was the deposition of newly formed barriers. These typical host reactions were found beyond the sites of potential fungal penetration. Wall appositions contained large amounts of callose and infiltrations of cellulose. The wall-bound chitin in Trichoderma hyphae was preserved, even when the hyphae had undergone substantial disorganization. Biochemical analyses revealed that inoculation with Trichoderma initiated increased peroxidase and chitinase activities within 48 and 72 h, respectively. These results were observed for both the roots and the leaves of treated seedlings, providing evidence that T. harzianum may induce systemic resistance mechanisms in cucumber plants.
In the past few years, Trichoderma spp., the most common saprophytic fungi in the rhizosphere, have received considerable attention as potential biocontrol agents for a number of soilborne pathogens (9, 35). The mechanisms by which Trichoderma isolates control pathogenic populations in the rhizosphere have been extensively studied. Recent progress in the purification and identification of Trichoderma metabolites has led to the notion that, in most cases, the antagonistic process relies on the production of antibiotics (16) and/or hydrolytic enzymes (17, 18, 20) associated with possible competition for nutrients in the rhizosphere (37). In spite of the increasing amount of research devoted to the antimicrobial activity of Trichoderma spp. in vitro (19, 23), knowledge of the exact mechanisms responsible for the observed reduction of disease incidence following soil treatment with Trichoderma propagules is still incomplete. Indeed, most studies have focused on microbial interactions and not on the possible involvement of the host plant, although circumstantial evidence correlating increased plant growth response with penetration of Trichoderma harzianum into the root system has been reported (25).
In recent studies, substrate amendment with T. harzianum resulted in enhanced plant growth throughout the growing season (1, 19). Biocontrol of minor pathogens in the rhizosphere, improved mineral uptake, nutrient release from the soil and organic matter, and enhanced plant hormone production (6, 19, 22) may partially explain the Trichoderma-mediated enhanced growth response. However, the possibility that Trichoderma spp. interact with root tissues and induce host plant resistance to pathogens has seldom been raised. This latter concept, along with the recent demonstration that infection with beneficial fungi, such as endomycorrhizal fungi, causes host plants to respond more rapidly and efficiently to pathogen attack (12, 29), raises the following question: to what extent might nonpathogenic fungi, such as Trichoderma spp., stimulate the plant defense response, leading to the activation of genes and ultimately to the accumulation of defense molecules?
A growing body of evidence from various studies indicates that increased resistance of arbuscular mycorrhizal roots may be associated in part with marked metabolic changes in the host, including enhanced production of peroxidases and phenolic compounds (39); accumulation of hydrolases, such as chitinases and β-1,3-glucanases, with antimicrobial potential (12, 38); and deposition of structural polymers, such as lignin (7) and hydroxyproline-rich glycoproteins (2). If one considers that the increased production of peroxidases and phenolic compounds may be of key importance in the resistance process (10) and that the accumulation of structural substances may increase the mechanical strength of the host cell walls, the induction of such defense mechanisms by beneficial fungi would likely inhibit or at least restrict pathogen invasion. However, at present, the situation is not clearly defined, and additional research is needed to confirm the effective stimulation of the plant defense system upon infection by nonpathogenic fungi. Nevertheless, these earlier observations, together with the finding that Trichoderma spp. are able to promote plant growth (22), raise key questions. (i) Does Trichoderma penetrate the root tissues, and if so, what are the pattern of fungal colonization and the relationship with the host plant? (ii) Is Trichoderma capable of stimulating the plant to defend itself through the accumulation of defense molecules?
In an attempt to answer these important questions, the objectives of the research described here were (i) to determine whether or not T. harzianum penetrates the epidermis of cucumber roots and develops in the inner tissues, (ii) to delineate the biological events associated with the cucumber-Trichoderma interaction, and (iii) to biochemically investigate the induction of some potential defense molecules. As a result, we present the first conclusive evidence that T. harzianum penetrates and grows in the epidermis and outer cortex and stimulates the plant defense system, leading to the production of biochemical and structural compounds.
MATERIALS AND METHODS
Plant material.
Seeds of cucumber (Cucumis sativus L. cv. Delila) from Gedera Seeds, Gedera, Israel, were used in this experiment. Plant growth medium (PGM) consisted of, per liter, 0.24 g of MgSO4, 0.04 g of K2HPO4, 0.17 g of K2SO4, 0.344 g of CaSO4 · H2O, 0.64 g of NH4NO3, and 1 ml of the following trace elements (per liter of stock solution): 0.05 g of FeCl3, 0.728 g of KCl, 1.546 g of H3BO3, 0.846 g of MnSO4 · H2O, 0.375 g of ZnSO4 · 7H2O, 0.125 g of CuSO4 · 5H2O, 0.081 g of H2MoO4, and 0.001 g of CoCl2 · 6H2O (pH 7.0).
Fungal material.
T. harzianum T-203 (13) was grown on potato dextrose agar (Difco). Synthetic medium for T. harzianum consisted of, per liter, 15 g of glucose, 0.2 g of MgSO4 · 7H2O, 0.6 g of K2HPO4, 0.15 g of KCl, 1 g of NH4NO3, and 1 ml of the following trace elements (per liter of stock solution: 0.005 g of FeSO4 · 7H2O, 0.006 g of MnSO4 · H2O, 0.004 g of ZnSO4 · H2O, and 0.002 g of CoCl2 (31). One milliliter (109 to 1010 spores, as counted with a hemocytometer) of 7-day-old T. harzianum cultured on potato dextrose agar was used as the inoculum for a 250-ml flask containing 100 ml of synthetic medium. The flask was shaken at 150 rpm for 24 h at 30°C to allow spore germination. After 24 h, the mycelial inoculum was separated from the growth medium by centrifugation at 10,000 × g and 4°C and washed twice in 100 ml of distilled water.
Axenic growth system.
Growth chambers were made of bicomponent, autoclavable, transparent polycarbonate boxes (Biological Industries, Beit Haemek, Israel). The top part was 13.7 cm high (10.7 by 10.7 cm), and the bottom part was 5.2 cm high (10.7 by 10.7 cm), holding up to 500 ml. Seeds were surface disinfected in 70% ethanol for 2 min, followed by 2.0% NaOCl for 2 min, and thoroughly washed with sterile distilled water. Seeds (24 per box) were placed on a sterile gauze sheet over a sterile stainless steel screen, which held them 1 cm above 300 ml of PGM. Plants were grown in a controlled environment: 26°C, 80% relative humidity, and a circadian cycle of 14 h of light and 10 h of dark. A Tygon autoclavable tube was inserted into the bottom part through a breach and hooked to an oxygen stone to aerate the liquid medium with atmospheric air filtered through a 0.45-μm-pore-size filter.
Plant inoculation with T. harzianum.
Mycelial inoculum was added under aseptic conditions to the PGM of 7-day-old seedlings to a final concentration of ±105 germinated spores/ml. The concentration of the Trichoderma inoculum was determined to be 10−3 times the concentration used for biocontrol under greenhouse conditions (107 to 108 CFU/g of soil). Thus, ±105 germinated spores/ml of PGM were used. Control plants were treated with sterile distilled water.
Tissue processing for electron microscopic studies.
Root samples (2 mm3) collected from the crown and the main root at sites of attempted fungal penetration 5 days after inoculation were embedded in 1% (wt/vol) aqueous Bacto Agar prior to fixation by immersion in 3% (vol/vol) glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 2 h at room temperature. Root samples were postfixed in 1% (wt/vol) osmium tetroxide in the same buffer for 1 h at 4°C, dehydrated in a graded ethanol series, and embedded in Epon 812. Ultrathin sections (0.1 μm) were cut with a diamond knife and collected on Formvar-coated nickel grids. The sections were either treated with uranyl acetate and lead citrate for immediate examination in a JEOL 1200 EX transmission electron microscope operating at 80 kV or further processed for cytochemical labeling. For each treatment, an average of five samples from five different roots were investigated. For each sample, 10 to 15 ultrathin sections were examined.
Preparation of the gold-complexed probes.
Colloidal gold with particles averaging 12 nm in diameter was prepared as described by Frens (14) with sodium citrate as the reducing agent. To localize cellulosic compounds, a β-1,4-exoglucanase (β-1,4-glucan cellobiohydrolase) purified from a cellulase produced by T. harzianum was directly complexed to colloidal gold at pH 9.0 (5). Callose, a polymer of β-1,3-glucans, was localized with β-1,3-glucanase extracted and purified from tobacco plants reacting hypersensitively to tobacco mosaic virus (24). The enzyme was complexed to gold particles at pH 5.5 (4).
N-Acetylglucosamine residues (chitin) were localized by a two-step procedure (3) with wheat germ agglutinin (WGA) as the first-step reagent and gold-complexed ovomucoid as the second-step reagent. The ovomucoid was complexed to the gold at pH 5.4. All gold-conjugated probes were stored at 4°C and centrifuged at 2,600 × g for 2 min before use.
Cytochemical labeling.
Labeling with the gold-complexed β-1,4-exoglucanase and β-1,3-glucanase was performed by first incubating the ultrathin root sections for 5 to 10 min in 1 drop of 0.01 M sodium phosphate-buffered saline (PBS) containing 0.02% (wt/vol) polyethylene glycol 20000 at pH 6.0, corresponding to the optimal activity of the enzyme. Sections were transferred to 1 drop of gold-complexed probe for 30 to 60 min at room temperature in a moist chamber. After careful washing with PBS (pH 7.2) and rinsing with distilled water, sections were treated with uranyl acetate and lead citrate and observed in the JEOL 1200 EX transmission electron microscope.
For the indirect labeling of chitin, sections were first incubated in 1 drop of PBS (pH 7.2), transferred to 1 drop of WGA diluted 1:60 in PBS (pH 7.2), and finally incubated in 1 drop of ovomucoid-gold complex (3). Sections were treated as already described. The specificity of the different labeling procedures was assessed by the following control tests: (i) addition of the corresponding substrate to each gold-complexed probe for a competition experiment (β-1,4-glucans from barley [1 mg/ml] for the β-1,4-exoglucanase–gold complex, laminarin [1 mg/ml] for the β-1,3-glucanase–gold complex, and N-N′-N"-triacetylchitotriose [1 mg/ml] for WGA; (ii) substitution of the gold complex under study with a bovine serum albumin-gold complex to assess nonspecific adsorption of the protein-gold complex to the tissue sections; (iii) incubation of the tissue sections with the enzyme-gold complexes under nonoptimal conditions for biological activity; and (iv) incubation of the tissue sections with colloidal gold alone to assess nonspecific adsorption of the gold particles to the tissue sections.
Protein extraction.
Seedlings were divided into portions, stems were excluded, and roots and leaves were separated, washed under running tap water for 5 min, dried gently, weighed, and ground with a mortar and pestle under liquid nitrogen. The ground matter was homogenized (2 min, 4°C) in phosphate buffer (1:2 w/v, pH 6, 0.05 M) by use of Corex tubes and an ULTRA-TURRAX apparatus (TP 18/10; IKA-WERK, Staufen, Germany). The homogenate was centrifuged twice at 10,000 × g and 4°C, and the supernatant was collected and kept at −20°C.
Detection of chitinase and peroxidase activities.
The total chitinase activity assay was based on the colorimetric determination of p-nitrophenyl cleaved from a chitin-analogous substrate, p-nitrophenyl-β-d-N,N′-diacetylchitobiose (PNP) (20, 33). A crude enzyme preparation and 10 μl of PNP stock solution (2 mg/ml) were added to 50 mM acetate buffer (pH 5.0) to a total volume of 0.5 ml and incubated for 2 h in a water bath at 37°C. The reaction was terminated with 0.5 ml of 0.2 M Na2CO3. An extinction coefficient of 7 × 103 mM−1 cm−1 at 410 nm was used to determine p-nitrophenyl release from the substrate (Varian Techtron DMS100 UV-visible spectrophotometer). Chitinase activity was expressed as millimoles of PNP produced per gram of fresh tissue per hour.
Peroxidase activity was assayed spectrophotometrically at 610 nm with phenol red as a substrate. The complete reaction mixture (1 ml, 37°C) contained 10 to 20 μl of a crude enzyme preparation, 50 μl of 0.2% (wt/vol) phenol red, and 50 mM sodium citrate (pH 4.2). Reactions were initiated with 10 μl of 1 mM hydrogen peroxide and stopped after 3 min with 40 μl of 2 N sodium hydroxide. The optical density was detected at 610 nm as described above. The absorbance was recorded at 610 nm and calculated with a molar extinction coefficient of 22,000 M−1 cm−1 for the oxidized product (34). Peroxidase activity was expressed as millimoles of phenol red oxidized per gram of fresh tissue per minute.
Enzymatic assays consisted of 15 plants per treatment and were repeated in at least four independent experiments, which showed similar results. The average of two representative experiments is shown (see Fig. 5 and 6).
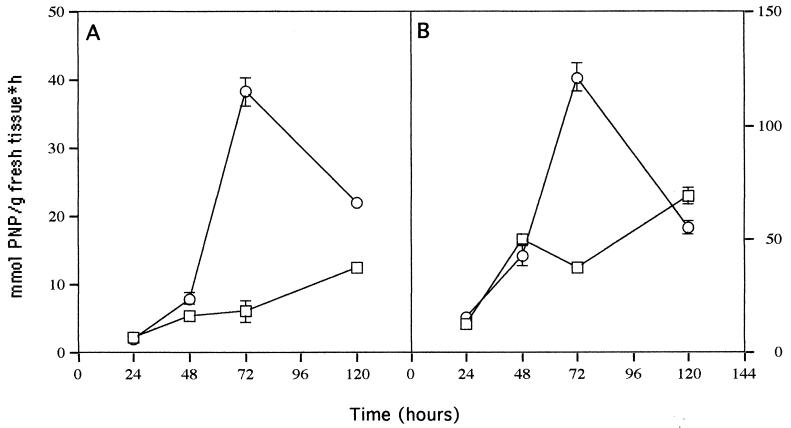
FIG. 5.
Chitinase activity in roots (A) and leaves (B) of 7-day-old cucumber seedlings inoculated with Trichoderma (○) at 24 to 120 h and uninoculated controls (□). Bars represent 1 standard deviation.
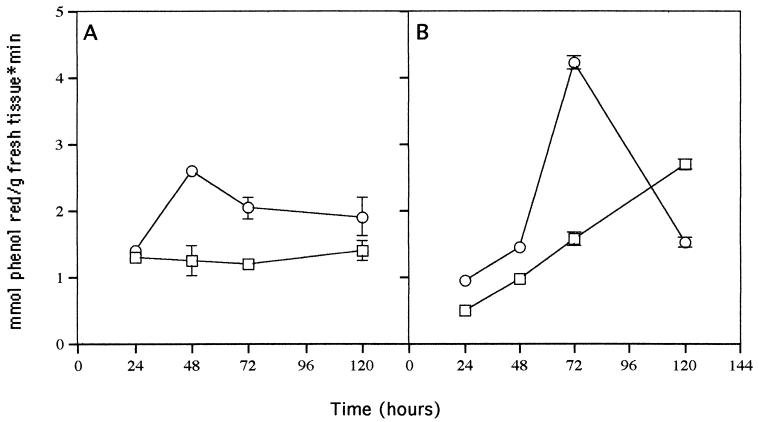
FIG. 6.
Peroxidase activity in roots (A) and leaves (B) of 7-day-old cucumber seedlings inoculated with Trichoderma (○) at 24 to 120 h and uninoculated controls (□). Bars represent 1 standard deviation.
RESULTS
Cytology of infection of cucumber root tissues by T. harzianum.
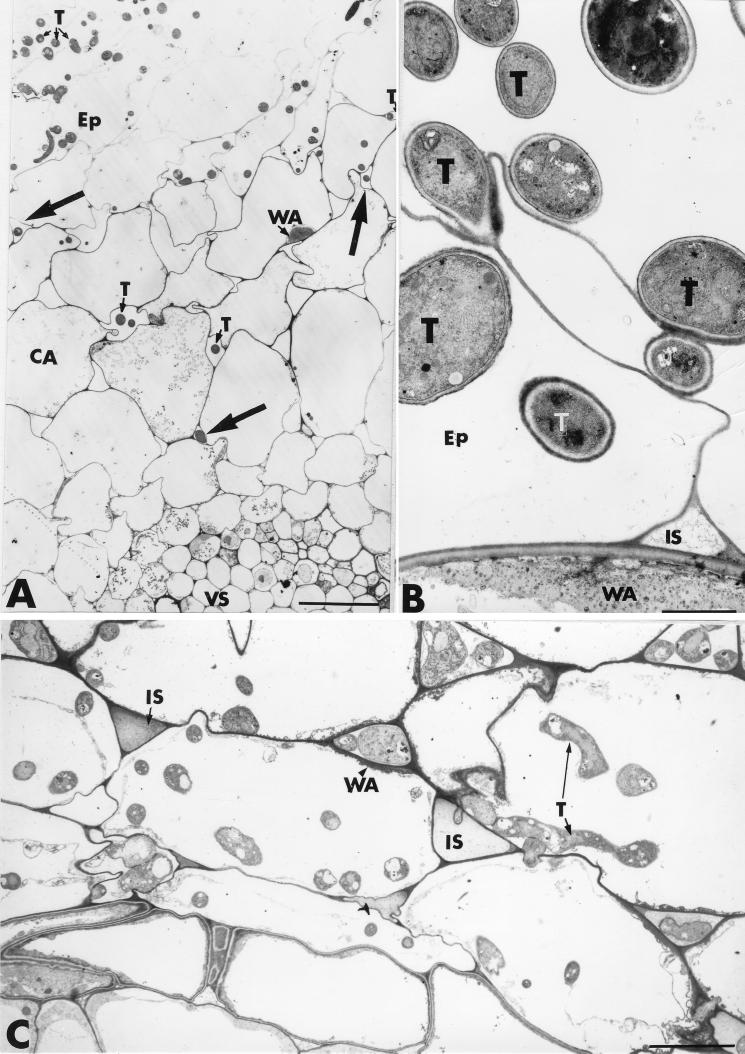
A hydroponic growth system was developed to control growth conditions and medium composition and to eliminate unwanted microorganisms. The growth chambers allowed the development of cucumber seedlings for up to 21 days. An increased growth response was observed during the growth period in the Trichoderma-treated plants. This experimental approach confirms, for the first time, the direct impact of the Trichoderma fungus on treated plants. Visual monitoring of the progression of the fungal-plant interaction indicated complete attachment of the fungal hyphae to the root surface as early as 48 h postinoculation. Transverse sections of root samples taken from cucumber seedlings grown in the presence of T. harzianum revealed a large number of fungal hyphae growing on the root surface and establishing intimate contact with the host exodermis (Fig. 1A and B). About 30 root sections were observed, providing evidence that the fungus penetrates the root epidermis without extensive host cell wall degradation (Fig. 1B). The fungus then multiplies in the epidermis and progresses toward the cortical area, mainly by intercellular growth (Fig. 1A, arrows). Fungal ingress into the innermost root tissues was seldom observed, except in some localized areas, where Trichoderma cells could be seen in the endodermis (Fig. 1C).
FIG. 1.
Transmission electron micrographs of T. harzianum-inoculated cucumber root tissues. A large number of fungal hyphae (T) develop at the root surface. Trichoderma hyphae penetrate the root epidermis (Ep) and progress toward the cortical area (CA), mainly by intercellular growth (arrows in A). Wall appositions (WA) are seen in noninvaded host cells beneath the colonized areas. Fungal colonization of the epidermis and cortex is not associated with host cell alterations or cell wall digestion (C). IS, intercellular spaces; VS, vascular stele. Bars: A, 10 μm; B, 1.5 μm; C, 5 μm.
Fungal colonization of the epidermis and cortex usually involved only a few hyphae, characterized by the high electron density of their cytoplasm, and was not associated with the host cell alterations or cell wall digestion reported for various other host-pathogen interactions (Fig. 1A and C).
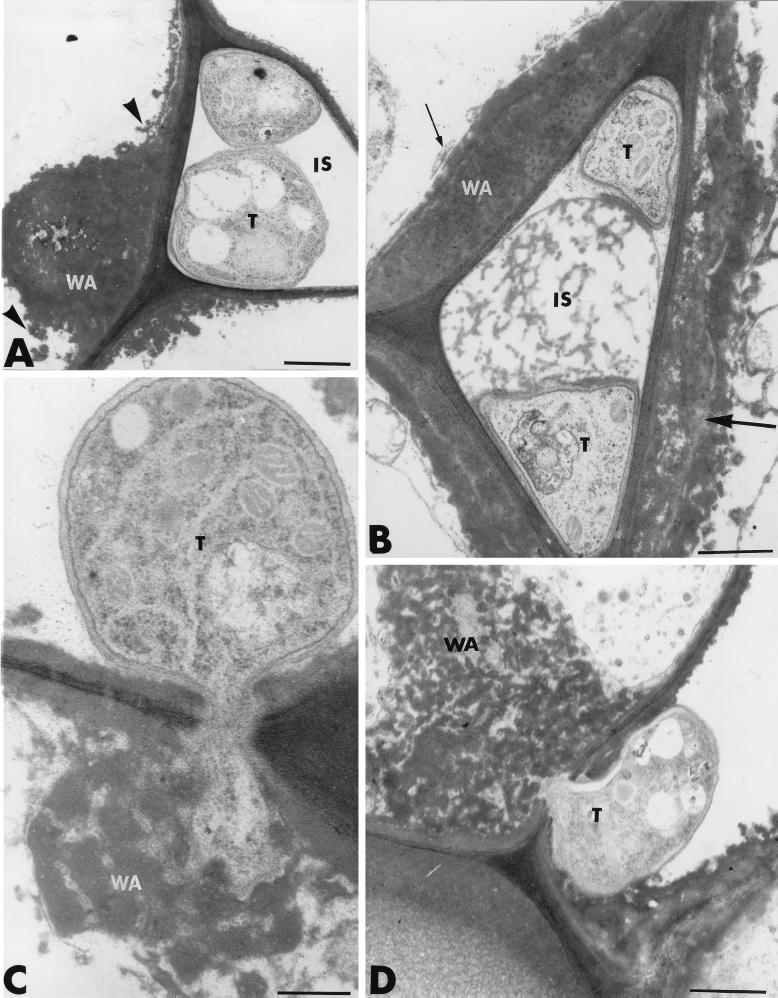
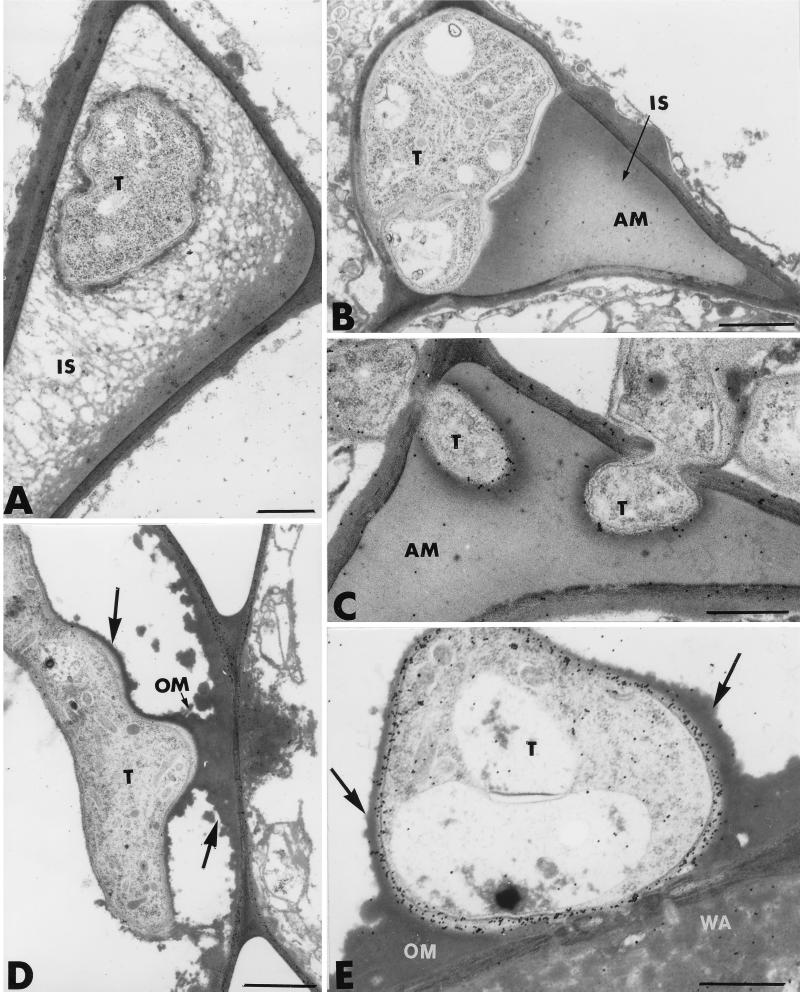
A closer examination of the invaded areas revealed, however, the occurrence of host reactions at sites of attempted fungal penetration. One of the most striking of these was the formation of heterogeneous wall appositions in the noninfected host cells adjacent to invaded cells (Fig. 2A and B). These appositions varied in size and shape, ranging from small, hemispherical or dome-like protuberances (Fig. 2A) to elongated deposits along a large portion of the host cell wall (Fig. 2B). The extensive heterogeneity of the newly formed wall appositions was clearly demonstrated in some areas, where the material accumulating at the junction of adjacent host cells appeared ultrastructurally different in terms of arrangement (Fig. 2B). The deposited material was either stratified and bordered by a layer of aggregated material (Fig. 2B, large arrow) or granular and of very high electron density (Fig. 2B, small arrow). Frequently, the appositions were delimited by a band of osmiophilic material that released small droplets into the cell lumen (Fig. 2A, arrowheads). Whether this densely stained material originated from an aggregation of the host cytoplasm or was newly synthesized as a response to infection remains unclear.
FIG. 2.
Transmission electron micrographs of T. harzianum-inoculated cucumber root tissues. (A and B) Heterogeneous wall appositions (WA) are formed in noninfected host cells adjacent to invaded cells. The material accumulating at the junction of adjacent host cells was either stratified and bordered by a layer of aggregated material (large arrow in B) or granular and of very high electron density (small arrow in B). Frequently, the appositions were delimited by a band of osmiophilic material that released small droplets into the cell lumen (arrowheads in A). (C and D) Unsuccessful attempts by the fungus to penetrate WA are observed. Trichoderma hyphae (T) penetrating such appositions show marked signs of alteration characterized by morphological changes and even necrosis of the penetration peg. IS, intercellular spaces. Bars: A and D, 1 μm; B, 0.5 μm; C, 0.25 μm.
The host cell wall itself displayed a higher electron density than controls, strongly suggesting infiltration of structural molecules (Fig. 2A). Unsuccessful attempts of fungi to penetrate wall appositions were frequently recorded (Fig. 2C and D). Hyphae penetrating such appositions showed marked signs of alteration, characterized by morphological changes and even necrosis of the penetration peg (Fig. 2C and D).
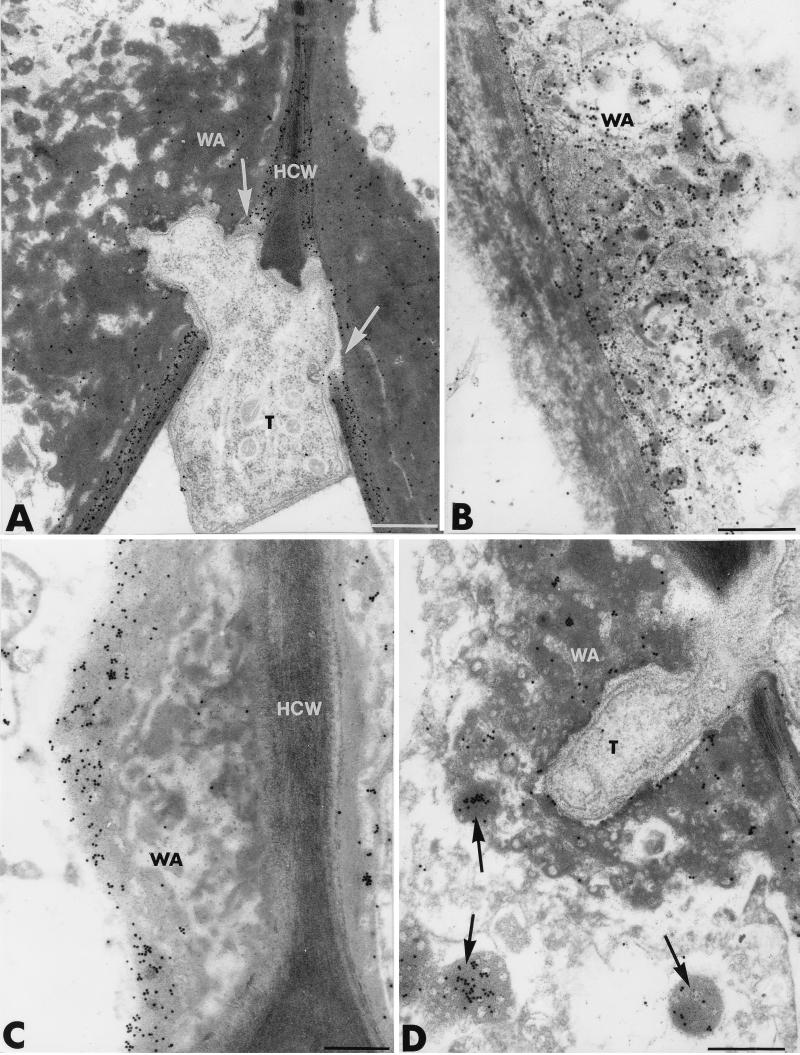
As expected, application of the β-1,4-exoglucanase–gold complex to sections of colonized cucumber root tissues resulted in the heavy deposition of gold particles over the host cell walls (Fig. 3A). In contrast, labeling was nearly absent over the host cytoplasm and organelles. Labeling also occurred over the wall appositions but was irregular and usually involved only a few randomly distributed gold particles (Fig. 3A). Interestingly, Trichoderma hyphae displayed the ability to locally disrupt cellulose-enriched host cell walls (Fig. 3A, arrows), although they appeared to be halted by the wall appositions. Control tests, including preincubation of the enzyme-gold complex with β-1,4-glucans prior to section labeling, resulted in the absence of labeling over both cell walls and wall appositions (data not shown). Upon incubation of sections with tobacco β-1,3-glucanase, many gold particles were detected over the wall appositions (Fig. 3B). A qualitative evaluation of the labeling clearly showed that young appositions, mainly characterized by their loose arrangement and their low density (Fig. 3B), were more intensely labeled than more mature ones, on which osmiophilic flecks were deposited (Fig. 3C). In such cases, labeling occurred preferentially over the outermost layer (Fig. 3C). Electron-opaque vesicles, either enclosed in the wall appositions or apparently free in the cytoplasm, were frequently seen (Fig. 3D, arrows). Such vesicles were always labeled with a substantial number of gold particles. Control tests, including incubation of the enzyme-gold complex with laminarin prior to section labeling, yielded negative results (data not shown).
FIG. 3.
Transmission electron micrographs of T. harzianum-inoculated cucumber root tissues. (A) Labeling with the β-1,4-exoglucanase–gold complex to localize cellulose. The host cell walls (HCW) are heavily labeled. Labeling also occurs over the wall appositions (WA) but is irregularly distributed. Trichoderma hyphae (T) display the ability to locally disrupt the cellulose-enriched HCW (arrows). Bar, 0.25 μm. (B to D) Labeling with the tobacco β-1,3-glucanase to localize callose. Young WA, mainly characterized by their loose arrangement and their low density (B), are more intensely labeled than the more mature ones, labeling of which occurs preferentially over the outermost layer (C). Electron-opaque vesicles, either enclosed in the WA (arrow pointing up in D) or apparently free in the cytoplasm (arrows pointing down in D), are labeled by a substantial number of gold particles. Bars, 0.25 μm.
Another prominent response to Trichoderma infection in cucumber plants was the occlusion of most intercellular spaces in the epidermis and outer root cortex with a dense material that showed various degrees of compactness and electron opacity (Fig. 4A to C). Fungal cells trapped in this material exhibited some morphological alterations (Fig. 4A) and were apparently halted in their development (Fig. 4B). Incubation with tobacco β-1,3-glucanase did not result in substantial labeling of the electron-opaque material, except next to the fungal cells, where a few gold particles could be seen (Fig. 4C). The material was similarly not labeled with the β-1,4-exoglucanase–gold complex (data not shown).
FIG. 4.
Transmission electron micrographs of T. harzianum-inoculated cucumber root tissues. Most intercellular spaces (IS) in the epidermis and outer root cortex are occluded with a dense material (AM) that shows various degrees of compactness and electron opacity. Fungal cells trapped in this material are morphologically altered. Upon incubation with the tobacco β-1,3-glucanase, this material appears unlabeled, except next to the fungal cells, where a few gold particles can be seen (C). An osmiophilic material (OM) coating the host cell walls surrounds the invading hyphae (arrows in D). Upon labeling with WGA–ovomucoid-gold complex for the localization of chitin, regular deposition of gold particles occurs over the coated fungal cell walls; arrows show the OM coating (E). T, Trichoderma hyphae; WA, wall appositions. Bars: A, D, and E, 0.5 μm; B and C, 1 μm.
In addition to the formation of wall appositions and the occlusion of intercellular spaces at sites of attempted fungal penetration, other host cell reactions included the deposition of an amorphous, osmiophilic material which coated the host cell walls and surrounded the invading hyphae by means of elongated strands of aggregated material (Fig. 4D and E, arrows). Sections labeled with the WGA–ovomucoid-gold complex revealed a regular deposition of gold particles over the coated fungal cell walls (Fig. 4E).
Activation of defense mechanisms.
Chitinase and peroxidase activities are commonly expressed during plant-fungus interactions (10, 21). These activities in roots and leaves were measured at 24, 48, 72, and 120 h postinoculation. Chitinase activity peaked at 72 h in both leaves and roots of treated plants (Fig. 5), while in nontreated plants, activity increased gradually with time. At peak chitinase activity, treated roots showed a 6.4-fold increase and treated leaves showed a 3.2-fold increase compared with nontreated plants. Peroxidase activity peaked at 48 h after inoculation in treated roots (Fig. 6), whereas it remained constant with time in nontreated plants. In treated leaves, peroxidase activity peaked at 72 h after inoculation, while nontreated plants showed a gradual increase with time. From 72 to 120 h, a two- to threefold decrease in both activities in the leaves and roots of treated plants was observed.
DISCUSSION
In recent years, interest in the ability of beneficial microorganisms to induce resistance in plants has grown, particularly with respect to their use as environmentally safe controllers of plant diseases (27, 29, 42). Among these microorganisms, saprophytic fungi have received only little attention as potential inducers of resistance (11). Nevertheless, saprophytic isolates that induced resistance also promoted the growth of cucumber plants (28, 30). In the present study, a hydroponic growth system was used to inoculate the roots of a host plant with monocultures of Trichoderma under predefined conditions. This approach enabled us to visualize the progression of the interaction to complete attachment of the fungal hyphae to the outer root tissue of cucumber seedlings. The interaction was also expressed in terms of biochemical and morphological changes induced in the cucumber host by the fungus, among which the increased growth response was the most salient, as described in earlier studies with soil (1, 19, 22, 25). Several hypotheses, including the control of minor pathogens (26), have been put forward to explain the effects of Trichoderma on plants. The results of our study in an aseptic environment with Trichoderma monocultures suggest that a direct plant-fungus interaction is responsible for the increased growth response as well as other responses in the plant.
In soils, plant disease suppression by Trichoderma spp. used as biocontrol agents has been widely documented (8, 9, 19, 37): it is considered to be a multifaceted process that requires the synergistic contribution of several mechanisms, which may include activation of the plant defense system (25). In the present study, we examined the effect of a fungal biocontrol agent on plants. The synthesis of pathogenesis-related (PR) proteins is one of the most common defense mechanisms triggered in plants following infection with inducing agents (10, 21, 40). Our work shows increased production of PR proteins in cucumber seedlings following Trichoderma application. Increased activities of chitinase and peroxidase were observed in both the roots and the leaves of Trichoderma-treated plants relative to nontreated ones as early as 48 h postinoculation. Moreover, the increase in enzyme activities in the leaves suggests a systemic defense response to the presence of Trichoderma in the rhizosphere. Strikingly, both enzymes displayed a similar trend of decreasing PR protein activities 120 h postinoculation, resembling a “mycorrhiza-like” pattern. This decrease was temporally correlated with colonization of the roots by Trichoderma. Plant infection with mycorrhizal fungi initiates some plant defense responses, although these do not reach their full potential, as the latter would probably prevent colonization. Transient increases in chitinase (38) and peroxidase (39) activities have been detected in leek roots during early stages of colonization by vesicular arbuscular fungi.
Vesicular arbuscular fungi are characterized by their ability to promote growth, penetrate and colonize plant roots, and initiate marked metabolic changes in roots (12, 39). To determine whether the observed biochemical changes correlated with structural modifications, Trichoderma-treated root tissues were examined at the ultrastructural level. Based on our cytological observations, root colonization by T. harzianum involves a sequence of events which include fungal proliferation along the elongating root and local penetration of the epidermis. The formation of fungal colonies at the junctions of adjacent epidermal cell walls showing localized signs of alterations suggests that these areas are preferential sites for replication and subsequent penetration. Penetration of the epidermis and subsequent ingress into the outer cortex suggest that at least small amounts of cell wall hydrolytic enzymes, such as cellulases, are produced by the fungus to locally weaken or loosen epidermal cell walls, thereby facilitating fungal spread into the root tissues. However, the regular pattern of cellulose distribution in the internal root tissues was taken as an indication that cellulases were produced at low levels, if at all, inside the plant.
Cellular changes characterized by the deposition onto the inner cell wall surface of callose-enriched wall appositions were typical reactions of epidermal and cortical cells of Trichoderma-colonized cucumber roots. This phenomenon was even amplified by the impregnation of osmiophilic substances in the host cell walls and in the intercellular spaces of reacting host cells. The massive deposition of such structures at sites of attempted fungal entry and the accumulation of osmiophilic deposits suggested that epidermal and cortical host cells were signaled to mobilize a number of defense strategies. Such cellular changes were apparently efficient in preventing fungal ingress into the vascular stele, since the fungus was seldom seen in the innermost root tissues. Fungal cells near wall appositions frequently appeared disorganized, suggesting a fungitoxic environment (32). Our cytochemical results provided evidence that callose and, to a lesser extent, cellulose occurred in the wall appositions. While callose appeared to be widely distributed over the underlying matrix of wall appositions, cellulose was seen mainly as a few randomly distributed molecules. In light of these observations, the early events leading to the development of complete appositions may involve splitting of the host cell walls followed by gradual deposition of polysaccharides, such as callose, between the split walls.
In an attempt to determine whether enzyme-mediated wall hydrolysis was associated with the frequent disorganization of fungal hyphae colonizing the outer tissues in Trichoderma-infected cucumber roots, chitin was ultrastructurally localized. An examination of the labeling pattern revealed fungal cell disorganization at a time when chitin still occurred in the cell walls. This observation suggests that, although chitinases are produced, they are not a primary determinant in the expression of plant resistance to Trichoderma infection. It is more likely that the formation of structural barriers and the synthesis of toxic substances, such as phenolic compounds and phytoalexins, precede the production of chitinases and other PR proteins. In agreement with this concept, the enhanced electron density of the host cell wall and the deposition of an osmiophilic material coating the invading hyphae in Trichoderma-treated plants correlated well with the presence of phenolic compounds known to stain densely upon reaction of O-dihydroxy groups with osmium tetroxide (36). Several studies have convincingly shown that phenolic structures can confer high rigidity to host cell walls through peroxidase-mediated cross-linking with preexisting wall carbohydrates, such as hemicellulose, pectin, and callose (15).
The results presented here demonstrate that striking modifications of epidermal and cortical cell walls, as well as deposition of newly formed barriers, are triggered in cucumber root tissues by colonization. These cellular changes, characterized by the deposition of callose-enriched wall appositions onto the inner surface of the cell walls, are apparently efficient in preventing fungal ingress into the vascular stele. In agreement with these results, Trichoderma treatment initiated a marked increase in peroxidase activity within 48 h after inoculation. As a general rule, peroxidase activity increases earlier than chitinase activity in Trichoderma-treated cucumber plants. Peroxidase may be rapidly involved in the peroxidation of substrate molecules, leading to the accumulation of highly toxic compounds (i.e., phenolic compounds), which may contribute to resistance via their antifungal potential (41). However, these compounds may, to some extent, be toxic to the plant itself, and it seems reasonable to assume that mechanisms designed to repress peroxidase expression are activated during the resistance process in order to maintain phenolic compounds below phytotoxic levels. In that context, the decrease in peroxidase activity observed at 120 h postinoculation may reflect a process elaborated by the plant to protect itself until such activity is needed, such as upon pathogenic attack. Like a symbiotic mycorrhizal association, the interaction between Trichoderma and the plant may involve molecular recognition between the two partners, resulting in the establishment of a beneficial partnership that subsequently leads to a decrease in the synthesis and accumulation of defense molecules. This concept is supported by the observation that plant growth was promoted in Trichoderma-treated plants, obviously indicating the beneficial effect of the association.
In the present study, application of the saprophytic fungus T. harzianum to the rhizosphere of young cucumber seedlings initiated in the plants a series of morphological as well as biochemical changes which are considered to be part of the plant defense response. To our knowledge, this study is the first to provide evidence that T. harzianum penetrates the root system without causing extensive damage and triggers the transient elaboration of host defense reactions. As with immunization, Trichoderma-inoculated plants may be sensitized to respond faster and to a greater extent to potential pathogen attacks. Further studies, designed to assess the role of T. harzianum in the induction of resistance against pathogens, are in progress.
ACKNOWLEDGMENT
This research was supported by Binational Agricultural Research and Development Fund (BARD) project IS-2880-97.
REFERENCES
- 1.Baker R. Improved Trichoderma spp. for promoting crop productivity. Trends Biotech. 1989;7:34–38. [Google Scholar]
- 2.Benhamou N. Immunocytochemistry of plant defense mechanisms induced upon microbial attack. Microsc Res Tech. 1995;31:63–78. doi: 10.1002/jemt.1070310106. [DOI] [PubMed] [Google Scholar]
- 3.Benhamou N. Preparation and application of lectin-gold complexes. In: Hayat M A, editor. Colloidal gold, principles, methods, and applications. Vol. 1. New York, N.Y: Academic Press, Inc.; 1989. pp. 95–143. [Google Scholar]
- 4.Benhamou N. Ultrastructural detection of β-1,3-glucans in tobacco root tissues infected by Phytophthora parasitica var. nicotianae using a gold-complexed tobacco β-1,3-glucanase. Physiol Mol Plant Pathol. 1992;41:351–370. [Google Scholar]
- 5.Benhamou N, Chamberland H, Ouellette G B, Pauzé F J. Ultrastructural localization of β-1,4-d-glucans in two pathogenic fungi and in their host tissues by means of an exoglucanase-gold complex. Can J Microbiol. 1987;33:405–417. [Google Scholar]
- 6.Beyrle H. The role of phytohormones in the function and biology of mycorrhizas. In: Varma A, Hock B, editors. Mycorrhiza. Berlin, Germany: Springer-Verlag KG; 1995. pp. 365–391. [Google Scholar]
- 7.Campbell M M, Ellis B E. Fungal elicitor-mediated responses in pine cell cultures: cell wall-bound phenolics. Phytochemistry. 1992;31:737–742. [Google Scholar]
- 8.Chet I. Biological control of soilborne pathogens with fungal antagonists in combination with soil treatment. In: Hornby D, Cook R J, Henis Y, Ko W H, Rovira A D, Schippers B, Scott P R, editors. Biological control of soilborne pathogens. New York, N.Y: CAB Publishing House; 1990. pp. 15–25. [Google Scholar]
- 9.Chet I. Trichoderma—application, mode of action, and potential as biocontrol agent of soilborne plant pathogenic fungi. In: Chet I, editor. Innovative approaches to plant disease control. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 137–160. [Google Scholar]
- 10.Dalisay R F, Kuc J A. Persistence of induced resistance and enhanced peroxidase and chitinase activities in cucumber plants. Physiol Mol Plant Pathol. 1995;47:315–327. [Google Scholar]
- 11.De Meyer G, Bigirimana J, Elad Y, Hofte M. Induced systemic resistance in Trichoderma harzianum T39 biocontrol of Botrytis cinerea. Eur J Plant Pathol. 1998;104:279–286. [Google Scholar]
- 12.Dumas-Gaudot E, Grenier J, Furlan V, Asselin A. Chitinase, chitosanase and β-1,3 glucanase activities in Allium and Pisum roots colonized by Glomus species. Plant Sci. 1984;84:17–24. [Google Scholar]
- 13.Elad Y, Chet I, Henis Y. Degradation of plant pathogenic fungi by Trichoderma harzianum. Can J Microbiol. 1982;28:719–725. [Google Scholar]
- 14.Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold solution. Nat Phys Sci. 1973;241:20–22. [Google Scholar]
- 15.Fry S C. Polymer-bound phenols as natural substrates of peroxidases. In: Greppin H, Penel C, Gaspar T, editors. Molecular and physiological aspects of plant peroxidase. Geneva, Switzerland: Université de Genève; 1986. pp. 169–182. [Google Scholar]
- 16.Ghisalberti E L, Sivasithamparam K. Anti fungal antibiotics produced by Trichoderma spp. Soil Biol Biochem. 1991;23:1011–1020. [Google Scholar]
- 17.Haran S, Schickler A, Chet I. Differential expression of Trichoderma harzianum chitinases during mycoparasitism. Phytopathology. 1996;86:980–985. [Google Scholar]
- 18.Haran S, Schickler H, Oppenheim A, Chet I. New components of the chitinolytic system of Trichoderma harzianum. Mycol Res. 1995;99:441–446. [Google Scholar]
- 19.Harman G E, Bjorkman T. Potential and existing uses of Trichoderma and Gliocladium for plant disease control and plant growth enhancement. In: Kubicek C K, Harman G E, editors. Trichoderma and Gliocladium. London, England: Taylor and Francis; 1998. pp. 229–265. [Google Scholar]
- 20.Harman G E, Hayes C K, Lorito M, Broadway R M, Di P A, Peterbauer C, Tronsmo A. Chitinolytic enzymes of Trichoderma harzianum: purification of chitobiosidase and endochitinase. Phytopathology. 1993;83:313–318. [Google Scholar]
- 21.Heath M C. Plant resistance to fungi. Can J Plant Pathol. 1996;18:469–475. [Google Scholar]
- 22.Inbar J, Abramsky M, Cohen D, Chet I. Plant growth enhancement and disease control by Trichoderma harzianum in vegetable seedlings grown under commercial conditions. Eur J Plant Pathol. 1994;100:337–346. [Google Scholar]
- 23.Inbar J, Chet I. Lectins and biocontrol. Crit Rev Biotechnol. 1997;17:1–20. doi: 10.3109/07388559709146604. [DOI] [PubMed] [Google Scholar]
- 24.Kauffmann S, Legrand M, Geoffroy P, Fritig B. Biological function of “pathogenesis-related” proteins. Four PR proteins of tobacco have 1,3-β-glucanase activity. EMBO J. 1987;6:3209–3212. doi: 10.1002/j.1460-2075.1987.tb02637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleifeld O. Ph.D. thesis. Jerusalem, Israel: Hebrew University of Jerusalem; 1990. [Google Scholar]
- 26.Kleifeld O, Chet I. Trichoderma harzianum—interaction with plants and effect on growth response. Plant Soil. 1992;144:267–272. [Google Scholar]
- 27.Liu L, Kloepper W, Tuzun S. Induction of systemic resistance in cucumber against Fusarium wilt by plant growth promoting rhizobacteria. Phytopathology. 1995;85:695–698. [Google Scholar]
- 28.Meera M S, Shivanna M B, Kageyama K, Hyakumachi M. Persistence of induced systemic resistance in cucumber in relation to root colonization by plant growth promoting fungal isolates. Crop Prot. 1995;14:123–130. [Google Scholar]
- 29.Meera M S, Shivanna M B, Kageyama K, Hyakumachi M. Plant growth promoting fungi from Zoysiagrass rhizosphere as potential inducers of systemic resistance in cucumbers. Phytopathology. 1994;84:1399–1406. [Google Scholar]
- 30.Meera M S, Shivanna M B, Kageyama K, Hyakumachi M. Responses of cucumber to induction of systemic resistance against anthracnose by plant growth promoting fungi. Eur J Plant Pathol. 1995;101:421–430. [Google Scholar]
- 31.Okon Y, Chet I, Henis Y. Effects of lactose, ethanol and cycloheximide on the translation pattern of radioactive compounds and on Sclerotium rolfsii. J Gen Microbiol. 1973;74:251–258. [Google Scholar]
- 32.Peng M, Kuc J A. Peroxidase generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology. 1992;82:696–699. [Google Scholar]
- 33.Roberts W K, Selitrennikoff C P. Plant and bacterial chitinases differ in antifungal activity. J Gen Microbiol. 1988;134:169–176. [Google Scholar]
- 34.Ruttimann C, Schwember E, Salas L, Cullen D, Vicuna R. Ligninolytic enzymes of the white rot basidiomycetes Phlebia breviospora and Ceriporiopsis subvermispora. Biotechnol Appl Biochem. 1992;16:64–76. [Google Scholar]
- 35.Samuels G J. Trichoderma: a review of biology and systematics of the genus. Mycol Res. 1996;100:923–935. [Google Scholar]
- 36.Scalet M, Crivaletto E, Mallardi F. Demonstration of phenolic compounds in plant tissue by an osmium-iodide post fixation procedure. Stain Technol. 1989;64:273–290. doi: 10.3109/10520298909107018. [DOI] [PubMed] [Google Scholar]
- 37.Sivan A, Chet I. Integrated control of Fusarium crown and root rot of tomato with Trichoderma harzianum in combination with methyl bromide or soil solarization. Crop Prot. 1993;12:380–386. [Google Scholar]
- 38.Spanu P, Boller T, Ludwig A, Wiemken A, Faccio A, Bonafante-Fasolo P. Chitinases in roots of mycorrhizal Allium porrum: regulation and localization. Planta. 1989;177:447–455. doi: 10.1007/BF00392612. [DOI] [PubMed] [Google Scholar]
- 39.Spanu P, Bonafante-Fasolo P. Cell wall bound peroxidase activity in roots of mycorrhizal Allium porrum. New Phytol. 1988;109:119–124. [Google Scholar]
- 40.van Loon L C. Pathogenesis related proteins. Plant Mol Biol. 1985;4:111–116. doi: 10.1007/BF02418757. [DOI] [PubMed] [Google Scholar]
- 41.Ward E W B. Biochemical mechanisms involved in resistance of plants to fungi. In: Baily J A, editor. Biology and molecular biology of plant-pathogen interactions. Berlin, Germany: Springer-Verlag KG; 1986. pp. 107–131. [Google Scholar]
- 42.Wei G, Kloepper J W, Tuzun S. Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth promoting rhizobacteria. Phytopathology. 1992;81:1508–1512. [Google Scholar]