Abstract
Chlorella meal is a potential protein source for aquafeeds. However, the physiological response of carnivorous fish fed Chlorella meal remains elusive. This study evaluated the effects of replacing dietary fish meal with Chlorella meal on growth performance, pigmentation, and liver health in largemouth bass. Five diets were formulated to replace dietary fish meal of 0% (C0, control), 25% (C25), 50% (C50), 75% (C75), and 100% (C100) with Chlorella meal, respectively. Total 300 fish (17.6 ± 0.03 g) were randomly assigned to 15 tanks (3 tanks/group). Fish were fed the experimental diet twice daily for 8 weeks. The increased dietary Chlorella meal quadratically influenced the final body weight (FBW), weight gain rate (WGR), specific growth rate (SGR), and feed intake (FI), which were significantly lower in the C100 group than in the other groups (P < 0.05). The feed conversion ratio (FCR) increased linearly or quadratically with dietary Chlorella meal. Dietary Chlorella meal linearly or quadratically increased the lutein content of plasma, liver, and dorsal muscle of largemouth bass (P < 0.05). Compared to the C0 group, all supplemented Chlorella meal groups significantly improved the yellowness (b∗) of the dorsal body (1.5 to 2.0 fold), abdominal body (1.5 to 1.8 fold), and dorsal muscle (3.8 to 5.4 fold) of largemouth bass (P < 0.05). In addition, compared to the C0 group, the liver vacuolation area of fish was significantly increased in the C75 and C100 groups (P < 0.05). Transcriptional levels of apoptosis-related genes of β-cell lymphoma-2 (bcl2), caspase-9-like (casp9), and caspase-3a (casp3) were markedly upregulated (0.9 to 1.6 fold) in the C100 group compared to the C0 group (P < 0.05). Based on the quadratic regression analysis between FBW, WGR, or SGR and dietary Chlorella meal level, largemouth bass had the best growth when replacing 31.7% to 32.6% of fish meal with 15.03% to 15.43% dietary Chlorella meal. The present results indicated that dietary supplementation with Chlorella meal (11.85% to 47.45%) significantly enhanced the pigmentation; however, total replacement of fish meal (40%) with Chlorella meal (47.45%) caused growth retardation, apoptosis, and liver damage in largemouth bass.
Keywords: Chlorella meal, Growth, Liver health, Micropterus salmoides, Pigmentation
1. Introduction
In aquafeeds, fish meal is an excellent protein source because of its relatively balanced nutrient composition, low levels of anti-nutritional factors, and good palatability (Zhou et al., 2005). However, the high price and limited resources of fish meal seriously restrict its use in aquafeeds (Li et al., 2021a). Therefore, the exploitation of new alternative protein sources for fish meal is pressing. At present, numerous protein sources have been studied to replace fish meal, including plant protein sources (Arriaga-Hernandez et al., 2021; Huang et al., 2017; Zhang et al., 2020a), animal protein sources (Cao et al., 2020; Jones et al., 2021; Psofakis et al., 2020; Stenberg et al., 2019; Terova et al., 2021), and single-cell protein sources (Hansen et al., 2019; Valente et al., 2019; Yang et al., 2021). However, many plant protein sources contain anti-nutritional factors (Francis et al., 2001; Jannathulla et al., 2019), imbalanced amino acid composition (Espe et al., 2006, 2007), and display poor palatability (Luo et al., 2010). Some animal protein sources also have an imbalanced amino acid composition (Bureau et al., 2000), poor digestibility (Luthada-Raswiswi et al., 2021), and high saturated fatty acid content (Liland et al., 2021). These disadvantages largely limit the use of them to replace fish meal in aquafeeds.
In recent years, microalgae have been recognized as a single-cell protein source in aquafeeds, which contain balanced amino acid composition, high polyunsaturated fatty acid, and display good palatability (Ahmad et al., 2020; Alagawany et al., 2021; Radhakrishnan et al., 2014; Liu et al., 2019). The utilization of microalgae has less adverse effects on the growth performance of some aquatic animals (Kousoulaki et al., 2016; Man et al., 2020; Safavi et al., 2019; Watanabe et al., 1990). Moreover, the utilization of microalgae in aquafeeds improved the body color of aquatic species (Guroy et al., 2012; Roohani et al., 2019; Ju et al., 2012; Pulcini et al., 2020), which may be attributed to the pigments present in microalgae, such as lutein (a carotenoid) (Liu et al., 2019). As natural pigment sources, microalgae are different from artificial pigments because they are non-toxic and have certain nutritional or pharmacological effects. As one of the well-studied genera of microalgae, Chlorella exhibits abundant nutritional elements, biological safety, and ease of culture, which make it a potential alternative protein source for fish meal (Liu and Chen, 2016; Chen et al., 2018). At present, replacing fish meal with Chlorella meal in rainbow trout (Oncorhynchus mykiss) (Chen et al., 2021), African catfish (Clarias gariepinus) (Raji et al., 2020), zebrafish (Danio rerio) (Carneiro et al., 2020), and crucian carp (Carassius auratus) (Shi et al., 2017b) did not cause negative growth performance. These results indicate that fish meal could potentially be replaced with Chlorella meal. However, the effects of dietary inclusion of Chlorella meal on the growth, pigmentation, and liver health in carnivorous fish, which normally depend on large amounts of fish meal in feeds, have remained elusive.
In China, largemouth bass (Micropterus salmoides) has been widely cultured with an annual production of more than 0.6 million metric tons (Jiang et al., 2018; Zhang et al., 2019). Furthermore, as a typical carnivorous fish, there is a high content of fish meal (about 40% to 54%) in the feeds of largemouth bass (Li et al., 2020a; Wu et al., 2021; Zhang et al., 2020b), which indicate that further development might encounter more challenges from unsustainable fish meal sources. Therefore, there is an urgent requirement to find new alternative proteins to replace fish meal for largemouth bass. However, the replacement of fish meal with Chlorella meal in largemouth bass has not been reported. The present study aimed to investigate the effects of dietary Chlorella meal to replace fish meal on the growth performance, pigmentation, and liver health of largemouth bass.
2. Materials and methods
All fish experiments were conducted according to the Guiding Principles for Care and Use of Laboratory Animals and were approved by the Institute of Hydrobiology, Chinese Academy of Science (IHB, CAS, Protocol No. 2016–018).
2.1. Apparent digestibility coefficients (ADC) determination
ADC of fish meal and Chlorella meal were determined according to the method of Liu et al. (2020) with minor modification. Briefly, Y2O3 (0.1%) was added as an indicator and mixed with different ingredients as a reference diet (RD) (Table 1). RD and 2 test diets, in which tested ingredients (fish meal and Chlorella meal) substituted at 30% of the RD were formulated, and made into puffed pellets (diameter: 2 mm). Largemouth bass (mean weight: 71.25 g, 18 fish/tank, 3 tanks/diet) were fed with different diets twice a day in the recycling water system (water temperature: 28 °C). Feces used for ADC determination were collected one week after the first feeding of the experimental diets, which allowed sufficient time to clear all intestinal contents from any previous diet. The feces were carefully collected (about 2 to 3 h after feeding) by siphon and dried on filter paper, and then immediately stored at - 20 °C until enough samples were collected. Subsequently, samples were frozen dried for chemical analyses. The ADC in dry matter and crude protein of the RD and test diets were calculated as follows:
| ADC of nutrients (%) = 100 × [1 - (fecal nutrient/dietary nutrient) × (Y2O3 in diet)/(Y2O3 in feces)] . |
Table 1.
Formulation and chemical composition of the reference diet (% dry matter).
| Item | Content |
|---|---|
| Ingredients | |
| Fish meal1 | 42 |
| Gluten2 | 20 |
| Cassava starch3 | 15 |
| Fish oil4 | 5 |
| Vitamin and mineral additives5 | 1.4 |
| Monocalcium phosphate6 | 2 |
| Choline chloride7 | 2 |
| Guar gum8 | 1.5 |
| Microcrystalline cellulose9 | 11 |
| Y2O310 | 0.1 |
| Total | 100 |
| Proximate composition | |
| Moisture, % wet weight | 4.76 |
| Crude protein | 44.31 |
Fish meal: from Superprime, TASA Fish Product Co., Ltd., Peru.
Gluten: from Henan Midaner Trading Co., Ltd., Xinzheng, Henan, China.
Cassava starch: from Wuhan Yiteng Starch Co., Ltd., Wuhan, Hubei, China.
Fish oil: from Coland Feed Co., Ltd., Wuhan, Hubei, China.
Vitamin and mineral additives: from Guangdong Nutriera Group, Guangzhou, China. Vitamin additives, mg/kg diet: vitamin A 10; vitamin B1 6; vitamin B2 5; vitamin B6 7.5; vitamin B12 (1%) 4; niacinamide 50; ascorbyl calcium phosphate (35%) 500; calcium pantothenate 20; biotin (2%) 2.5; folic acid 5; vitamin E (50%) 200; vitamin K3 10; vitamin D3 5; inositol 100; corn protein powder 75. Mineral additives, mg/kg diet: CuSO4·5H2O 10; FeSO4·H2O 300; ZnSO4·H2O 200; MnSO4·H2O 100; KIO3 (10%) 80; Na2SeO3 (10% Se) 67; CoCl2·6H2O (10% Co) 5; NaCl 100; zeolite 638.
Monocalcium phosphate: from Sinopharm Chemical Reagent Co., Ltd., China.
Choline chloride: from Guangdong Nutriera Group, Guangzhou, China.
Guar gum: from Henan Wanbang Chemical Technology Co., Ltd., China.
Microcrystalline cellulose: from Shandong Liujia Pharmaceutical Excipients Co., Ltd., Jining, Shandong, China.
Y2O3: from Sinopharm Chemical Reagent Co., Ltd., China.
The ADC in dry matter and crude protein of the test ingredients (fish meal and Chlorella meal) were calculated as the following formulas:
| ADCi (%) = ADt + [(ADt - ADr) × (0.7 × Dr/0.3 × Di)] , |
where ADCi is the apparent digestibility coefficient of the ingredient, ADt is the apparent digestibility of the nutrient in the test diet, ADr is the apparent digestibility of nutrient in the reference diet, Dr is the nutrient content in the reference diet, Di is the nutrient content in the test ingredient.
2.2. Experimental diets
Chlorella meal (Demeter Bio-Tech Co., Ltd., Wuhan, China) and fish meal were used as the main protein sources in the experimental diets. The biochemical composition of fish meal and Chlorella meal was shown in Table 2. In the present study, Chlorella meal was used at concentrations of 11.85%, 23.7%, 35.6%, and 47.45% to replace dietary fish meal at levels of 25% (C25), 50% (C50), 75% (C75), and 100% (C100). A basal diet containing 40% fish meal was used as the control group (C0). Five isonitrogenous and isolipidic experimental diets were formulated according to Table 3. The five extruded pellet diets were made at the Feed Research Institute, Chinese Academy of Agricultural Sciences (Beijing, China). First, all ingredients of each diet were ground through a 100 mesh. Second, they were completely mixed to pass through a twin-screw extruder (EXT50A, Yanggong Machine, Beijing, China) into 2-mm diameter pellets under the following extrusion conditions: feeding section (100 °C), compression section (109 °C) and metering section (124 °C). Third, all pellets were dried in an oven at 65 °C. All the diets were stored at 4 °C for the feeding experiment. The proximate composition of the diets was shown in Table 3, and their amino acid composition was presented in Table 4.
Table 2.
Biochemical composition and apparent digestibility coefficients of fish meal and Chlorella meal.
| Item | Fish meal1 | Chlorella meal2 | |
|---|---|---|---|
| Proximate composition, % dry matter | |||
| Moisture, % wet weight | 6.80 | 2.67 | |
| Crude protein | 68.21 | 57.50 | |
| Crude lipid | 9.00 | 10.20 | |
| Crude ash | 14.05 | 4.71 | |
| Crude fiber | 1.61 | 0.89 | |
| Carbohydrate (crude fiber free)3 | 0.33 | 24.0.03 | |
| Gross energy, kJ/g | 19.98 | 19.00 | |
| Lutein, mg/kg | 0.00 | 79.73 | |
| Apparent digestibility coefficient (ADC) of ingredient, % | |||
| ADC of dry matter | 81.29 | 72.94 | |
| ADC of protein | 92.62 | 87.38 | |
| Indispensable amino acids (IAA), % dry matter | |||
| Methionine | 1.65 | 0.87 | |
| Lysine | 4.44 | 2.80 | |
| Threonine | 2.42 | 2.05 | |
| Isoleucine | 2.63 | 1.89 | |
| Histidine | 1.82 | 0.93 | |
| Valine | 2.53 | 2.34 | |
| Leucine | 4.50 | 4.31 | |
| Arginine | 2.19 | 2.12 | |
| Phenylalanine | 2.33 | 2.48 | |
| ∑IAA | 24.52 | 19.80 | |
| Dispensable amino acids (DAA), % dry matter | |||
| Aspartic acid | 4.55 | 4.03 | |
| Serine | 2.13 | 1.84 | |
| Glutamic acid | 7.99 | 7.56 | |
| Glycine | 3.69 | 2.95 | |
| Alanine | 3.85 | 4.43 | |
| Cysteine | 0.33 | 0.34 | |
| Proline | 2.61 | 2.64 | |
| Tyrosine | 1.87 | 1.59 | |
| ∑DAA | 27.02 | 25.38 | |
| ∑TAA | 51.54 | 45.18 | |
| Fatty acid composition, % total fatty acids | |||
| C14:0 | 8.12 | 0.32 | |
| C15:0 | 0.55 | 0.26 | |
| C16:0 | 14.13 | 14.11 | |
| C17:0 | 0.64 | 0.31 | |
| C18:0 | 7.04 | 1.54 | |
| C20:0 | 0.18 | 0.17 | |
| C24:0 | 0.41 | 0.28 | |
| ∑SFA | 31.40 | 17.12 | |
| ∑MUFA | 21.08 | 21.25 | |
| C18:3n-3 | 0.56 | 19.23 | |
| C20:3n-3 | 0.09 | 0.00 | |
| C20:5n-3 | 15.37 | 0.00 | |
| C22:6n-3 | 21.02 | 0.00 | |
| C18:2n-6c | 1.08 | 46.90 | |
| C18:3n-6 | 0.21 | 0.15 | |
| C20:2n-6 | 0.20 | 0.00 | |
| C20:3n-6 | 0.14 | 0.00 | |
| C20:4n-6 | 1.20 | 0.00 | |
| C22:2n-6 | 7.61 | 0.00 | |
| n-3 PUFA | 37.03 | 19.23 | |
| n-6 PUFA | 10.45 | 47.05 | |
| ∑HUFA | 47.48 | 66.28 | |
∑IAA = sum of indispensable amino acids; ∑DAA = sum of dispensable amino acids; ∑TAA = sum of total amino acids; ∑SFA = sum of saturated fatty acids; ∑MUFA = sum of monounsaturated fatty acids; n-3 PUFA = sum of n-3 polyunsaturated fatty acids; n-6 PUFA = sum of n-6 polyunsaturated fatty acids; ∑HUFA = sum of highly unsaturated fatty acids.
Fish meal: from Superprime, TASA Fish Product Co., Ltd., Peru.
Chlorella meal: from Demeter Bio-Tech Co., Ltd., Wuhan, Hubei, China.
Carbohydrate (crude fiber free) = 100 – (Moisture + Crude protein + Crude lipid + Crude ash + Crude fiber).
Table 3.
Formulation and proximate composition of the experimental diets (% dry matter).
| Item | Diets1 |
||||
|---|---|---|---|---|---|
| C0 | C25 | C50 | C75 | C100 | |
| Ingredients | |||||
| Fish meal2 | 40.00 | 30.00 | 20.00 | 10.00 | 0.00 |
| Chlorella meal3 | 0.00 | 11.85 | 23.70 | 35.60 | 47.45 |
| Blood meal4 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Gluten5 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Soybean meal6 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Soybean protein concentrate7 | 13.00 | 13.00 | 13.00 | 13.00 | 13.00 |
| Cassava starch8 | 11.00 | 11.00 | 11.00 | 11.00 | 11.00 |
| Fish oil9 | 3.50 | 3.35 | 3.19 | 3.04 | 2.88 |
| Soybean oil10 | 3.50 | 3.35 | 3.19 | 3.04 | 2.88 |
| Vitamin and mineral additives11 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Monocalcium phosphate12 | 1.50 | 1.50 | 1.50 | 1.50 | 1.50 |
| Choline chloride13 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Microcrystalline cellulose14 | 7.40 | 5.85 | 4.32 | 2.72 | 1.19 |
| Proximate composition | |||||
| Moisture, % wet weight | 4.25 | 4.36 | 4.55 | 4.71 | 3.37 |
| Crude protein | 49.93 | 50.10 | 50.30 | 50.16 | 49.64 |
| Crude lipid | 12.66 | 12.72 | 12.96 | 11.44 | 11.32 |
| Crude ash | 9.61 | 8.66 | 7.47 | 6.46 | 5.61 |
| Crude fiber | 1.43 | 1.40 | 1.30 | 1.24 | 1.21 |
| Carbohydrate (crude fiber free) | 22.12 | 22.76 | 23.42 | 25.99 | 28.85 |
| Gross energy, kJ/g dry matter | 20.63 | 20.99 | 21.16 | 21.57 | 21.81 |
| Lutein, mg/kg dry matter | 0.00 | 8.03 | 11.39 | 15.87 | 22.28 |
C0, C25, C50, C75, and C100 represent diets in which Chlorella meal replaced fish meal levels of 0%, 25%, 50%, 75%, and 100%, respectively.
Fish meal: from Superprime, TASA Fish Product Co., Ltd., Peru.
Chlorella meal: from Demeter Bio-Tech Co., Ltd., Wuhan, Hubei, China.
Blood meal: from Beijing Yangyuan Veterinary Medicine Technology Co., Ltd., Beijing, China.
Gluten: from Henan Midaner Trading Co., Ltd., Xinzheng, Henan, China.
Soybean meal: from Qingdao Bohai Agricultural Development Co., Ltd., Qingdao, Shandong, China.
Soybean protein concentrate: from Yihai grain and oil industry Co., Ltd., Taizhou, Jiangsu, China. Proximate composition (dry matter basis): 64% crude protein and 1.05% crude lipid.
Cassava starch: from Wuhan Yiteng Starch Co., Ltd., Wuhan, Hubei, China.
Fish oil: from Coland Feed Co., Ltd., Wuhan, Hubei, China.
Soybean oil: from Yihai Kerry Arawana Holdings Co., Ltd., Shanghai, China.
Vitamin and mineral additives: from Guangdong Nutriera Group, Guangzhou, China. Vitamin additives, mg/kg diet: vitamin A 10; vitamin B1 6; vitamin B2 5; vitamin B6 7.5; vitamin B12 (1%) 4; niacinamide 50; ascorbyl calcium phosphate (35%) 500; calcium pantothenate 20; biotin (2%) 2.5; folic acid 5; vitamin E (50%) 200; vitamin K3 10; vitamin D3 5; inositol 100; corn protein powder 75. Mineral additives, mg/kg diet: CuSO4·5H2O 10; FeSO4·H2O 300; ZnSO4·H2O 200; MnSO4·H2O 100; KIO3 (10%) 80; Na2SeO3 (10% Se) 67; CoCl2·6H2O (10% Co) 5; NaCl 100; zeolite 638.
Monocalcium phosphate: from Sinopharm Chemical Reagent Co., Ltd., China.
Choline chloride: from Guangdong Nutriera Group, Guangzhou, China.
Microcrystalline cellulose: from Shandong Liujia Pharmaceutical Excipients Co., Ltd., Jining, Shandong, China.
Table 4.
Amino acid composition of the experimental diets (% dry matter).
| Item | Diets1 |
||||
|---|---|---|---|---|---|
| C0 | C25 | C50 | C75 | C100 | |
| Methionine | 0.89 | 0.89 | 0.80 | 0.73 | 0.64 |
| Lysine | 3.09 | 3.29 | 3.04 | 2.94 | 2.47 |
| Threonine | 1.73 | 1.86 | 1.85 | 1.84 | 1.72 |
| Isoleucine | 1.92 | 2.00 | 1.99 | 1.93 | 1.73 |
| Histidine | 1.33 | 1.38 | 1.33 | 1.22 | 1.10 |
| Valine | 1.97 | 2.18 | 2.21 | 2.24 | 2.10 |
| Leucine | 3.66 | 3.97 | 4.00 | 4.11 | 3.88 |
| Arginine | 1.94 | 2.13 | 2.12 | 2.14 | 1.99 |
| Phenylalanine | 2.10 | 2.21 | 2.23 | 2.26 | 2.24 |
| Aspartic acid | 3.64 | 3.86 | 3.83 | 3.76 | 3.43 |
| Serine | 1.85 | 1.97 | 1.97 | 1.93 | 1.80 |
| Glutamic acid | 7.61 | 8.08 | 8.13 | 7.97 | 7.24 |
| Glycine | 2.31 | 2.45 | 2.48 | 2.40 | 2.21 |
| Alanine | 2.58 | 2.80 | 2.89 | 3.02 | 2.86 |
| Cysteine | 0.35 | 0.31 | 0.31 | 0.33 | 0.32 |
| Proline | 2.51 | 2.56 | 2.61 | 2.68 | 2.54 |
| Tyrosine | 1.36 | 1.48 | 1.42 | 1.49 | 1.37 |
C0, C25, C50, C75, and C100 represent diets in which Chlorella meal replaced fish meal levels of 0%, 25%, 50%, 75%, and 100%, respectively.
2.3. Fish and the feeding experiment
The largemouth bass were obtained from a fish farm (Ezhou, Hubei, China). All fish were then transferred into fiberglass cylinders (1,500 L). During the acclimation period of 2 weeks, fish were fed a commercial feed (≥49% crude protein and ≥5% crude lipid, Largemouth bass 1#, from Wuhan CP Aquatic Co., Ltd., Wuhan, Hubei, China) twice a day at 08:30 and 16:30. The feeding experiment was performed in an indoor recirculating water system containing 15 fiberglass cylinders (rearing volume of 200 L). Residual feed was removed immediately after each feeding, and a siphon was used to remove feces from each tank to maintain the stable operation of the rearing system and to control the water quality. One-fifth of the water in each tank was re-recruited every day. The recycled water was removed from the suspended particulates through a microfiltration machine, trapped the impurities through a sand filter, and eliminated pathogenic bacteria through an ultraviolet sterilizer.
After 24 h of fasting, a total of 300 healthy fish (initial weight: 17.6 ± 0.03 g, n = 15) were randomly distributed into 15 tanks with 20 fish per tank (semidiameter: 30 cm; height: 70 cm; with 167 L of actual water volume). One of the five experimental diets was fed to three tanks (3 tanks per group). Fish were fed twice a day at 08:30 to 09:30 and 16:30 to 17:30 for 8 weeks. Every feeding lasted for 1 h until all fish in the tank were in apparent satiation (there were a few uneaten pellets in each tank). The remaining pellets of each tank were collected, dried, and weighed to calculate the daily feed intake.
During the experiment, continuous aeration was provided to each tank except for the feeding time, and the water flow rate into each tank was approximately 1.4 L/min. The water temperature was maintained at 28 ± 2 °C, and the photoperiod was 12-h light to 12-h dark with a light period from 08:00 to 20:00. The water quality parameters were monitored twice a week, and the values were as follows: pH 7.0 to 7.8, dissolved oxygen ≥5.0 mg/L, ammonia-nitrogen < 0.2 mg/L.
2.4. Sampling
After the feeding experiment was completed, the fish were fasted for 24 h, and then all fish from each tank were weighed and counted. Before sampling, 10 fish from each tank were randomly selected and anesthetized with 10 mg/L MS-222 (Sigma, St Louis, MO, USA). Three fish in each tank (9 fish per group) were randomly collected to measure individual body weight, body length, and liver weight to calculate the condition factor (CF) and hepatosomatic index (HSI). Two fish (6 fish per group) were weighed and stored at - 20 °C for chemical analysis. Two fish (6 fish per group) were used for the dorsal body and abdominal body colorness measurement, then the fish were killed from the abdomen using scissors and the dorsal body skin was peeled with a scalpel and forceps for the colorness measurement of dorsal muscle. Three fish (9 fish per group) were collected for blood sampling (from the caudal vein using heparinized syringes) and 0.2% heparin sodium (configured with 0.68% NaCl) (CAS 9041-08-1, Solarbio, China) was prepared to rinse the syringes and centrifuge tubes before blood sampling. Then, blood samples were centrifuged at 2,054 × g at 4 °C for 10 min to acquire plasma, which was stored at - 80 °C for further analysis. After blood sampling, the fish were killed from the abdomen using scissors and most of the liver and dorsal muscle from each fish were immediately dissected on ice and frozen in liquid nitrogen, then stored at - 80 °C for further analysis. A small part of each liver was fixed by 4% paraformaldehyde for histological analysis.
2.5. Biochemical analysis
The proximate composition of moisture, crude protein, crude lipid, crude ash, and gross energy content of the fish meal, Chlorella meal, experimental diets, whole fish, and dorsal muscle tissues were determined according to the methods of AOAC (2000). Briefly, moisture content was determined by drying the samples to a constant weight at 105 °C for 24 h and calculated as the percentage of water loss. Lipid content was determined using the chloroform-methanol (2:1) method (the samples were crushed and sonicated before the extraction process). Crude protein content (N × 6.25) was determined after acid digestion using an auto Kjeldahl system (Kjeltec-8400, FOSS Tecator, Haganas, Sweden). Crude ash content was determined by incineration in a muffle furnace at 550 °C for 12 h. Gross energy was determined using a Philips Microbomb Calorimeter (Gentry Instruments Inc., Aiken, USA). The crude fiber of fish meal, Chlorella meal, and experimental diets were analyzed using an automatic fiber analyzer (A2000i, ANKOM, USA). The amino acid composition of fish meal, Chlorella meal, and experimental diets were analyzed using an A300 amino acid analyzer (membraPure, Germany) after using the acid hydrolysis method to process all samples (Liu et al., 2016). Fatty acid composition of fish meal and Chlorella meal was analyzed as described by Fei et al. (2020) using a gas chromatograph-mass spectrometer (GC–MS, 7890A, Agilent Technologies, USA). Plasma glucose content was measured using commercial assay kits (638-50971, Wako Pure Chemical Industries, Tokyo, Japan). Each test was performed with at least 2 replicates.
2.6. qRT-PCR analysis
Total RNA was extracted using TRIzol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, USA). Agarose gel electrophoresis and NanoDrop ND-2000 UV–Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) analysis were performed to detect RNA quality and quantity, respectively, and the total RNA was stored at −80 °C for cDNA synthesis. cDNA was synthesized using cDNA synthesis kit (TransGen Biotech, AE311-03) according to the manufacturer's instructions. Real-time quantitative PCR was carried out with SYBR Green I Master Mix (Roche, Germany) on a Light-Cycler 480 System (Roche). All reactions were performed in a 6 μL volume containing 2 μL cDNA template, 0.24 μL each of forward and reverse primers, 3 μL LightCycle 480 SYBR Green I Master, and 0.52 μL ddH2O. The template was replaced with ddH2O and amplified in the same plate as the negative controls. The parameters were as follows: pre-incubation at 95 °C for 5 min, followed by 40 cycles of 10 s at 95 °C, 20 s at 60 °C, and 10 s at 72 °C. β-actin was used as a housekeeping gene, and the primers used in this study were listed in Table 5. Each sample was run in triplicate, and the results were expressed according to the method described by Bustin et al. (2009).
Table 5.
Primer sequences used for qRT-PCR analysis.
| Gene | Accession number | Forward primer (5′-3′) | Reverse primer (5′-3′) | Size, bp | PCR efficiency |
|---|---|---|---|---|---|
| β-actin | XM_038695351.1 | AAAGGGAAATCGTGCGTGAC | AAGGAAGGCTGGAAGAGGG | 184 | 1.99 |
| igf-1 | XM_038738328.1 | CTTCAAGAGTGCGATGTGC | GCCATAGCCTGTTGGTTTACTG | 187 | 2.01 |
| ira | XM_038717604.1 | CCCTTGTATCCCTCTCGTTT | CCAATTTCCTGTTCCTCTCC | 64 | 2.02 |
| irs1 | XM_038730647.1 | TAGTGGTGGTGTCAGCGGT | GGAGGTGGAAGTAAAGGAT | 301 | 2.04 |
| gck | XM_038703173.1 | CAGCGTGAGATGGACAGAGG | GGGGGTGGAGCAGACATAAG | 87 | 1.99 |
| pk | XM_038700626.1 | CTCTTTCATCCGCAAAGC | AATTCCCAGGTCACCACG | 172 | 2.01 |
| g6pc | XM_038735542.1 | AGAAAGCACAGAAGTGGTG | CTTGGTCTCGGTGTAGAGG | 137 | 2.05 |
| pepck | XM_038692953.1 | GGAAACGGCCAACATTCT | GCCAACCAGCAGTTCTCAT | 81 | 2.03 |
| bcl2 | XM_038695757.1 | CATCCTCCTTGGCTCTGG | GGGTCTGTTTGCCTTTGG | 141 | 2.01 |
| casp8 | XM_038718636.1 | GAGACAGACAGCAGACAACCA | TTCCATTTCAGCAAACACATC | 195 | 1.96 |
| casp9 | XM_038723308.1 | CTGGAATGCCTTCAGGAGACGGG | GGGAGGGGCAAGACAACAGGGTG | 125 | 1.99 |
| casp3 | XM_038713063.1 | GCTTCATTCGTCTGTGTTC | CGAAAAAGTGATGTGAGGTA | 98 | 2.00 |
| tor | XM_038723321.1 | TCAGGACCTCTTCTCATTGGC | CCTCTCCCACCATGTTTCTCT | 208 | 2.01 |
| s6 | XM_038713349.1 | GCCAATCTCAGCGTTCTCAAC | CTGCCTAACATCATCCTCCTT | 156 | 1.97 |
| eif2α | XM_038727791.1 | TAAGTCCAGCCCATCCAAAA | CACCCGAGGAGGCCATCAAG | 233 | 2.01 |
| atf4 | XM_038712791.1 | GGAGACCAGGAAGATGCGTAG | TGTCCAGCAGCAGTGATGACA | 209 | 1.95 |
β-actin = actin, beta 2; igf-1 = insulin-like growth factor 1; ira = insulin receptor a; irs1 = insulin receptor substrate 1; gck = glucokinase (hexokinase 4); pk = pyruvate kinase; g6pc = glucose-6-phosphatase catalytic subunit; pepck = phosphoenolpyruvate carboxykinase; bcl2 = β-cell lymphoma-2; casp8 = caspase-8-like; casp9 = caspase-9-like; casp3 = caspase-3a; tor = target of rapamycin; s6 = ribosomal protein s6; eif2α = elongation initiation factor 2α; atf4 = activating transcription factor 4.
2.7. Photographing and colorness determination
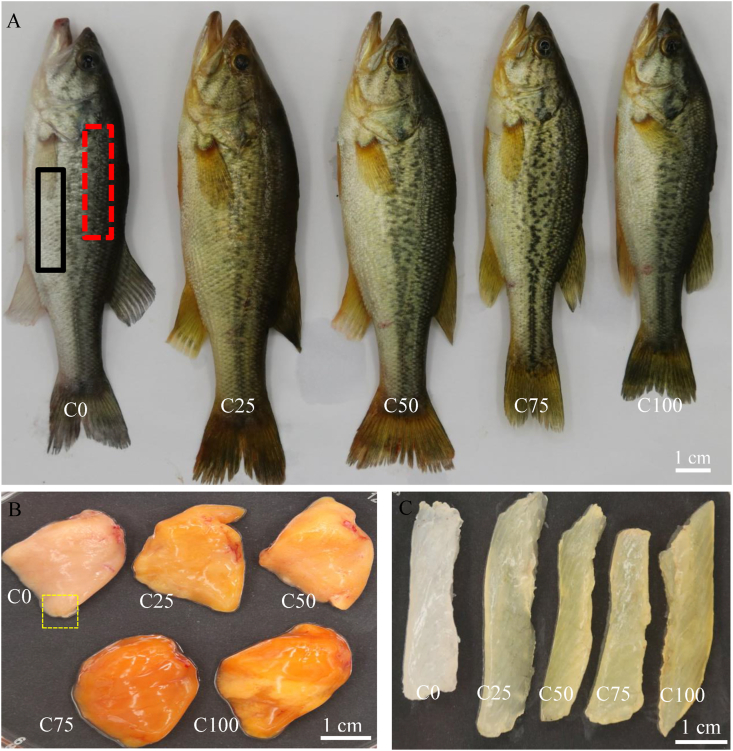
A Canon EOS 80D camera (Japan) was used for photographing the whole fish, liver, and dorsal muscle of the fish after largemouth bass fed different experimental diets for 8 weeks. The color of the dorsal body, abdominal body, and dorsal muscle of the fish were measured using a Konica Minolta CR-400 tristimulus colorimeter (Minolta, Osaka, Japan). Dorsal body and abdominal body areas were indicated by the red dashed box and black solid box, respectively (Fig. 1A). The dorsal muscle area was where the dorsal body skin was peeled. The L∗ value represents lightness (0 for black, 100 for white), a∗ represents the red/green dimension (redness), and b∗ represents the yellow/blue dimension (yellowness), according to the recommendations of the International Commission on Illumination (Pauli, 1976).
Fig. 1.
Photographing the whole fish, liver and dorsal muscle of largemouth bass fed different experimental diets for 8 weeks. C0, C25, C50, C75, and C100 represent diets in which Chlorella meal replaced fish meal levels of 0%, 25%, 50%, 75%, and 100%, respectively. (A) Representative images of whole fish; The red dashed box and black solid box area in the picture represent dorsal body and abdominal body, respectively. (B) Representative images of liver tissues; The yellow dashed box part in the picture was sampled for histological analysis. (C) Representative images of dorsal muscle. Scale bar (1 cm) was presented in the bottom right corner in every picture.
2.8. Lutein determination
The pretreatments of samples were conducted as previously described: fish meal, Chlorella meal, and diets (Ferreira et al., 2007), plasma (Breithaupt et al., 2003), and liver and dorsal muscle tissues (Liu et al., 2019). After pretreatment, the obtained samples were redissolved in 1 mL extract liquid (methanol: dichloromethane = 3:1, vol:vol), filtered, and immediately used for HPLC (Waters e2695, USA) analyses (Liu et al., 2019). All the steps were performed under dim light. Lutein was identified according to the peak time of the lutein standard product (PHL89723-5 MG, Supelco, USA), and the lutein content was quantified according to the standard curve (Y = 5E-08X, R2= 0.9998).
2.9. Histological analysis
Liver samples were dehydrated in a graded ethanol series after fixation for 24 h in 4% paraformaldehyde. They were then embedded in paraffin and cut into 4 μm sections that were stained with hematoxylin and eosin (H&E), terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), and 40, 6-diamidino-2-phenylindole (DAPI), respectively. H&E staining sections were pictured (Nikon Eclipse CI, Japan) and scored (1 to 2 = severe damage, 3 to 4 = moderate, 5 to 6 = mild, 7 to 8 = slight, 9 to 10 = normal) according to their vacuolation area. TUNEL and DAPI staining were pictured (Nikon Eclipse Ti-SR, Japan), and the color area was calculated to determine the apoptosis rate (%) = [100 × green area (TUNEL)/blue area (DAPI)]. Image Pro Plus 6.0 software (Media Cybernetics, USA) was used to quantify the above parameters.
2.10. Statistical analysis
Results were shown as the means ± the standard error of the mean (SEM). All data were analyzed using SPSS 24.0 software (IBM, USA). Levene's test was applied to the homogeneity of variances. One-way analysis of variance (ANOVA) was performed to test the effect of dietary Chlorella meal, and Duncan's multiple range test with P < 0.05 was used to detect the significance of differences among all groups. Orthogonal polynomial contrasts were used to determine whether there was a linear and/or quadratic regression (Wei et al., 2019). A quadratic equation was used to calculate the dietary inclusion level of Chlorella meal when fish achieved maximum values (final body weight, weight gain rate, specific growth rate, or plasma lutein concentrations). R2 (0.7 ≤ R2 ≤ 1) indicated a good fit of the regression equation to the data (Xu et al., 2018).
3. Results
3.1. The ADC of fish meal and Chlorella meal
The ADC of dry matter in fish meal and Chlorella meal were 81.29% and 72.94%, respectively. The ADC of protein in fish meal and Chlorella meal were 92.62% and 87.38%, respectively (Table 2).
3.2. Growth, feed utilization, and morphological indices
Growth, feed utilization, and morphological indices were listed in Table 6. Increased levels of Chlorella meal significantly influenced final body weight (FBW), weight gain rate (WGR), specific growth rate (SGR), feed intake (FI), feed conversion ratio (FCR), and HSI (P < 0.05). However, survival rate (SR) and CF were not affected by dietary Chlorella meal levels. There were significant linear or quadratic regressions regarding dietary Chlorella meal levels for FBW, WGR, SGR, FCR, and HSI (P < 0.05). The lowest FBW, WGR, and SGR values were found in the C100 group among all treatments (P < 0.05). There was a significant quadratic regression regarding the dietary Chlorella meal levels for FI, which was significantly lower in the C100 group than in the other groups (P < 0.05). Higher FCR and HSI values were found in the C75 and C100 groups than in the other groups (P < 0.05). Based on the quadratic regression analysis between FBW, WGR, or SGR and dietary Chlorella meal level, largemouth bass had the best growth when adding 15.03% to 15.43% dietary Chlorella meal (replace 31.7% to 32.6% of fish meal).
Table 6.
Growth, feed utilization, and morphological indices of largemouth bass fed different experimental diets for 8 weeks (mean ± SEM, n = 3 for IBW, FBW, WGR, SGR, FI, FCR, and SR; n = 9 for HSI and CF).
| Item | Diets1 |
Pr > F2 |
||||||
|---|---|---|---|---|---|---|---|---|
| C0 | C25 | C50 | C75 | C100 | ANOVA | Linear trend | Quadratic trend | |
| IBW3, g/fish | 17.65 ± 0.08 | 17.68 ± 0.07 | 17.60 ± 0.09 | 17.68 ± 0.09 | 17.57 ± 0.07 | 0.804 | 0.474 | 0.718 |
| FBW4, g/fish | 66.85 ± 2.03bc | 71.70 ± 2.11c | 68.93 ± 1.43bc | 64.83 ± 2.14b | 51.21 ± 1.97a | 0.000 | 0.003 | 0.000 |
| WGR5, % | 278.80 ± 13.09bc | 305.56 ± 10.47c | 291.77 ± 9.43bc | 266.64 ± 10.62b | 191.54 ± 11.48a | 0.000 | 0.003 | 0.000 |
| SGR6, %/d | 2.22 ± 0.06b | 2.33 ± 0.04b | 2.27 ± 0.04b | 2.16 ± 0.05b | 1.78 ± 0.06a | 0.000 | 0.003 | 0.000 |
| FI7, g/fish | 41.39 ± 1.24b | 45.22 ± 1.43b | 44.49 ± 0.79b | 43.49 ± 1.63b | 36.58 ± 1.79a | 0.010 | 0.106 | 0.001 |
| FCR8 | 0.84 ± 0.01a | 0.84 ± 0.01a | 0.87 ± 0.01a | 0.92 ± 0.01b | 1.09 ± 0.01c | 0.000 | 0.000 | 0.000 |
| SR9, % | 91.67 ± 4.41 | 96.67 ± 3.33 | 98.33 ± 1.67 | 96.67 ± 1.67 | 96.67 ± 1.67 | 0.540 | 0.259 | 0.226 |
| HSI10, % | 3.02 ± 0.11c | 3.37 ± 0.12c | 3.54 ± 0.10c | 4.32 ± 0.27b | 4.94 ± 0.23a | 0.000 | 0.000 | 0.000 |
| CF11, g/cm3 | 2.32 ± 0.05 | 2.24 ± 0.02 | 2.28 ± 0.03 | 2.29 ± 0.03 | 2.22 ± 0.05 | 0.174 | 0.238 | 0.496 |
| Quadratic regressions | R2 | Ymax12, % | Replace fish meal level13, % | |||||
| YFBW = - 0.0002X2 + 0.0601X + 66.8621 | 0.8577 | 15.03 | 31.7 | |||||
| YWGR = - 0.0011X2 + 0.338X + 278.8271 | 0.8576 | 15.43 | 32.6 | |||||
| YSGR = - 5E-06X2 + 0.0016X + 2.2125 | 0.8633 | 15.03 | 31.7 | |||||
C0, C25, C50, C75, and C100 represent diets in which Chlorella meal replaced fish meal levels of 0%, 25%, 50%, 75%, and 100%, respectively. Duncan's multiple range test was used to detect the significance of differences among all groups. Different superscripts on the same row indicate significant differences (P < 0.05).
Significance probability associated with the F-statistic.
IBW = initial body weight.
FBW = final body weight.
Weight gain rate (WGR) = 100 × (Final body weight- Initial body weight)/Initial body weight).
Specific growth rate (SGR) = 100 × [ln (Final body weight) − ln (Initial body weight)]/Days.
FI = feed intake (total amount of the dry feed consumed).
Feed conversion ratio (FCR) = Feed intake/(Total final body weight – Total initial body weight).
Survival rate (SR) = 100 × (Final fish number/Initial fish number).
Hepatosomatic index (HIS) = 100 × (Liver weight/Whole body weight).
Condition factor (CF) = 100 × (Body weight/Body length3).
Based on the quadratic regression analysis between the parameters and dietary Chlorella meal level to calculate the optimal Chlorella meal content.
Based on the Ymax to calculate the replace fish meal level by Chlorella meal.
3.3. Biochemical indicators
The plasma glucose content and proximate composition of the whole fish and dorsal muscle were shown in Table 7. The increasing levels of Chlorella meal significantly affected the plasma glucose and crude protein of the whole fish and dorsal muscle (P < 0.05). No significant effects of dietary Chlorella meal levels were observed on whole fish and dorsal muscle, including moisture, crude lipid, crude ash, and gross energy. With increasing dietary Chlorella meal levels, plasma glucose increased linearly or quadratically, whereas plasma glucose significantly increased in the C75 and C100 groups compared to the other groups (P < 0.05). With increasing dietary Chlorella meal levels, the crude protein of the whole fish and dorsal muscle decreased linearly and quadratically, with the lowest content in the C100 group (P < 0.05).
Table 7.
Plasma glucose and proximate composition of whole fish and dorsal muscle of largemouth bass fed different experimental diets for 8 weeks (mean ± SEM, n = 6 for glucose content; n = 3 for the proximate composition of whole fish and dorsal muscle parameters).
| Item | Diets1 |
Pr > F2 |
||||||
|---|---|---|---|---|---|---|---|---|
| C0 | C25 | C50 | C75 | C100 | ANOVA | Linear trend | Quadratic trend | |
| Plasma | ||||||||
| Glucose content, mg/dL | 60.21 ± 2.31a | 57.97 ± 3.78a | 62.70 ± 3.73a | 81.24 ± 5.52b | 91.14 ± 3.96b | 0.000 | 0.000 | 0.000 |
| Whole fish, % wet weight | ||||||||
| Moisture | 71.44 ± 0.65 | 70.35 ± 0.38 | 71.00 ± 0.17 | 71.60 ± 0.14 | 71.65 ± 0.62 | 0.311 | 0.339 | 0.334 |
| Crude protein | 17.04 ± 0.19ab | 17.09 ± 0.03b | 16.93 ± 0.16ab | 16.54 ± 0.22ab | 16.50 ± 0.09a | 0.024 | 0.002 | 0.007 |
| Crude lipid | 8.01 ± 0.37 | 8.91 ± 0.30 | 8.59 ± 0.17 | 8.37 ± 0.21 | 8.40 ± 0.51 | 0.443 | 0.821 | 0.426 |
| Crude ash | 3.78 ± 0.11 | 3.84 ± 0.11 | 3.93 ± 0.08 | 3.82 ± 0.06 | 3.66 ± 0.06 | 0.329 | 0.383 | 0.100 |
| Gross energy3, kJ/g | 23.16 ± 0.40 | 24.44 ± 0.66 | 23.65 ± 0.12 | 23.46 ± 0.15 | 23.74 ± 0.41 | 0.320 | 0.895 | 0.709 |
| Dorsal muscle, % wet weight | ||||||||
| Moisture | 77.89 ± 0.23 | 78.17 ± 0.03 | 78.14 ± 0.08 | 78.78 ± 0.37 | 78.75 ± 0.11 | 0.542 | 0.503 | 0.623 |
| Crude protein | 19.09 ± 0.13b | 19.00 ± 0.07b | 19.03 ± 0.08b | 18.59 ± 0.19ab | 18.33 ± 0.05a | 0.000 | 0.000 | 0.000 |
| Crude lipid | 2.47 ± 0.17 | 2.49 ± 0.19 | 2.52 ± 0.14 | 2.47 ± 0.18 | 2.51 ± 0.15 | 0.999 | 0.896 | 0.990 |
| Crude ash | 1.37 ± 0.02 | 1.32 ± 0.02 | 1.39 ± 0.03 | 1.35 ± 0.02 | 1.41 ± 0.04 | 0.238 | 0.225 | 0.247 |
| 4Gross energy, kJ/g | 21.84 ± 0.03 | 21.85 ± 0.09 | 21.73 ± 0.09 | 21.76 ± 0.07 | 21.72 ± 0.04 | 0.537 | 0.114 | 0.297 |
C0, C25, C50, C75, and C100 represent diets in which Chlorella meal replaced fish meal levels of 0%, 25%, 50%, 75%, and 100%, respectively. Duncan's multiple range test was used to detect the significance of differences among all groups. Different superscripts on the same row indicate significant differences (P < 0.05).
Significance probability associated with the F-statistic.
Gross energy in whole fish (dry matter basis).
Gross energy in dorsal muscle (dry matter basis).
3.4. Transcriptional expression in liver tissues
As shown in Table 8, increasing levels of Chlorella meal significantly affected the liver transcriptional levels of insulin-like growth factor 1 (igf-1), insulin pathway-related genes of insulin receptor a (ira) and insulin receptor substrate 1 (irs1), glycolysis-related genes of glucokinase (gck) and pyruvate kinase (pk), gluconeogenesis-related genes of glucose-6-phosphatase catalytic subunit (g6pc) and phosphoenolpyruvate carboxykinase (pepck), apoptosis-related genes of β-cell lymphoma-2 (bcl2), caspase-9-like (casp9), and caspase-3a (casp3), and amino acid response pathway-related genes of activating transcription factor 4 (atf4) (P < 0.05). However, the dietary Chlorella meal did not significantly affect caspase-8-like (casp8), elongation initiation factor 2α (eif2α), and target of rapamycin (TOR) pathway-related genes of target of rapamycin (tor) and ribosomal protein s6 (s6). The liver transcriptional levels of igf-1, g6pc, and pepck significantly decreased linearly or quadratically (P < 0.05), whereas igf-1 and pepck significantly decreased in the C75 and C100 groups compared with the C0 group (P < 0.05), and g6pc significantly decreased in the C50 and C100 groups compared with the C0 group (P < 0.05). Moreover, compared to the C0 group, the liver transcriptional levels of bcl2, casp9, and casp3 in the C100 group were markedly upregulated 1.1-fold, 0.9-fold, and 1.6-fold, respectively.
Table 8.
The transcriptional level of genes in the liver of largemouth bass fed different experimental diets for 8 weeks (mean ± SEM, n = 6).
| Gene1 | Diets2 |
Pr > F3 |
||||||
|---|---|---|---|---|---|---|---|---|
| C0 | C25 | C50 | C75 | C100 | ANOVA | Linear trend | Quadratic trend | |
| igf-1 | 1.06 ± 0.16b | 1.20 ± 0.13b | 0.87 ± 0.03ab | 0.43 ± 0.10a | 0.67 ± 0.07a | 0.001 | 0.001 | 0.004 |
| ira | 1.05 ± 0.14a | 1.62 ± 0.25ab | 1.08 ± 0.17a | 1.35 ± 0.10ab | 2.39 ± 0.56b | 0.021 | 0.023 | 0.026 |
| irs1 | 1.32 ± 0.44a | 1.23 ± 0.17a | 2.22 ± 0.51a | 3.20 ± 0.12ab | 9.19 ± 2.73b | 0.003 | 0.001 | 0.000 |
| gck | 1.24 ± 0.29ab | 1.17 ± 0.10a | 1.66 ± 0.33ab | 1.00 ± 0.19a | 3.62 ± 1.20b | 0.021 | 0.030 | 0.018 |
| pk | 1.19 ± 0.32a | 1.06 ± 0.18a | 0.71 ± 0.03a | 2.13 ± 0.42ab | 3.47 ± 1.23b | 0.006 | 0.005 | 0.001 |
| g6pc | 1.03 ± 0.12b | 1.18 ± 0.12b | 0.64 ± 0.04a | 0.66 ± 0.10ab | 0.61 ± 0.04a | 0.000 | 0.000 | 0.001 |
| pepck | 1.07 ± 0.18b | 0.77 ± 0.14ab | 0.65 ± 0.15ab | 0.50 ± 0.12a | 0.58 ± 0.07a | 0.055 | 0.007 | 0.009 |
| bcl2 | 1.13 ± 0.28a | 1.14 ± 0.13a | 0.87 ± 0.19a | 1.03 ± 0.11a | 2.39 ± 0.70b | 0.039 | 0.055 | 0.013 |
| casp8 | 1.04 ± 0.35 | 1.17 ± 0.16 | 0.96 ± 0.15 | 1.03 ± 0.09 | 1.45 ± 0.30 | 0.488 | 0.311 | 0.315 |
| casp9 | 1.16 ± 0.30a | 0.90 ± 0.07a | 0.99 ± 0.15a | 1.23 ± 0.08a | 2.97 ± 0.23b | 0.000 | 0.003 | 0.000 |
| casp3 | 1.04 ± 0.21a | 1.12 ± 0.07a | 0.94 ± 0.15a | 1.17 ± 0.06a | 1.94 ± 0.09b | 0.001 | 0.005 | 0.000 |
| tor | 1.09 ± 0.22 | 1.19 ± 0.09 | 1.07 ± 0.13 | 0.97 ± 0.14 | 1.04 ± 0.06 | 0.846 | 0.444 | 0.749 |
| s6 | 1.10 ± 0.22 | 1.21 ± 0.06 | 0.87 ± 0.09 | 0.85 ± 0.11 | 0.96 ± 0.19 | 0.358 | 0.160 | 0.326 |
| eif2α | 1.11 ± 0.26 | 1.20 ± 0.10 | 0.96 ± 0.09 | 0.81 ± 0.15 | 1.23 ± 0.14 | 0.322 | 0.777 | 0.441 |
| atf4 | 1.01 ± 0.23ab | 0.93 ± 0.10a | 1.26 ± 0.11ab | 1.18 ± 0.08ab | 2.22 ± 0.82b | 0.044 | 0.013 | 0.015 |
igf-1 = insulin-like growth factor 1; ira = insulin receptor a; irs1 = insulin receptor substrate 1; gck = glucokinase (hexokinase 4); pk = pyruvate kinase; g6pc = glucose-6-phosphatase catalytic subunit; pepck = phosphoenolpyruvate carboxykinase; bcl2 = β-cell lymphoma-2; casp8 = caspase-8-like; casp9 = caspase-9-like; casp3 = caspase-3a; tor = target of rapamycin; s6 = ribosomal protein s6; eif2α = elongation initiation factor 2α; atf4 = activating transcription factor 4.
β-actin was used as a housekeeping gene.
C0, C25, C50, C75, and C100 represent diets in which Chlorella meal replaced fish meal levels of 0%, 25%, 50%, 75%, and 100%, respectively. Duncan's multiple range test was used to detect the significance of differences among all groups. Different superscripts on the same row indicate significant differences (P < 0.05).
Significance probability associated with the F-statistic.
3.5. Colorness and lutein content
Compared to the C0 group, all other groups showed visual color changes in the whole fish (Fig. 1A), liver (Fig. 1B), and dorsal muscle tissues (Fig. 1C) of largemouth bass. As shown in Table 9, the a∗ (redness) in the dorsal body, abdominal body, and b∗ (yellowness) in the dorsal body, abdominal body, and dorsal muscle were significantly affected by the dietary Chlorella meal levels (P < 0.05). No significant effects of dietary Chlorella meal levels were observed on the L∗ (lightness) of the dorsal body, abdominal body, and dorsal muscle. There were significant linear or quadratic trends regarding the dietary Chlorella meal levels for the a∗ in the dorsal body and abdominal body and for the b∗ in the dorsal body, abdominal body, and dorsal muscle (P < 0.05). Moreover, the b∗ value of the dorsal body, abdominal body, and dorsal muscle in all supplemented Chlorella meal groups were significantly increased 1.5 to 2.0 fold, 1.5 to 1.8 fold, and 3.8 to 5.4 fold, respectively.
Table 9.
The color parameters of the dorsal body, abdominal body, and dorsal muscle of largemouth bass fed different experimental diets for 8 weeks (mean ± SEM, n = 6).
| Item | Diets1 |
Pr > F2 |
||||||
|---|---|---|---|---|---|---|---|---|
| C0 | C25 | C50 | C75 | C100 | ANOVA | Linear trend | Quadratic trend | |
| Dorsal body3 | ||||||||
| L∗ | 42.15 ± 2.43ab | 41.06 ± 1.54a | 43.79 ± 1.21ab | 47.01 ± 1.57b | 41.63 ± 1.89a | 0.155 | 0.417 | 0.377 |
| a∗ | −4.85 ± 0.30c | −6.74 ± 0.33bc | −6.76 ± 0.20bc | −7.28 ± 0.33a | −6.20 ± 0.45b | 0.000 | 0.023 | 0.000 |
| b∗ | 5.34 ± 0.28a | 13.73 ± 0.59bc | 13.36 ± 1.10b | 16.02 ± 1.29c | 14.65 ± 0.76bc | 0.000 | 0.000 | 0.000 |
| Abdominal body4 | ||||||||
| L∗ | 76.02 ± 0.68 | 78.18 ± 1.06 | 79.84 ± 1.25 | 78.47 ± 1.23 | 76.23 ± 2.16 | 0.267 | 0.875 | 0.073 |
| a∗ | −6.72 ± 0.21b | −8.95 ± 0.20a | −8.88 ± 0.30a | −9.27 ± 0.49a | −8.60 ± 0.19a | 0.000 | 0.004 | 0.000 |
| b∗ | 8.64 ± 0.53a | 21.83 ± 1.0b | 21.85 ± 1.74b | 24.47 ± 1.76b | 21.30 ± 0.77b | 0.000 | 0.000 | 0.000 |
| Dorsal muscle5 | ||||||||
| L∗ | 42.99 ± 0.51 | 42.09 ± 1.07 | 41.17 ± 1.44 | 42.05 ± 0.74 | 41.64 ± 1.10 | 0.785 | 0.386 | 0.523 |
| a∗ | −3.31 ± 0.08b | −3.32 ± 0.46b | −3.90 ± 0.40ab | −4.48 ± 0.16a | −3.68 ± 0.34ab | 0.090 | 0.086 | 0.103 |
| b∗ | 1.38 ± 0.14a | 6.63.00 ± 0.69b | 7.41 ± 0.83bc | 7.70 ± 0.54bc | 8.89 ± 0.65c | 0.000 | 0.000 | 0.000 |
L∗ = lightness; a∗ = redness; b∗ = yellowness.
C0, C25, C50, C75, and C100 represent diets in which Chlorella meal replaced fish meal levels of 0%, 25%, 50%, 75%, and 100%, respectively. Duncan's multiple range test was used to detect the significance of differences among all groups. Different superscripts on the same row indicate significant differences (P < 0.05).
Significance probability is associated with the F-statistic.
Refer to Fig. 1A for the specific measured position.
Refer to Fig. 1A for the specific measured position.
Refer to Fig. 1A for the specific measured position, measured after peeled the skin of dorsal body.
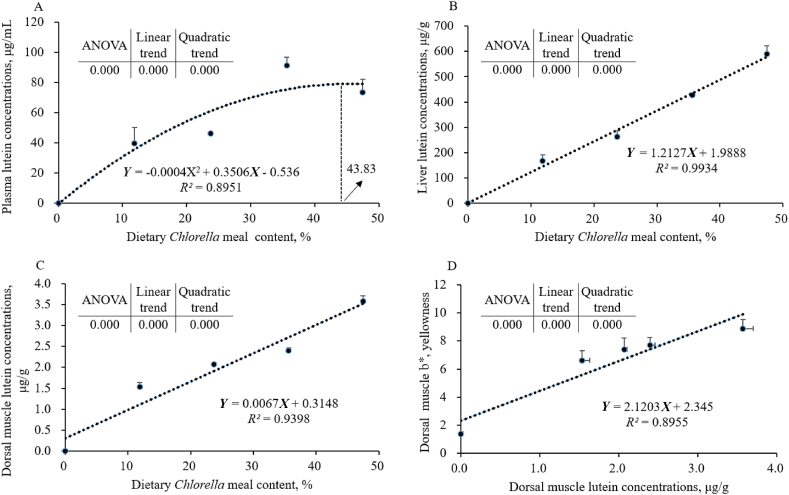
Linear and quadratic regressions were analyzed by orthogonal polynomial contrasts, and then the equation with a higher R2 score was selected to make the figures shown in Fig. 2. The lutein concentrations in plasma (Fig. 2A), liver (Fig. 2B), and dorsal muscle (Fig. 2C) were significantly affected by dietary Chlorella meal levels (P < 0.05), and there were significant linear or quadratic trends between the increasing levels of Chlorella meal (P < 0.05). Based on quadratic regression analysis between plasma lutein concentrations and dietary Chlorella meal levels, largemouth bass had the maximum plasma lutein concentration when adding 43.83% of Chlorella meal to the diet. Similarly, there were significant linear or quadratic relationships between b∗ and lutein concentrations in the dorsal muscles (Fig. 2D) (P < 0.05).
Fig. 2.
The regression analyses between lutein concentrations and different parameters of largemouth bass fed different experimental diets for 8 weeks (mean ± SEM, n = 3). (A) The quadratic regression analysis between the plasma lutein concentrations and dietary Chlorella meal content. (B) The linear regression analysis between the liver lutein concentrations and dietary Chlorella meal content. (C) The linear regression analysis between the dorsal muscle lutein concentrations and dietary Chlorella meal content. (D) The linear regression analysis between the dorsal muscle lutein concentrations and b∗ of dorsal muscle. (Interpretation: Linear and quadratic trends were analyzed by orthogonal polynomial contrasts, and then the equation with a higher R2 score was selected to make the figures shown above).
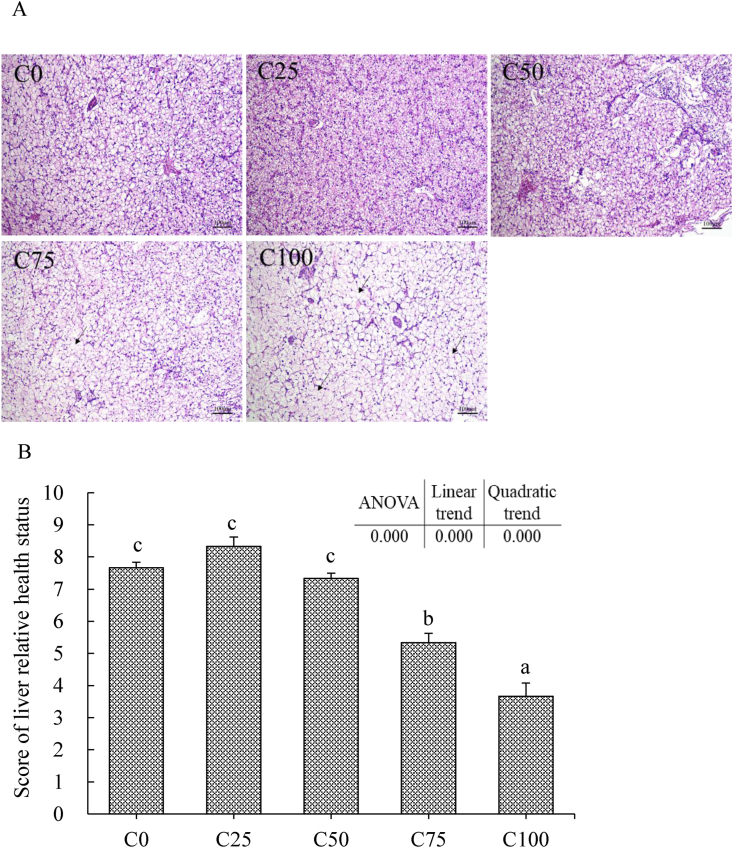
3.6. Histological changes
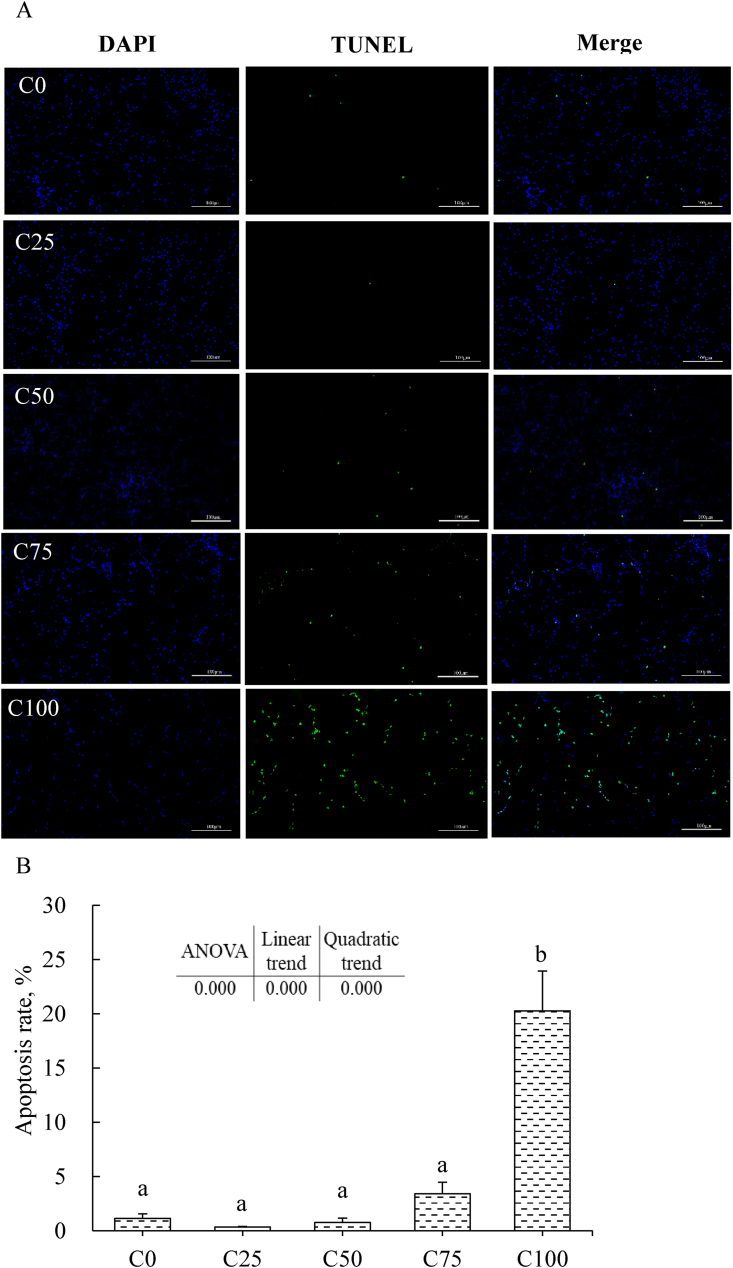
The fish liver in the C25 group showed the best health status compared to the other groups (Fig. 3). However, with the increased inclusion of dietary Chlorella meal, liver cells became bigger, and many nuclei disappeared in cells, especially in the C100 group (Fig. 3A). Fish fed the C75 and C100 diets exhibited much more severe liver vacuolization than those fed the other diets (Fig. 3B) (P < 0.05). The results of liver DAPI and TUNEL staining were shown in Fig. 4. The C100 group showed the highest apoptosis rate in the liver compared to the other groups (Fig. 4B) (P < 0.05).
Fig. 3.
Liver H&E staining analysis of largemouth bass fed different experimental diets for 8 weeks. C0, C25, C50, C75, and C100 represent diets in which Chlorella meal replaced fish meal levels of 0%, 25%, 50%, 75%, and 100%, respectively. (A) Representative images (100×, scale bar = 100 μm); The black arrows represent the cell nucleus disappear and the cytoplasmic vacuolization. (B) Images' statistical analysis (mean ± SEM, n = 9); Liver relative health status of largemouth bass was scored according to the degree of its vacuolization (1 to 2: severe damage, 3 to 4: moderate, 5 to 6: mild, 7 to 8: slight, 9 to 10: normal). Duncan's multiple range test used to detect its significance. Values marked with different superscripts above the bars indicate significant differences (P < 0.05).
Fig. 4.
Liver DAPI and TUNEL staining analyses of largemouth bass fed different experimental diets for 8 weeks. C0, C25, C50, C75, and C100 represent diets in which Chlorella meal replaced fish meal levels of 0%, 25%, 50%, 75%, and 100%, respectively. (A) Representative images (200 × , scale bar = 100 μm). Under the fluorescence microscope, the blue color represents the nucleus and the green color represents apoptotic cells. (B) Images' statistical analysis (mean ± SEM, n = 9). Apoptosis rate (%) = 100 × [Green color area (TUNEL area)]/[Bule color area (DAPI area)]. TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling; DAPI = 40, 6-diamidino-2-phenylindole. Duncan's multiple range test used to detect its significance. Values marked with different superscripts above the bars indicate significant differences (P < 0.05).
4. Discussion
4.1. Effects of dietary Chlorella meal on growth performance of fish
In the present study, compared to the control group (40% dietary fish meal), Chlorella meal replacing 31.7% to 32.6% of the dietary fish meal had the best growth (FBW, WGR, and SGR) in largemouth bass and Chlorella meal, replacing up to 50% of dietary fish meal did not have a negative influence on growth and FCR. This is in agreement with many previous studies showing that dietary Chlorella inclusion had no negative impact on growth in rainbow trout (Gouveia et al., 1998), sterlet (Acipenser ruthenus) (Sergejevova and Masojidek, 2012), gilthead seabream (Sparus aurata) (Gouveia et al., 2002), zebrafish (Carneiro et al., 2020) and Nile tilapia (Oreochromis niloticus) (Teuling et al., 2017). In olive flounder (Paralichthys olivaceus), supplementation with 10% to 15% dietary Chlorella vulgaris enhanced the growth performance of fish after 8-week feeding (Rahimnejad et al., 2017). In rainbow trout, dietary inclusion of 10% Chlorella sorokiniana meal (containing 26.6% dietary fish meal) did not significantly influence the FBW, WGR, SGR, and FCR compared with the control diet (containing 30% dietary fish meal) after a 90-day feeding experiment (Chen et al., 2021). Similarly, the SGR of rainbow trout was not influenced by the dietary inclusion of 4% C. vulgaris after 9-week feeding (Gouveia et al., 1996). These results indicate that Chlorella meal is an effective alternative protein source for fish meal in aquatic animals, including largemouth bass. This may be due to the abundant nutrients of Chlorella, such as balanced amino acids, high levels of highly unsaturated fatty acids, antioxidants, pigments, vitamins, and a growth substance known as the Chlorella growth factor (CGF), which made Chlorella have a similar nutritional contribution as fish meal (Alagawany et al., 2021; Kotrbacek et al., 2015).
However, the growth performance of largemouth bass was significantly decreased when the fish meal was completely replaced by 47.45% dietary Chlorella meal. This could be due to the significantly decreased FI. The digestibility, especially the protein digestibility, was lower in Chlorella meal than in the fish meal, which might lead to inadequate utilization of the nutrients in Chlorella meal by largemouth bass, and decrease the growth in totally replacement group. The inadequate utilization of the nutrients could be caused by the thick cell walls of Chlorella meal (Shah et al., 2018). This was further evidenced by the significantly increased FCR in the C75 and C100 groups. In addition, the balanced dietary amino acid composition is essential for the growth of aquaculture species (Canada et al., 2016; Oliveira et al., 2021). For largemouth bass, the optimal dietary level of methionine is 1.22% (Chen et al., 2010). In the present study, compared to fish meal, the low level of methionine in Chlorella meal led to the low dietary level of methionine (0.64%) in the C100 group, which is 50% of the requirement of largemouth bass. Furthermore, the protein digestibility was lower in Chlorella meal than fish meal, which meant the available amino acid would be even lower in the C100 group. This might be one key reason for the decreased growth in the C100 group. Li et al. (2021b) proved that compared to the control diet (65.3% of fish meal), supplementation with 0.5% of methionine to the low fish meal diet (14.5% of fish meal) significantly improved the growth of largemouth bass.
Besides, excessive dietary Chlorella meal could not be efficiently utilized because of excessive supplementation of Chlorella meal led to high dietary carbohydrate, which was 28.85% carbohydrate (crude fiber-free) in the C100 group. Zhang et al. (2019) reported that high dietary starch levels (24 to 36%) could cause significant adverse effects on largemouth bass. This could cause glucose intolerance, abnormal glucose metabolism, and retarded growth of largemouth bass, as evidenced by the present results of significantly increased plasma glucose level, decreased growth, and glucose metabolism in the C100 group. Furthermore, the crude protein content was significantly decreased in the C100 group, which might be a further subject along with the decreased growth in the C100 group.
In addition, the growth of fish is mainly regulated by some pathways, including Growth Hormone-IGF-1 and insulin signals. Igf-1 is an essential regulator of fish growth (Dai et al., 2020). It has been reported that total replacement of fish meal (70.37% fish meal in control diet) by a mixture of plant proteins significantly decreased the growth and down-regulated the expression of igf-1 in the liver of gilthead sea bream (S. aurata) (Gomez-Requeni et al., 2004). In Japanese flounder (P. olivaceus), a 3.7% ultrafiltration diet significantly increased the growth and upregulated the expression of igf-1 in the fish liver (Zheng et al., 2012). In the present study, significantly reduced growth was accompanied by downregulation of liver igf-1 in the C100 group. This could partly explain the suppressed growth of largemouth bass in the C100 group.
The insulin pathway is important in maintaining glucose homeostasis. Activation of the insulin pathway usually stimulates glycolysis and inhibits gluconeogenesis (Wang et al., 2020). In the present study, the C100 group significantly induced the transcriptional levels of ira and irs1 in the liver, indicating activation of the insulin pathway. Meanwhile, a significant upregulation of gck and pk was found in the liver of the C100 group, which plays a key role in glycolysis. This was supported by previous results showing an obvious upregulation of gk and pk in the liver of largemouth bass fed high-carbohydrate diets (24% to 36% dietary starch) (Zhang et al., 2019). In the present study, the transcriptional levels of pepck and g6pc, key enzymes in gluconeogenesis, were significantly downregulated in the largemouth bass liver of the C100 group. This was also supported by the fact that high dietary corn starch (20%) could suppress gluconeogenesis by downregulating the expression of pepck and g6pc in the liver of largemouth bass (Zhang et al., 2020c). All these activated glucose metabolism genes indicated that the C100 group received the effects of increased dietary carbohydrate, which would cause the suppressed growth of largemouth bass.
Many studies have shown that dietary Chlorella does not affect the crude protein content of the whole body or muscle of fish (Kim et al., 2002; Rahimnejad et al., 2017; Luo et al., 2018). Similarly, our results showed that replacing up to 75% of fish meal with Chlorella meal did not significantly affect the crude protein content of the whole body and dorsal muscle of largemouth bass. However, crude protein content was significantly decreased in the C100 group. In fish, the TOR pathway mainly regulates protein synthesis (Song et al., 2016). In the present study, no significant changes in tor and s6, two key factors in the TOR pathway, were found in the liver tissues of largemouth bass fed different Chlorella meal diets. In contrast to the TOR pathway, the amino acid response pathway is key to regulating protein catabolism through the activation of atf4 (Kilberg et al., 2005; Dai et al., 2020). In the present study, atf4 was significantly upregulated in the C100 group, indicating the induction of protein catabolism. This was responsible for the reduced crude protein content of the whole body and dorsal muscle and finally suppressed the growth of largemouth bass in the C100 group.
4.2. Dietary inclusion of Chlorella meal improves the pigmentation of fish
The carotenoid is a pigment commonly found in some microalgae, such as Dunaliella salina, Haematococcus pluvialis, C. vulgaris, and Porphyridium cruentum (Ahmad et al., 2020; Gong and Bassi, 2016). Feed supplemented carotenoids (including natural-source carotenoids of microalgae) improved fish pigmentation because fish cannot de novo synthesis of carotenoids (Alagawany et al., 2021). Chlorella is a type of microalgae rich in carotenoids (0.4% on a dry basis) (Gouveia et al., 2002), which can improve the health and body color of fish (Zhu and Mai, 2003). The present study observed that dietary Chlorella meal could enhance the pigmentation of largemouth bass. In agreement with our study, the inclusion of Chlorella in the feed also enhanced the pigmentation of sterlet (Sergejevova and Masojidek, 2012), rainbow trout (Gouveia et al., 1996, 1997, 1998), and gilthead seabream (Gouveia et al., 2002). It has been reported that the lutein (a primary carotenoid) content in Chlorella was about ten times higher than that in marigold, where marigold is the main plant species that produces lutein in the industry (Chen et al., 2018; Chen and Liu, 2018; Gong and Bassi, 2017). This indicates that Chlorella contains abundant lutein and has been directly used as a source of natural pigments in medicine, food, and cosmetics (Ahmad et al., 2020). In the present study, rich lutein content was found in the plasma, liver, and dorsal muscle tissues of largemouth bass fed different Chlorella meal diets. This is mainly caused by the high lutein content in Chlorella meal (79.73 mg/kg) because no lutein content was detected in the control diet, and fish could not synthesize lutein by themselves. This result was supported by previous studies showing that dietary lutein could accumulate in the fillets of channel catfish (Ictalurus punctatus) (Li et al., 2007), skin and liver tissues of yellow catfish (Pelteobagrus fulvidraco) (Liu et al., 2019). Moreover, Meilisza et al. (2017) found that the dietary inclusion of 0.1% lutein could significantly increase the muscle carotenoid (including lutein) content in the Lake Kurumoi rainbowfish (Melanotaenia parva). These studies indicate that lutein accumulates in different tissues of different fish species. Yellow body color is determined by xanthophores containing carotenoids (Kimler and Taylor, 2002). b∗ is an important parameter used to analyze coloration in fish (Wei et al., 2019; Liu et al., 2019). Storebakken et al. (2004) used several mathematical models to describe the relationship between instrumentally assessed coloration and carotenoid concentration, indicating that instrument color analysis under standard conditions can accurately predict carotenoid levels. Liu et al. (2019) found a positive correlation between b∗ and lutein content in the abdominal and dorsal skin and suggested that lutein could be a key indicator for judging the pigmentation of yellow catfish. Similarly, in the present study, the b∗ of the dorsal and abdominal body and dorsal muscle were significantly increased in the dietary Chlorella groups compared with the control group. There was a positive correlation between b∗ and lutein content of the dorsal muscle, which indicated that lutein was also an important indicator for judging the pigmentation of largemouth bass.
4.3. Excess replacing fish meal with Chlorella meal leads to liver damage of fish
Histological changes in the liver are crucial for understanding nutrition-related pathological alterations in fish (Shi et al., 2017a, b). Dietary inclusion of Chlorella meal has been reported to lead to karyopyknosis and enlarged hepatocyte size in the liver tissues of crucian carp (Shi et al., 2017b). Another study found that replacing fish meal with a combination of soybean meal and Chlorella meal also caused the disappearance of the nuclear membrane in cells, karyopyknosis, and hepatocyte necrosis in juvenile crucian carp (Shi et al., 2017a). Similarly, in the present study, replacing 75% or 100% dietary fish meal with Chlorella meal led to severe vacuolation in the liver of largemouth bass. The reason could be that the high dietary inclusion of Chlorella meal (35.6% to 47.45%) increased dietary carbohydrate (crude fiber-free) content (25.99% in the C75 group and 28.85% in the C100 group), which caused glucose intolerance and liver impairment of largemouth bass, which was evidenced by the increased plasma glucose levels and liver apoptosis signals in the C75 and C100 groups. This was also supported by high dietary carbohydrates (24% to 36% of dietary starch) causing hyperglycemia and severe liver vacuolation in largemouth bass.
Apoptosis is essential for the development and maintenance of cellular homeostasis (Kumar et al., 2016). However, the over-activation of these signals induces irreparable apoptosis, which is detrimental to the liver health of fish (Li et al., 2020b; Lu et al., 2021). The C100 group significantly induced the liver transcriptional level of bcl2, one of the major markers of anti-apoptosis, which implied that feedback regulation was stimulated under adverse conditions to maintain cellular homeostasis. The caspase family plays a very important role in mediating apoptosis (Elmore, 2007). Many studies have suggested that there is a close relation between proteolysis and apoptotic pathways (Yin et al., 2019). Caspase-8 and caspase-9 are two essential proteases of the extrinsic and intrinsic apoptotic pathways, respectively (Wang and Lenardo, 2000). In the present study, the relative expressions of liver caspase-9 and caspase-3 were significantly upregulated in the C100 group. These together with TUNEL staining results suggested that apoptotic pathways might be induced in the liver of largemouth bass. A recent study also found that caspase-9 and caspase-3 were significantly upregulated when more than 50% fish meal (40% fish meal in the control diet) was replaced by a single-cell protein, Clostridium autoethanogenum, in the largemouth bass liver (Lu et al., 2021). The significantly increased liver transcriptional levels of caspase-9 and caspase-3 and significantly increased liver apoptosis rate (TUNEL and DAPI) in the C100 group implied that apoptosis induced by caspases might cause irreversible liver damage.
5. Conclusions
In the present study, based on the quadratic regression analysis between FBW, WGR, SGR, and dietary Chlorella meal, largemouth bass had the best growth when replacing 31.7% to 32.6% of fish meal with 15.03% to 15.43% dietary Chlorella meal. Dietary inclusion of Chlorella meal (11.85% to 47.45%) significantly improved the pigmentation of largemouth bass. Total replacement of fish meal (40%) by Chlorella meal (47.45%) caused suppressed growth, decreased feed utilization, and liver damage in largemouth bass. In future, further studies could be focused on the bioactive substances in Chlorella meal on the immune response of fish and the specific mechanism of Chlorella meal in modulating fish pigmentation. Furthermore, whether supplementation with amino acids (such as methionine) in Chlorella meal could alleviate the negative effects on fish under high replacement of fish meal also needs to be considered.
Author contributions
Longwei Xi: writing–original draft, methodology, software, data curation, formal analysis, investigation. Qisheng Lu: methodology, software, formal analysis, investigation. Yulong Liu: methodology, software, formal analysis, investigation. Jingzhi Su: methodology, software, formal analysis, investigation. Wen Chen: data curation, investigation. Yulong Gong: formal analysis, investigation. Dong Han: conceptualization, writing-review & editing, supervision, funding acquisition. Yunxia Yang: resources. Zhimin Zhang: resources. Junyan Jin: resources. Haokun Liu: resources. Xiaoming Zhu: conceptualization, funding acquisition, resources. Shouqi Xie: conceptualization, funding acquisition, resources.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgements
This research was funded by National Natural Science Foundation of China (U21A20266, 31972771, 31972805, 31672670), China Agriculture Research System of MOF and MARA (CARS-46), National Key R&D Program of China (2018YFD0900400), Fund Project in State Key Laboratory of Freshwater Ecology and Biotechnology (2019FBZ02, 2019FBZ05), and Hubei High-tech Innovation and Business Incubation Center (2019-02-055). The authors are grateful to Guanghan Nie for his technical help. We would like to thank Jun Men and Yanxia Zuo at the Analysis and Testing Center of Institute of Hydrobiology, Chinese Academy of Sciences for the composition of amino acids and fatty acids analysis. We would like to thank Prof. Xia Wan at Oil Crops Research Institute, Chinese Academy of Agricultural Sciences for assistance with the crude fiber analysis.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Ahmad M.T., Shariff M., Md Yusoff F., Goh Y.M., Banerjee S. Applications of microalga Chlorella vulgaris in aquaculture. Rev Aquacult. 2020;12:328–346. [Google Scholar]
- Alagawany M., Taha A.A., Noreldin A., El-Tarabily K.A., El-Hank M.E.A. Nutritional applications of species of Spirulina and Chlorella in farmed fish: a review. Aquaculture. 2021;542:736841. [Google Scholar]
- Aoac . 17th ed. USA Association of Official Analytical Chemists Inc.; Arlington, Virginia: 2000. Official method of analysis. [Google Scholar]
- Arriaga-Hernandez D., Hernández C., Martínez-Montaño E., Ibarra-Castro L., Lizárraga-Velázquez E., Leyva-López N., et al. Fish meal replacement by soybean products in aquaculture feeds for white snook, Centropomus viridis: effect on growth, diet digestibility, and digestive capacity. Aquaculture. 2021;530:735823. [Google Scholar]
- Breithaupt D.E., Weller P., Grashorn M.A. Quantification of carotenoids in chicken plasma after feeding free or esterified lutein and capsanthin using high-performance liquid chromatography and liquid chromatography-mass spectrometry analysis. Poultry. 2003;82(3):395–401. doi: 10.1093/ps/82.3.395. [DOI] [PubMed] [Google Scholar]
- Bureau D.P., Harris A.M., Bevan D.J., Simmons L.A., Azevedo P.A., Cho C.Y. Feather meals and meat and bone meals from different origins as protein sources in rainbow trout (Oncorhynchus mykiss) diets. Aquaculture. 2000;181:281–291. [Google Scholar]
- Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cao S.H., Li P.Y., Huang B.S., Zhang Z.D., Hao T.T., Wang C.Q., et al. Assessing feasibility of replacement of fishmeal with enzyme-treated feather meal in the diet of juvenile turbot (Scophthalmus maximus L.) Aquacult Nutr. 2020;26:1340–1352. [Google Scholar]
- Carneiro W.F., Castro T.F.D., Orlando T.M., Meurer F., Paula D.A.D., Virote B.D.R., et al. Replacing fish meal by Chlorella sp. meal: effects on zebrafish growth, reproductive performance, biochemical parameters and digestive enzymes. Aquaculture. 2020;528:735612. [Google Scholar]
- Canada P., Engrola S., Richard N., Lopes A.F., Pinto W., Valente L.M.P., et al. Dietary indispensable amino acids profile affects protein utilization and growth of Senegalese sole larvae. Fish Physiol Biochem. 2016;42:1493–1508. doi: 10.1007/s10695-016-0235-1. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Liu C.C. Optimization of lutein production with a two-stage mixotrophic cultivation system with Chlorella sorokiniana MB-1. Bioresour Technol. 2018;262:74–79. doi: 10.1016/j.biortech.2018.04.024. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Lu I.C., Nagarajan D., Chang C.H., Ng I.S., Lee D.J., et al. A highly efficient two-stage cultivation strategy for lutein production using heterotrophic culture of Chlorella sorokiniana MB-1-M12. Bioresour Technol. 2018;253:141–147. doi: 10.1016/j.biortech.2018.01.027. [DOI] [PubMed] [Google Scholar]
- Chen N.S., Ma J.Z., Zhou H.Y., Zhou J., Qiu X.J., Jin L.N., et al. Assessment of dietary methionine requirement in largemouth bass, Micropterus salmoides. J Fish China. 2010;34:1244–1253. [Google Scholar]
- Chen W.J., Luo L., Han D.X., Long F.P., Chi Q.L., Hu Q. Effect of dietary supplementation with Chlorella sorokiniana meal on the growth performance, antioxidant status, and immune response of rainbow trout (Oncorhynchus mykiss) J Appl Phycol. 2021;33:3113–3122. [Google Scholar]
- Dai M., Li S.L., Fu C.F., Qiu H.J., Chen N.S. The potential role of marine protein hydrolyzates in elevating nutritive values of diets for largemouth bass, Micropterus salmoides. Front Mar Sci. 2020;7:197. [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espe M., Lemme A., Petri A., El-Mowafi A. Can Atlantic salmon (Salmo salar) grow on diets devoid of fish meal? Aquaculture. 2006;255:255–262. [Google Scholar]
- Espe M., Lemme A., Petri A., El-Mowafi A. Assessment of lysine requirement for maximal protein accretion in Atlantic salmon using plant protein diets. Aquaculture. 2007;263:168–178. [Google Scholar]
- Ferreira A., Yeum K.J., Matsubara L.S., Matsubara B.B., Correa C.R., Pereira E.J., et al. Doxorubicin as an antioxidant: maintenance of myocardial levels of lycopene under doxorubicin treatment. Free Radical Bio Med. 2007;43(5):740–751. doi: 10.1016/j.freeradbiomed.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Fei S.Z., Chen Z., Xia Y., Liu H.K., Han D., Jin J.Y., et al. Effects of dietary arachidonic acid on reproduction performance, tissue fatty acid profile and gonadal steroidogenesis in female yellow catfish Pelteobagrus fulvidraco. Aquacult Nutr. 2020:1–12. 00. [Google Scholar]
- Francis G., Makkar H.P.D., Becker K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture. 2001;199:197–227. [Google Scholar]
- Gomez-Requeni P., Mingarro M., Calduch-Giner J.A., Medale F., Martin S.A.M., Houlihan D.F., et al. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata) Aquaculture. 2004;232:493–510. [Google Scholar]
- Gong M., Bassi A. Investigation of Chlorella vulgaris UTEX 265 cultivation under light and low temperature stressed conditions for lutein production in flasks and the Coiled Tree Photo-Bioreactor (CTPBR) Appl Biochem Biotechnol. 2017;183:652–671. doi: 10.1007/s12010-017-2537-x. [DOI] [PubMed] [Google Scholar]
- Gong M.Y., Bassi A. Carotenoids from microalgae: a review of recent developments. Biotechnol Adv. 2016;34:1396–1412. doi: 10.1016/j.biotechadv.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Gouveia L., Gomes E., Empis J. Potential use of a microalga (Chlorella vulgaris) in the pigmentation of rainbow trout (Oncorhynchus mykiss) muscle. Leben Unter Fors. 1996;202:75–79. [Google Scholar]
- Gouveia L., Gomes E., Empis J. Use of Chlorella vulgaris in rainbow trout, Onchorynchus mykiss, diets to enhance muscle pigmentation. J Appl Aquacult. 1997;7:61–70. [Google Scholar]
- Gouveia L., Choubert G., Gomes E., Rema P., Empis J. Use of Chlorella vulgaris as a carotenoid source for rainbow trout: effect of dietary lipid content on pigmentation, digestibility and retention in the muscle tissue. Aquacult Int. 1998;6:269–279. [Google Scholar]
- Gouveia L., Choubert G., Pereira N., Santinha J., Empis J., Gomes E. Pigmentation of gilthead seabream, Sparus aurata (L. 1875), using Chlorella vulgaris (Chlorophyta, Volvocales) microalga. Aquacult Res. 2002;33:987–993. [Google Scholar]
- Guroy B., Sahin I., Mantolu S., Kayali S. Spirulina as a natural carotenoid source on growth, pigmentation and reproductive performance of yellow tail cichlid Pseudotropheus acei. Aquacult Int. 2012;20(5):869–878. [Google Scholar]
- Hansen J.O., Hofossaeter M., Sahlmann C., Anestad R., Reveco-Urzua F.E., Press C.M., et al. Effect of Candida utilis on growth and intestinal health of Atlantic salmon (Salmo salar) parr. Aquaculture. 2019;511:734239. [Google Scholar]
- Huang F., Wang L., Zhang C.X., Song K. Replacement of fishmeal with soybean meal and mineral supplements in diets of Litopenaeus vannamei reared in low-salinity water. Aquaculture. 2017;473:172–180. [Google Scholar]
- Jannathulla R., Rajaram V., Kalanjiam R., Ambasankar K., Muralidhar M., Dayal J.S. Fishmeal availability in the scenarios of climate change: inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquacult Res. 2019;50:3493–3506. [Google Scholar]
- Jiang Y., Zhao P.F., Lin S.M., Tang R.J., Chen Y.J., Luo L. Partial substitution of soybean meal with fermented soybean residue in diets for juvenile largemouth bass, Micropterus salmoides. Aquacult Nutr. 2018;24:1213–1222. [Google Scholar]
- Jones C., Nel A., Adesola A., Shipton T., Kaiser H. The effect of fishmeal replacement with terrestrial protein sources on growth, body condition and intestinal microbiota of juvenile dusky kob Argyrosomus japonicus. Afr J Mar Sci. 2021;43:45–52. [Google Scholar]
- Ju Z.Y., Deng D.F., Dominy W. A defatted microalgae (Haematococcus pluvialis) meal as a protein ingredient to partially replace fishmeal in diets of Pacific white shrimp (Litopenaeus vannamei, Boone, 1931) Aquaculture. 2012;354:50–55. [Google Scholar]
- Kilberg M.S., Pan Y.X., Chen H., Leung-Pineda V. Nutritional control of gene expression: how mammalian cells respond to amino acid limitation. Annu Rev Nutr. 2005;25:59–85. doi: 10.1146/annurev.nutr.24.012003.132145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.M., Bai S.C.C., Koo J.W., Wang X.J., Kim S.K. Effects of dietary Chlorella ellipsoidea supplementation on growth, blood characteristics, and whole-body composition in juvenile Japanese flounder Paralichthys olivaceus. J World Aquacult Soc. 2002;33:425–431. [Google Scholar]
- Kimler V.A., Taylor J.D. Morphological studies on the mechanisms of pigmentary organelle transport in fish xanthophores and melanophores. Microsc Res Tech. 2002;58:470–480. doi: 10.1002/jemt.10165. [DOI] [PubMed] [Google Scholar]
- Kotrbacek V., Doubek J., Doucha J. The chlorococcalean alga Chlorella in animal nutrition: a review. J Appl Phycol. 2015;27:2173–2180. [Google Scholar]
- Kousoulaki K., Mørkøre T., Nengas I., Berge R.K., Sweetman J. Microalgae and organic minerals enhance lipid retention efficiency and fillet quality in Atlantic salmon (Salmo salar L.) Aquaculture. 2016;451:47–57. [Google Scholar]
- Kumar S., Stokes J., Singh U.P., Gunn K.S., Acharya A., Manne U., et al. Targeting Hsp70: a possible therapy for cancer. Cancer Lett. 2016;374:156–166. doi: 10.1016/j.canlet.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liland N.S., Araujo P., Xu X.X., Lock E.J., Radhakrishnan G., Prabhu A.J.P., et al. A meta-analysis on the nutritional value of insects in aquafeeds. J Insects Food Feed. 2021;7:743–759. [Google Scholar]
- Li X., Wei X., Guo X., Mi S.C., Hua X.M., Li N.Y., et al. Enhanced growth performance, muscle quality and liver health of largemouth bass (Micropterus salmoides) were related to dietary small peptides supplementation. Aquacult Nutr. 2020;26:2169–2177. [Google Scholar]
- Li H.Y., Xu W.J., Wu L.Y., Dong B., Jin J.Y., Han D., et al. Distinct dietary cadmium toxic effects and defense strategies in two strains of gibel carp (Carassius gibelio) revealed by a comprehensive perspective. Chemosphere. 2020;261:127597. doi: 10.1016/j.chemosphere.2020.127597. [DOI] [PubMed] [Google Scholar]
- Li M.H.H., Robinson E.H., Oberle D.F., Zimba P.V. Effects of various dietary carotenoid pigments on fillet appearance and pigment absorption in channel catfish, Ictalurus punctatus. J World Aquacult Soc. 2007;38:557–563. [Google Scholar]
- Li S.L., Dai M., Qiu H.J., Chen N.S. Effects of fishmeal replacement with composite mixture of shrimp hydrolysate and plant proteins on growth performance, feed utilization, and target of rapamycin pathway in largemouth bass. Micropterus salmoides. Aquaculture. 2021;533:736185. [Google Scholar]
- Li X.Y., Zheng S.X., Cheng K.M., Ma X.K., Wu G.Y. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part II: effects of supplementation with methionine or taurine on growth, feed utilization, and health. Amino Acids. 2021;53:49–62. doi: 10.1007/s00726-020-02922-4. [DOI] [PubMed] [Google Scholar]
- Liu C., Liu H.K., Xu W.J., Han D., Xie S.Q., Jin J.Y., et al. Effects of dietary Arthrospira platensis supplementation on the growth, pigmentation, and antioxidation in yellow catfish (Pelteobagrus fulvidraco) Aquaculture. 2019;510:267–275. [Google Scholar]
- Liu C., Zhou J.C., Liu H.K., Han D., Jin J.Y., Yang Y.X., et al. Apparent digestibility of nutrients in four animal protein ingredients with different processing technology for yellow catfish (Pelteobagrus fulvidraco) Acta Hydrobiol Sin. 2020;44:6. [Google Scholar]
- Liu H.K., Zhu X.M., Yang Y.X., Han D., Jin J.Y., Xie S.Q. Effect of substitution of dietary fishmeal by soya bean meal on different sizes of gibel carp (carassius auratus gibelio): nutrient digestibility, growth performance, body composition and morphometry. Aquacult Nutr. 2016;22:142–157. [Google Scholar]
- Liu J., Chen F. Biology and industrial applications of Chlorella: advances and prospects. Microalgae Biotechnology. 2016;153:1–35. doi: 10.1007/10_2014_286. [DOI] [PubMed] [Google Scholar]
- Luo L., Xue M., Wu X., Cai X., Cao H., Liang Y. Partial or total replacement of fishmeal by solvent-extracted cottonseed meal in diets for juvenile rainbow trout (Oncorhynchus mykiss) Aquacult Nutr. 2010;12:418–424. [Google Scholar]
- Luo Z., Ye H.M., Gao Y., Ling S.C., Wei C.C., Zhu X.M. Chlorella additive increased growth performance, improved appetite and immune response of juvenile crucian carp Carassius auratus. Aquacult Res. 2018;49:3329–3337. [Google Scholar]
- Luthada-Raswiswi R., Mukaratirwa S., O'Brien G. Animal protein sources as a substitute for fishmeal in aquaculture diets: a systematic review and meta-analysis. Appl Sci-Basel. 2021;11:3854. [Google Scholar]
- Lu Q.S., Xi Longwei, Liu Yulong, Gong Yulong, Su Jingzhi, Dong Han, et al. Effects of dietary inclusion of Clostridium autoethanogenum protein on the growth performance and liver health of largemouth bass (Micropterus salmoides) Front Mar Sci. 2021;8:764964. [Google Scholar]
- Man Y.B., Zhang F., Ma K.L., Mo W.Y., Kwan H.S., Chow K.L., et al. Growth and intestinal microbiota of Sabah giant grouper reared on food waste-based pellets supplemented with spirulina as a growth promoter and alternative protein source. Aquacult Rep. 2020;18:100553. [Google Scholar]
- Meilisza N., Jusadi D., Zairin M., Artika I.M., Utomo N.B.P., Kadarini T., et al. Digestibility, growth and pigmentation of astaxanthin, canthaxanthin or lutein diets in Lake Kurumoi rainbowfish, Melanotaenia parva (Allen) cultured species. Aquacult Res. 2017;48:5517–5525. [Google Scholar]
- Oliveira T.S., Khan K.U., Boaratti A.Z., Rodrigues A.T., Reis M.P., Sakomura N.K., et al. Evaluation of the optimum dietary essential amino acid pattern for adult pacu (Piaractus mesopotamicus) Aquaculture. 2021;540:736686. [Google Scholar]
- Pauli H. Proposed extension of CIE recommendation on “Uniform color spaces, color difference equations, and metric color terms”. J Opt Soc Am A. 1976;66:866–867. [Google Scholar]
- Psofakis P., Karapanagiotidis I.T., Malandrakis E.E., Golomazou E., Exadactylos A., Mente E. Effect of fishmeal replacement by hydrolyzed feather meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and growth-related gene expression of gilthead seabream (Sparus aurata) Aquaculture. 2020;521:735006. [Google Scholar]
- Pulcini D., Capoccioni F., Franceschini S., Martinoli M., Tibaldi E. Skin pigmentation in gilthead seabream (Sparus aurata L.) fed conventional and novel protein sources in diets deprived of fish meal. Animals. 2020;10:21–38. doi: 10.3390/ani10112138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan S., Bhavan P.S., Seenivasan C., Shanthi R., Muralisankar T. Replacement of fishmeal with Spirulina platensis, Chlorella vulgaris and Azolla pinnata on non-enzymatic and enzymatic antioxidant activities of Macrobrachium rosenbergii. J. Basic Appl. Zool. 2014;67:25–33. [Google Scholar]
- Rahimnejad S., Lee S.M., Park H.G., Choi J. Effects of dietary inclusion of Chlorella vulgaris on growth, blood biochemical parameters, and antioxidant enzyme activity in olive flounder, Paralichthys olivaceus. J World Aquacult Soc. 2017:103–112. [Google Scholar]
- Raji A.A., Jimoh W.A., Abu Bakar N.H., Taufek C.H.M., Muin H., Alisa Z., et al. Dietary use of Spirulina (Arthrospira) and Chlorella instead of fish meal on growth and digestibility of nutrients, amino acids and fatty acids by African catfish. J Appl Phycol. 2020;32:1763–1770. [Google Scholar]
- Roohani A.M., Kenari A.A., Kapoorchali M.F., Borani M.S., Zorriehzahra M.J., Smiley A.H., et al. Effect of spirulina Spirulina platensis as a complementary ingredient to reduce dietary fish meal on the growth performance, whole-body composition, fatty acid and amino acid profiles, and pigmentation of Caspian brown trout (Salmo trutta caspius) juveniles. Aquacult Nutr. 2019;25:633–645. [Google Scholar]
- Safavi S.V., Kenar A.A., Tabarsa M., Esmaeili M. Effect of sulfated polysaccharides extracted from marine macroalgae (Ulva intestinalis and Gracilariopsis persica) on growth performance, fatty acid profile, and immune response of rainbow trout (Oncorhynchus mykiss) J Appl Phycol. 2019;31:4021–4035. [Google Scholar]
- Sergejevova M., Masojidek J. Chlorella biomass as feed supplement for freshwater fish: sterlet, Acipenser ruthenus. Aquacult Res. 2012;44:157–159. [Google Scholar]
- Shah M.R., Lutzu G.A., Alam A., Sarker P., Chowdhury M.A.K., Parsaeimehr A., et al. Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol. 2018;30:197–213. [Google Scholar]
- Shi X., Luo Z., Chen G.H., Chen F., Zhang L.H., Zhu X.M., et al. Replacement of fishmeal by a mixture of soybean meal and chlorella meal in practical diets for juvenile crucian carp, Carassius auratus. J World Aquacult Soc. 2017;48:770–781. [Google Scholar]
- Shi X., Luo Z., Chen F., Wei C.C., Wu K., Zhu X.M., Liu X. Effect of fish meal replacement by Chlorella meal with dietary cellulase addition on growth performance, digestive enzymatic activities, histology and myogenic genes' expression for crucian carp Carassius auratus. Aquacult Res. 2017;48:3244–3256. [Google Scholar]
- Song F., Xu D.D., Mai K.S., Zhou H.H., Xu W., He G. Comparative study on the cellular and systemic nutrient sensing and intermediary metabolism after partial replacement of fishmeal by meat and bone meal in the diet of turbot (Scophthalmus maximus L.) PLoS One. 2016;11 doi: 10.1371/journal.pone.0165708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg O.K., Holen E., Piemontese L., Liland N.S., Lock E.J., Espe M., et al. Effect of dietary replacement of fish meal with insect meal on in vitro bacterial and viral induced gene response in Atlantic salmon (Salmo salar) head kidney leukocytes. Fish Shellfish Immunol. 2019;91:223–232. doi: 10.1016/j.fsi.2019.05.042. [DOI] [PubMed] [Google Scholar]
- Storebakken T., Sorensen M., Bjerkeng B., Hiu S. Utilization of astaxanthin from red yeast, Xanthophyllomyces dendrorhous, in rainbow trout, Oncorhynchus mykiss: effects of enzymatic cell wall disruption and feed extrusion temperature. Aquaculture. 2004;236:391–403. [Google Scholar]
- Terova G., Gini E., Gasco L., Moroni F., Antonini M., Rimoldi S. Effects of full replacement of dietary fishmeal with insect meal from Tenebrio molitor on rainbow trout gut and skin microbiota. J Anim Sci Biotechnol. 2021;12:30. doi: 10.1186/s40104-021-00551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuling E., Schrama J.W., Gruppen H., Wierenga P.A. Effect of cell wall characteristics on algae nutrient digestibility in Nile tilapia (Oreochromis niloticus) and African catfish (Clarus gariepinus) Aquaculture. 2017;479:490–500. [Google Scholar]
- Valente L.M.P., Custodio M., Batista S., Fernandes H., Kiron N. Defatted microalgae (Nannochloropsis sp.) from biorefinery as a potential feed protein source to replace fishmeal in European sea bass diets. Fish Physiol Biochem. 2019;45:1067–1081. doi: 10.1007/s10695-019-00621-w. [DOI] [PubMed] [Google Scholar]
- Wang A., Li S.L., Liu Y.J., Han Z.H., Chen N.S. The inclusion of fenugreek seed extract aggravated hepatic glycogen accumulation through reducing the expression of genes involved in insulin pathway and glycolysis in largemouth bass, Micropterus salmoides. Aquaculture. 2020;528:735567. [Google Scholar]
- Wang J., Lenardo M.J. Roles of caspases in apoptosis, development, and cytokine maturation revealed by homozygous gene deficiencies. J Cell Sci. 2000;113:753–757. doi: 10.1242/jcs.113.5.753. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Liao W.L., Takeuchi T., Yamamoto H. Effect of dietary Spirulina supplementation on growth performance and flesh lipids of cultured striped jack. J Tokyo Univ Fish. 1990;77:231–239. [Google Scholar]
- Wei Y.T., Shen H.H., Xu W.Q., Pan Y., Chen J., Zhang W.B., et al. Replacement of dietary fishmeal by Antarctic krill meal on growth performance, intestinal morphology, body composition and organoleptic quality of large yellow croaker Larimichthys crocea. Aquaculture. 2019;512:73. [Google Scholar]
- Wu Y.B., Ma H.J., Wang X.J., Ren X. Taurine supplementation increases the potential of fishmeal replacement by soybean meal in diets for largemouth bass Micropterus salmoides. Aquacult Nutr. 2021;27 691-669. [Google Scholar]
- Xu J., Feng L., Jiang W., Wu P., Liu Y., Jiang J., et al. Different dietary protein levels affect flesh quality, fatty acids and alter gene expression of Nrf2-mediated antioxidant enzymesin the muscle of grass carp (Ctenopharyngodon idella) Aquaculture. 2018;493:272–282. [Google Scholar]
- Yang P.X., Li X.Q., Song B.W., He M., Wu C.Y., Leng X.J. The potential of Clostridium autoethanogenum, a new single cell protein, in substituting fish meal in the diet of largemouth bass (Micropterus salmoides): growth, feed utilization and intestinal histology. Aquaculture and Fisheries. 2021 doi: 10.1016/j.aaf.2021.03.003. [in press] [DOI] [Google Scholar]
- Yin P., Xie S.W., Huo Y.J., Guo T.Y., Fang H.H., Zhang Y.M., et al. Effects of dietary oxidized fish oil on growth performance, antioxidant defense system, apoptosis and mitochondrial function of juvenile largemouth bass (Micropterus salmoides) Aquaculture. 2019;500:347–358. [Google Scholar]
- Zhang W., Liu K., Tan B.P., Liu H.Y., Dong X.H., Yang Q.H., et al. Transcriptome, enzyme activity and histopathology analysis reveal the effects of dietary carbohydrate on glycometabolism in juvenile largemouth bass, Micropterus salmoides. Aquaculture. 2019;504:39–51. [Google Scholar]
- Zhang X.D., Zhang H.Z., Zhang J.W., Lin B.B., Chen L.S., Wang Q.M., et al. Assessment of rapeseed meal as fish meal alternative in diets for juvenile Asian red-tailed catfish (Hemibagrus wyckioides) Aquacult Rep. 2020;18:100497. [Google Scholar]
- Zhang Y.M., Xie S.W., Guo T.Y., Liu Z.L., Fang H.H., Zheng L., et al. High dietary starch inclusion impairs growth and antioxidant status, and alters liver organization and intestinal microbiota in largemouth bass Micropterus salmoides. Aquacult Nutr. 2020;26:1806–1821. [Google Scholar]
- Zhang Y.M., Xie S.W., Wei H.L., Zheng L., Liu Z.L., Fang H.H., et al. High dietary starch impaired growth performance, liver histology and hepatic glucose metabolism of juvenile largemouth bass, Micropterus salmoides. Aquacult Nutr. 2020;26:1083–1095. [Google Scholar]
- Zheng K., Liang M., Yao H., Wang J., Chang Q. Effect of dietary fish protein hydrolysate on growth, feed utilization and IGF-I levels of Japanese flounder (Paralichthys olivaceus) Aquacult Nutr. 2012;18:297–303. [Google Scholar]
- Zhou Q.C., Mai K.S., Liu Y.J., Tan B.P. Advances in animal and plant protein sources in place of fish meal. J Fish China. 2005;29:404–410. [Google Scholar]
- Zhu Y.F., Mai K.S. A review of xarothenoid of pigmentation additives in fish feeds. Acta Hydrobiol Sin. 2003;27:196–200. [Google Scholar]