Highlights
-
•
All the studies we included are RCTs.
-
•
We conducted a meta-analysis on the effects of n-3 fatty acids as nutritional supplements on postoperative inflammation, immunity, nutrition, incidence of infectious related complications and length of hospital stay in patients with gastrointestinal cancer. Our results can provide a theoretical basis for the clinical application of nutritional supplements in patients with gastrointestinal cancer;
-
•
The risk of bias of an individual study was assessed using the Cochrane Risk of Bias Tool, built into the Review Manager software (version 5.3), using the following items:random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias sources.
Keywords: Omega-3 fatty acids, Gastrointestinal cancer, Immune function, Inflammatory response
Abstract
Background
Surgical resection remains the primary treatment for gastrointestinal (GI) cancer, omega-3 polyunsaturated fatty acids (n-3 PUFAs) have been reported to improve the prognosis of patients undergoing gastrointestinal tumor surgery. This meta-analysis aims to explore the efficacy of n-3 PUFAs on GI cancer patients undergoing surgery.
Methods
A systematic search of PubMed, Cochrane Library databases, EMBASE (until December 2021) was conducted. PRISMA checklist was followed. The data were analyzed by RevMan v5.3.0.
Results
A total of ten RCTs articles including 663 patients were studied. The analysis demonstrated that the n-3 PUFAs group significantly reduced levels of interleukin-6 (IL-6) (P = 0.001), C-reactive protein (CRP) (P < 0.00001), tumor necrosis factor-ɑ (TNF-α) (P = 0.0003) compared with the control group. and higher levels of CD4+T cells (P = 0.03), CD8+T cells (P = 0.02) and CD4+/CD8+ratio (P = 0.03) compared with the control group. but there was no significant difference in infection complications rate (P = 0.50) and the level of prealbumin (P = 0.80), albumin (P = 0.21), retinol-binding protein(P = 0.80) between the two groups. In addition, the n-3 PUFAs group significantly reduced the length of hospital stay (P = 0.007).
Conclusion
Our meta-analysis shows that n-3 PUFAs can effectively improve the immune function of patients undergoing gastrointestinal cancer surgery, reduce inflammatory response and reduce the length of hospital stay, But it has no significant impact on the incidence of infectious-related complications and the level of nutrient protein.
1. Introduction
GI cancers are the most common group of malignancies and it has become the leading cause of cancer deaths worldwide (Qu et al., 2016, Song et al., 2016). Surgery is the primary treatment for patients with early-stage GI cancer. However, patients undergoing selective GI cancer.
surgery will face the risk of developing various postoperative complications due to negative impact factors, such as malnutrition, tumor-induced immune suppression, surgical stress, and inflammation, resulting in prolonged hospital-stay and increased costs, which brings huge chalenges to the rehabilitation of patients after GI cancer surgery (Horgan, 2014).
As an important essential fatty acid, n-3 PUFAs have received increasing attention from researchers.The research on n-3 PUFAs mainly focuses on the prevention and treatment of cardiovascular and cerebrovascular diseases, n-3 PUFAs plays an active role in nutritional support for cardiovascular and cerebrovascular diseases through anti-thrombosis and vasodilation mechanisms (Pisaniello, Psaltis, & King, 2021). It has been reported in the literature that n-3 PUFAs has a positive effect on improving the nutritional status of tumor patients, reducing inflammation, and enhancing immune function (Cheng, Zhang, Ning, & Huo, 2021). However, other studies have shown that n-3 PUFAs cannot improve the nutritional status and clinical outcomes of cancer patients (Giger-Pabst, Lange, & Maurer, 2013), the impact of n-3 PUFAs on disease inflammation and nutritional status remains controversial. Considering the results and conclusions in these studies were not completely consistent because of limited sample size, different study designs, and potential bias, we conducted a meta-analysis of all relevant randomized controlled trials (RCTs), focusing on the effects of n-3 PUFAs on the nutritional status, inflammation and immune function of patients after gastrointestinal tumor surgery, provide a theoretical basis for the clinical standardized application of n-3 PUFAs in GI cancer patients.
2. Materials and methods
2.1. Search strategy
We searched the PubMed, EMBASE, and Cochrane library databases for articles published before December 2021 to collect randomized controlled trials of GI cancer surgery patients receiving n-3 PUFAs treatment and any control intervention. using various combinations of keywords including fatty acids, omega-3,gastrointestinal neoplasms and operation. The study was limited to published articles with no restrictions on language. The references of related articles were also searched. Two authors independently performed the study selection (LS and ZY).Full-text review was required where titles and abstracts were insufficient to determine if the study met the inclusion criteria. When there was any controversy, articles would be sent to the third author (TH) for assessment. If necessary, authors would be contacted to provide more accurate data from their researches.
2.2. Inclusion and exclusion criteria
The studies were included in our analysis if they met the following criteria: (1) research design: randomized controlled trials; (2) participants: the patients with gastrointestinal cancer; (3) intervention measures: n-3 fatty acid supplementation during perioperative period; (4) outcomes: postoperative infectious complications, length of hospital stay, immune indicators: CD4(%), CD8(%), CD4/CD8; Inflammation indicators: Interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF-α), C-reactive protein (CRP); nutritional indicators: Prealbumin (PAB), Albumin (ALB),Retinol-binding protein (RBP).
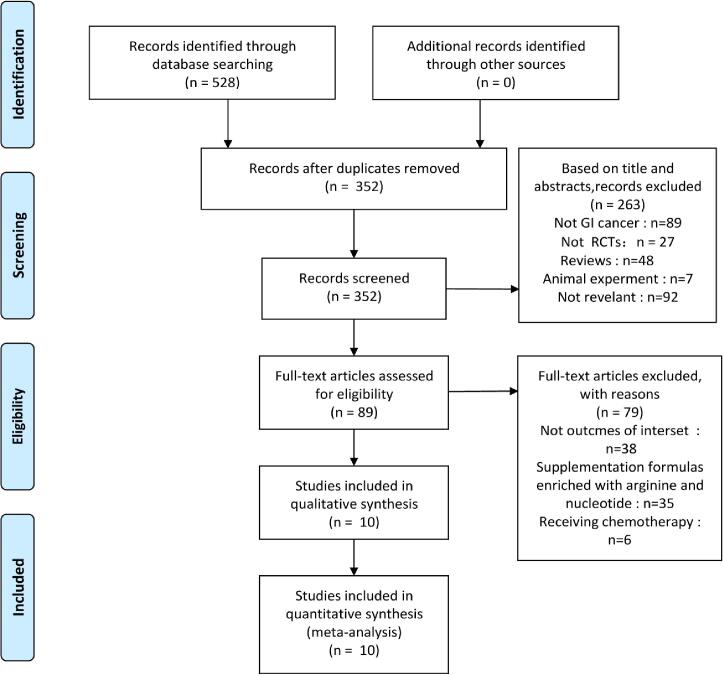
Exclusion criteria: (1) animal studies, in vitro studies, review, case report, conference summary and other non-clinical research literature; (2) incorrect or incomplete data could not be extracted; (3) The intervention group contains other immunonutritions such as glutamine or arginine; Two authors (LS and ZY) independently reviewed the literature and extracted all potentially eligible studies; the inconsistencies were discussed until a consensus was reached (Fig. 1).
Fig. 1.
The process of study selection according to Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guidelines. Our search strategy found 528 articles, 176 of which were deleted as duplicate documents retrieved from two or more databases. After carefully examining their abstracts and titles, we excluded 263 articles. Among the remaining 89 articles, 79 articles were excluded due to lack of valid data. Finally, 10 RCTs articles involving a total of 663 subjects (330 in the n-3 PUFAs group and 333 in the control group) were selected for this meta-analysis.
2.3. Quality assessment
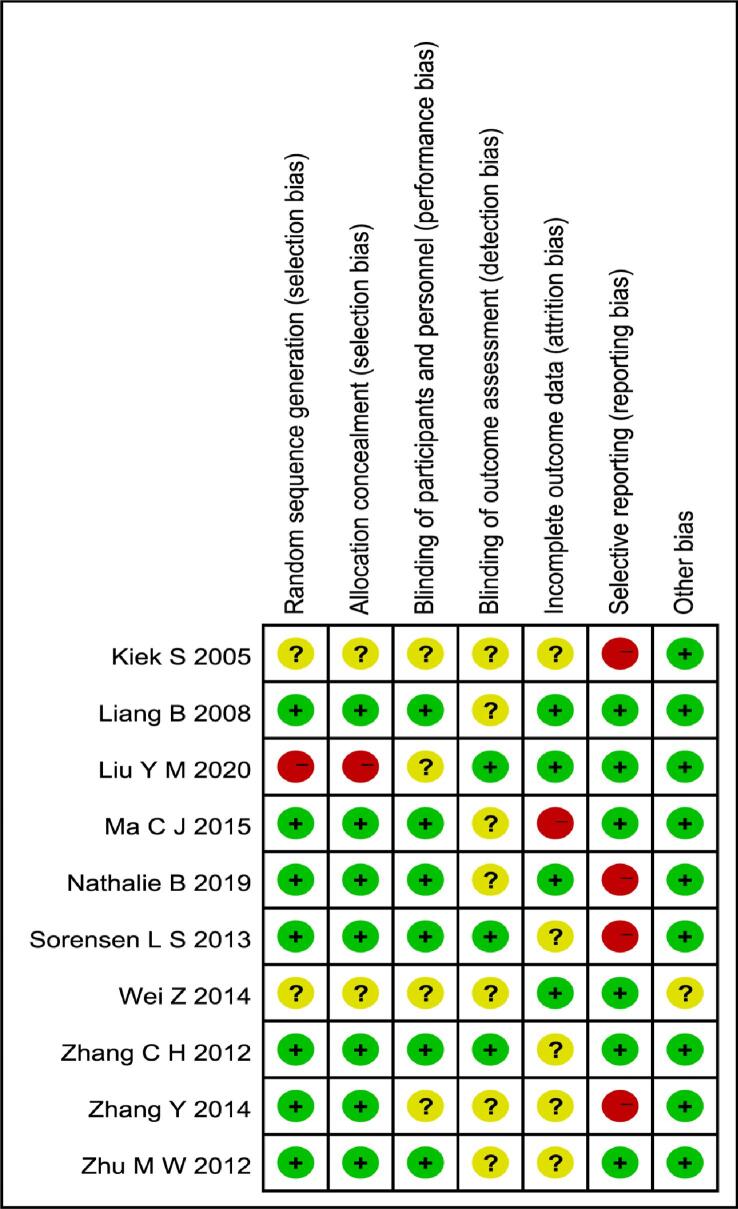
Quality assessment was performed by using the Cochrane bias-risk tool, which includes six domains: selection bias, performance bias, detection bias, attrition bias, reporting bias and other bias.The risk of each included study was rated as“high bias risk”,“unclear biasrisk”or“low bias risk”according to the information extracted. The graphical results of methodological quality are shown in Fig. 2.
Fig. 2.
Summary of the quality assessment of the included studies. All of the selected studies were RCTs. Among the included studies, four studies (Liang B, Zhu M W, Ma C J, Nathalie B) generated the random sequence by computer and two studies(Sorensen L S, Wei Z) generated from sealed opaque envelopes containing randomized numbers, one study (Zhang C H) generated from random number method and one study (Liu Y M) carried out the randomisation by medical record number, seven studies (Liang B, Liu Y M, Ma C J, Nathalie B, Sorensen L S, Zhang C H, Zhu M W) described their blind-ing method. Four studies (Liang B, Liu Y M, Nathalie B, Wei Z) had no follow-up bias, five studies(Kiek S,Sorensen L, Zhang C H, Zhang Y, Zhu M W) might have follow-up bias, and one study (Ma C J) had follow-up bias. Six studies (Liang B, Liu Y M, Ma C J, Wei Z, Zhang C H, Zhu M W) had no reporting bias and Four studies had reporting bias(Kiek S, Nathalie B, Sorensen L S, Zhang Y). Nine studies with no other bias and one study(Wei Z) may have other biases.
2.4. Data extraction
The following data were extracted independently by 2 authors (LS and ZY) from the included studies: name of first author, publication year, cancer types, number of participants, age and gender of the participant, body mass index (BMI), intervention measures, intervention time, and reported outcomes. Any disagreements in the results of data extraction were resolved through discussion with a third author (TH). If useful data were presented indirectly by figures and graphs or through different metrics, they were translated into correlative patterns by using get-data software or relevant formulae. If mean values or standard deviation (SD) for analysis were unavailable, they were calculated from medians and ranges using relevant formulae.
2.5. Statistical analysis
The analysis of comparable data was conducted by Review Manager 5.3 (Cochrane Collaboration). We assessed the continuous outcomes using mean difference (MD) and dichotomous outcomes using the odds ratio (OR).We estimated the comparable data using 95% confidence interval (CI). The I2 test would be accounted to evaluate statistical heterogeneity. A random-effects model would be adopted for the result if the I2 > 50%; otherwise, a fixed-effects model was chosen. p < 0.05would be considered statistically significant.
3. Results
3.1. Search results and study characteristics
The process of study selection according to Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guidelines is shown in Fig. 1,
Our search strategy found 528 articles, 176 of which were deleted as duplicate documents retrieved from two or more databases. After checking their abstracts and titles carefully, we excluded 263 articles. Among the remaining 89 articles, 79 articles were excluded due to lack of valid data. Finally, 10 RCTs (Bakker et al., 2020, Cheng-Jen and Jin-Ming, 2015, Kłek et al., 2005, Liang et al., 2008, Liu et al., 2020, Sorensen et al., 2014, Wei et al., 2014, Zhang et al., 2012, Zhang et al., 2014, Zhu et al., 2012) articles involving a total of 663 subjects (330 in the n-3 PUFAs group and 333 in the control group) were selected for this meta-analysis. The baseline characteristics of 10 studies are summarized in Table 1.
Table 1.
Characteristics of included randomized trials.
|
Study |
Cancer types |
Sample size --30480762-304807620 |
Mean age, years (SD or range) 520707620 |
Gender (Male/Female)--24130762-241307620 |
BMI--43180762-431807620 |
Treatment protocol--33655762-336557620 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp | Con | Exp | Con | Exp | Con | Exp | Con | Exp | Con | ||
| Kiek S |
GC | 30 | 30 | NA | NA | NA | NA | NA | NA | Standard PN + ω-3 PUFA after operation; (1-9d); |
Standard PN 9 days after operation;(1-9d); |
| Liang B | CRC | 20 | 21 | 55.80 ± 10.10 | 59.19 ± 10.61 | 10/10 | 15/6 | 23.38 ± 2.38 | 23.92 ± 2.84 | SO + FO after operation; (1-7d); | SO after operation; (1-7d) |
| Zhang C H | GIC | 32 | 32 | 56.6 ± 11.9 | 56.8 ± 12.6 | 20/12 | 24/8 | 23.1 ± 2.4 | 23.5 ± 2.6 | Lipoplus(MCT + LCT + ω-3 PUFA) after operation; (1-5d); | Lipofund (MCT + LCT) after operation; (1-5d); |
| Zhu M W |
CRC | 29 | 28 | 69.8 ± 10.5 | 70.8 ± 6.4 | 17/11 | 16/13 | 22.9 ± 3.1 | 23.2 ± 3.6 | 1.2 g/kg SO per day after operation; (1-7d); | 0.2 g/kg FO and 1.0 g/kg SO per day after operation; (1-7d); |
| Sorensen L S | CRC | 74 | 74 | 64 ± 11 | 71 ± 10 | 44/30 | 36/38 | 26 ± 5 | 26 ± 5 | ω-3PUFA enriched(ONS) (Supportan, 200 ml twice daily) providing 2 g EPA and 1 g DHA; (7 days before and 7 days after operation); |
Standard isocaloric and isonitrogenous ONS; (7 days before and 7 days after operation); |
| Zhang Y | GIC | 32 | 32 | 58.13 ± 11.32 | 56.40 ± 11.57 | 17/15 | 16/16 | 22.26 ± 2.27 | 23.88 ± 2.63 | LCT:MCT = 5:5 after operation; (1-5d); | LCT:MCT:ω-3PUFA = 5:4:1 after operation; (1-5d); |
| Wei Z | GC | 26 | 25 | 50.5(29–75) | 59(36–74) | 15/11 | 11/9 | 22.2(15.–28.1) | 22.5(17–29.7) | The fat emulsion used was omega-6 lipid (20% Intralipid) after operation; (≧6d); |
The omega-6 lipid content of TPN was partially replaced by ω-3PUFA(10%Omegave) up to 0.2 g/kg body weight daily after surgery; (≧6d); |
| Ma C J | GIC | 44 | 41 | 61.55 ± 9.78 | 62.85 ± 10.12 | 29/22 | 27/21 | 23.45 ± 3.44 | 23.91 ± 3.79 | ω-3PUFA enriched intravenous fat emulsion (IVFE) after operation; (1-7d); | lipid emulsion comprised of soybean oil and MCTs after operation; (1-7d); |
| Nathalie B | CRC | 18 | 23 | 65 (64–75) | 69 (63–74) | 13/5 | 15/8 | 26.9(25.1–27.8) | 25.7(23.5–29.4) | 2 intravenous ω-3PUFA infusions the night before and the morning after operation; |
2 intravenous saline control infusions the night before and the morning after operation; |
| Liu Y M | GC | 25 | 27 | 54.6 ± 10.3 | 56.1 ± 9.8 | 18/7 | 19/8 | NA | NA | Enteral nutrition preparations twice a day after operation, 500 ml each time, including 1.5 g ω-3PUFA;(1–3 months) | Routine enteral nutrition after surgery; (1–3 months) |
BMI,body mass index;CRC, colorectal cancer; GC, gastric cancer; GIC, gastrointestinal cancer; PUFA,polyunsaturated fatty acids;SO,soybean oil;FO, fish oil;DHA,docosahexaenoic acid;EPA,eicosapentaenoic acid; LCT,long-chain triglycerides;MCT,mediumchain triglycerides, Exp, experimental;Con, control; NA, no available.
3.2. Quality of the individual studies
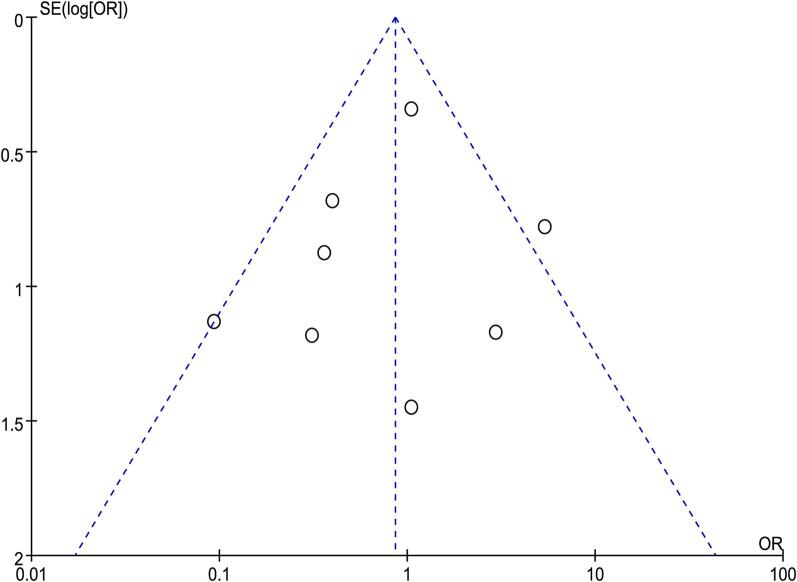
All of the selected studies were RCTs. Among all the literature studies, four generated the random sequence by computer and two generated from sealed opaque envelopes containing randomized numbers. One generated from random number method and one study carried out the randomisation by medical record number. Seven studies described their blind-ing method, The quality assessment is summarised in Fig. 2. Publication bias was assessed using a funnel plot regarding postoperative infectious complications, which contained the largest number of trials. The funnel plot of infectious complications was symmetrical based on visual inspection suggesting no evidence of publication bias Fig. 3.
Fig. 3.
Funnel plot of the studies included in our meta-analysis. Publication bias was assessed using a funnel plot regarding postoperative infectious complications, which contained the largest number of studies. The funnel plot of infectious complications was symmetrical based on visual inspection suggesting no evidence of publication bias.
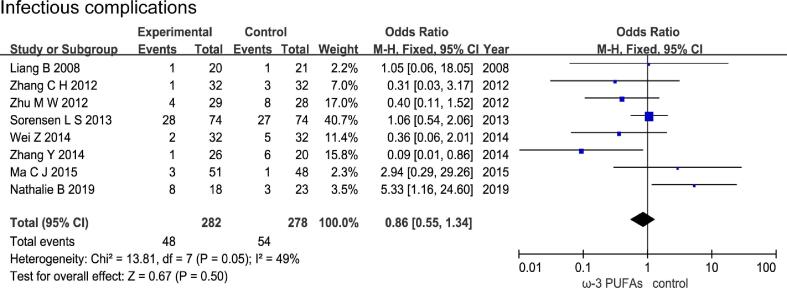
3.3. Effect of n-3 PUFAs on postoperative infectious complications
Eight RCTs with a total of 560 patients (282 in the n-3 PUFAs group and 278 in the control group) evaluated the incidence of infectious complications, The results of heterogeneity test were P = 0.05 and I2 = 49%. The forest plot indicated that there was no statistical significance in incidence of infectious complications between the n-3 PUFAs group and the control group (OR = 0.86, 95% CI 0.55–1.34, P = 0.50) (Fig. 4).
Fig. 4.
Effect of n-3 PUFAs on postoperative infectious complications Eight RCTs with a total of 560 patients evaluated the incidence of infectious complications. The forest plot indicated that there was no statistical significance in incidence of infectious complications between the n-3 PUFAs group and the control group.
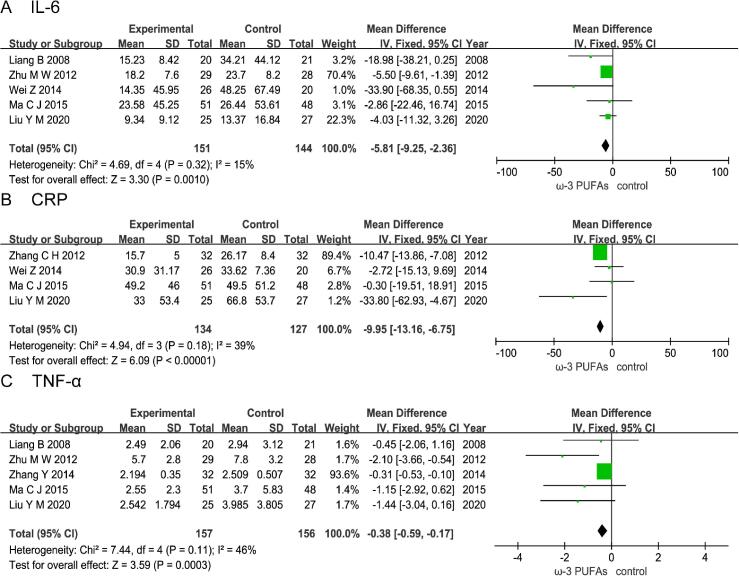
3.4. Effect of n-3 PUFAs on postoperative level of inflammatory factor
3.4.1. Il-6
Five RCTs enrolling 295 patients (151 in the n-3 PUFAs group and 144 in the control group) reported the level of serum IL-6. The results of heterogeneity test were P = 0.32 and I2 = 15%. The pooled results showed that the n-3 PUFAs group had a statistically lower than control group (MD = -5.81; 95%CI, −9.25 - −2.36; P = 0.0010) (Fig. 5A).
Fig. 5.
Effect of n-3 PUFAs on postoperative level of inflammatory factor Five RCTs enrolling 295 patients reported the level of serum IL-6. The pooled results showed that the n-3 PUFAs group was statistically lower than the control group. Four RCTs enrolling 261 patients reported the level of serum C-reactive protein (CRP). The pooled results showed that the n-3 PUFAs group had a statistically lower than control group. Five RCTs enrolling 313 patients reported the level of serum TNF-α. The pooled results showed that the n-3 PUFAs group had a statistically lower than control group.
3.4.2. Crp
Four RCTs enrolling 261 patients (134 in the n-3 PUFAs group and 127 in the control group) reported the level of serum C-reactive protein (CRP).The results of heterogeneity test were P = 0.18 and I2 = 39%. The pooled results showed that the n-3 PUFAs group had a statistically lower than control group (MD = -9.95; 95%CI, −13.16 - −6.75; P < 0.00001) (Fig. 5B).
3.4.3. Tnf-α
Five RCTs enrolling 313 patients (157 in the n-3 PUFAs group and 156 in the control group) reported the level of serum tumor necrosis factor-α (TNF-α).The results of heterogeneity test were P = 0.11 and I2 = 46%. The pooled results showed that the n-3 PUFAs group had a statistically lower than control group (MD = -0.38; 95%CI, −0.59 - −0.17; P = 0.0003) (Fig. 5C).
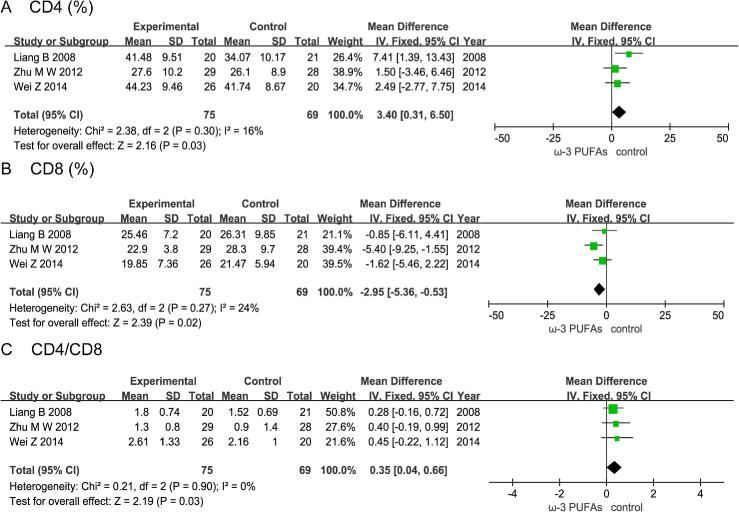
4. Effect of n-3 PUFAs on postoperative immune function
4.1. CD4+T cells
Three RCTs enrolling 144 patients (75 in the n-3 PUFAs group and 69 in the control group) reported the level of CD4+T cells.The results of heterogeneity test were P = 0.3 and I2 = 16%. The pooled results showed that the n-3 PUFAs group had a statistically higher than control group (MD = 3.40; 95%CI, 0.31–6.50; P = 0.03) (Fig. 6A).
Fig. 6.
Effect of n-3 PUFAs on postoperative immune function Three RCTs enrolling 144 patients reported the level of CD4+T cells. The pooled results showed that the n-3 PUFAs group had a statistically higher than control group. Three RCTs enrolling 144 patients reported the level of CD8+T cells. The pooled results showed that the n-3 PUFAs group had a statistically lower than control group. Three RCTs enrolling 144 patients reported the level of CD4+/CD8+T cells. The pooled results showed that the n-3 PUFAs group had a statistically higher than control group.
4.2. CD8+T cells
Three RCTs enrolling 144 patients (75 in the n-3 PUFAs group and 69 in the control group) reported the level of CD8+T cells.The results of heterogeneity test were P = 0.27 and I2 = 24%. The pooled results showed that the n-3 PUFAs group had a statistically lower than control group (MD = -2.95; 95%CI, −5.36 - −0.53; P = 0.02) (Fig. 6B).
4.3. CD4+/CD8+T cells
Three RCTs enrolling 144 patients (75 in the n-3 PUFAs group and 69 in the control group) reported the level of CD4+/CD8+T cells.The results of heterogeneity test were P = 0.90 and I2 = 0%. The pooled results showed that the n-3 PUFAs group had a statistically higher than control group (MD = 0.35; 95%CI, 0.04–0.66; P = 0.03) (Fig. 6C).
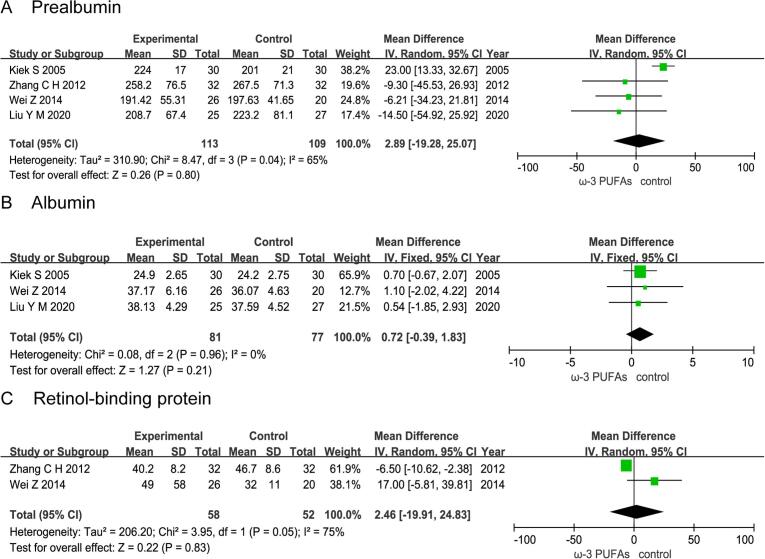
5. Effect of n-3 PUFAs on postoperative nutritional status
5.1. Prealbumin
Four RCTs enrolling 222 patients (113 in the n-3 PUFAs group and 109 in the control group) reported the level of prealbumin. The results of heterogeneity test were P = 0.04 and I2 = 65%. The forest plot indicated that there was no statistical significance in the level of prealbumin between the n-3 PUFAs group and the control group (MD = 2.89; 95%CI, −19.28–25.07; P = 0.80) (Fig. 7A).
Fig. 7.
Effect of n-3 PUFAs on postoperative nutritional status Four RCTs enrolling 222 patients reported the level of prealbumin. The forest plot indicated that there was no statistical significance in the level of prealbumin between the n-3 PUFAs group and the control group. Three RCTs enrolling 158 patients reported the level of albumin. The forest plot indicated that there was no statistical significance in the level of albumin between the n-3 PUFAs group and the control group. Two RCTs enrolling 110 patients reported the level of retinol-binding protein. The forest plot indicated that there was no statistical significance in the level of retinol-binding protein between the n-3 PUFAs group and the control group.
5.2. Albumin
Three RCTs enrolling 158 patients (81 in the n-3 PUFAs group and 77 in the control group) reported the level of albumin. The results of heterogeneity test were P = 0.96 and I2 = 0%. The forest plot indicated that there was no statistical significance in the level of albumin between the n-3 PUFAs group and the control group (MD = 0.72; 95%CI, −0.39–1.83; P = 0.21) (Fig. 7B).
5.3. Retinol-binding protein
Two RCTs enrolling 110 patients (58 in the n-3 PUFAs group and 52 in the control group) reported the level of retinol-binding protein. The results of heterogeneity test were P = 0.05 and I2 = 75%. The forest plot indicated that there was no statistical significance in the level of retinol-binding protein between the n-3 PUFAs group and the control group (MD = 2.46; 95%CI, −19.91–24.83; P = 0.83) (Fig. 7C).
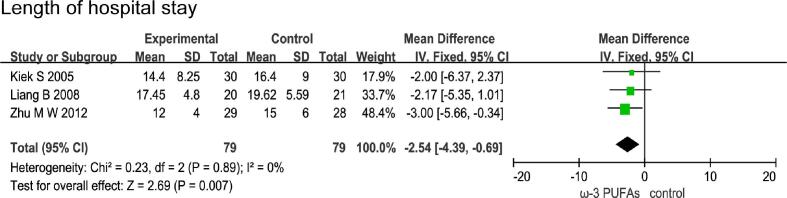
5.4. Effect of n-3 PUFAs on length of hospital stay
Three RCTs enrolling 158 patients (79 in the n-3 PUFAs group and 79 in the control group) reported the length of hospital stay. The results of heterogeneity test were P = 0.89 and I2 = 0%. The pooled results showed that the n-3 PUFAs group had a statistically lower than control group (MD = -2.54; 95%CI, −4.391 - −0.69; P = 0.007) (Fig. 8).
Fig. 8.
Effect of n-3 PUFAs on length of hospital stay Three RCTs enrolling 158 patients reported the length of hospital stay. The pooled results showed that the n-3 PUFAs group had a statistically lower than control group.
6. Discussion
n-3 PUFAs are essential fatty acids for the human body, which can not only oxidize and supply energy in the body, but also participate in changes in cell structure and metabolites, thereby affecting cell functions. Studies have shown that n-3 PUFAs play an important role in the host immune response and inflammatory response of gastrointestinal tumors, so compared with isocaloric nutrition, n-3 PUFAs are the best choice for postoperative treatment (Kew et al., 2004, Wallace et al., 2001, Yaqoob and Calder, 1995). Our meta-analysis evaluated 10 RCTs to assess the impact of n-3 PUFA nutritional support on postoperative infectious complications, levels of inflammatory cytokines, immune cells and nutrient proteins, and length of stay in patients with gastrointestinal cancer. The main result of this study is that n-3 PUFAs nutritional support effectively reduces the levels of serum IL-6, TNF-α, CRP, and CD8 + T lymphocytes and improved the number of CD4 + T lymphocytes and the ratio of CD4+/CD8 + in patients undergoing gastrointestinal cancer surgery. n-3 PUFAs reduce the patient's hospital stay by enhancing the body's immune function and reducing the body's inflammatory response.
Due to poor appetite, digestion and absorption disorders, tumor self-depletion and the production of immunosuppressive factors, most gastrointestinal tumor microenvironments are in an immunosuppressive state, and radical surgical resection further reduces the body's immune function. n-3 PUFAs improve the immune response by increasing the total number of peripheral blood lymphocytes (including T lymphocytes and CD4 + T cells) (Marano, Porfidia, & Pezzella, 2013). Different subsets of mature T cells carry out the functions of cell-mediated immunity, including killing virally infected cells and tumor cells (CD8 + Tcells) and providing help for and regulating components of the immune system (CD4 + T cells). Turbitt showed that n-3 PUFAs may induce an increase in the production of IL-2 and IFN-g in T cells, thereby driving the Th1 response and enhancing the anti-tumor immune function (Turbitt, Black, & Collins, 2015). By regulating Th1/Th2 differentiation and Th17 response, n-3 PUFAs play an important role in alleviating stress-induced immunosuppression after surgical resection (Suzuki, Furukawa, & Kimura, 2010).
IL-6 is one of the most sensitive and important inflammatory factors. During surgery, IL-6 induces the liver to synthesize the acute phase reactive protein-C reactive protein that promotes the phagocytic activity of neutrophils and macrophages, the release level of IL-6 and C-reactive protein in the body can reflect the body's stress situation. The platelet activating factor PAF has a powerful inflammatory effect by inducing the massive production of inflammatory factors to cause the cascade effect of inflammatory factors release. n-3 PUFAs can inhibit the release of IL-6, reduce the production of CRP and PAF, reduce the degree of inflammatory response, and enhance the immune function of the body (Koch and Heller, 2005, Liang et al., 2008, Matos et al., 2013). A number of previous studies have shown that n-3 PUFAs can down-regulate the levels of IL-6 and TNF-α in cancer patients after surgery (Ancrile et al., 2007, Don and Kaysen, 2004, Ghavami et al., 2009, Knüpfer and Preiss, 2010, Schneider et al., 2000), and shorten the use of ventilator and hospital stay in patients with major abdominal surgery (Tsekos, Reuter, & Stehle, 2004), this conclusion is consistent with the results of our meta-analysis. However, we did not observe the effect of n-3 PUFAs on the incidence of infectious complications in our analysis. Compared with Ma's study, taking supplements containing n-3 PUFAs, glutamine, arginine and nucleotides reduced the incidence of postoperative infection-related complications (Ma, Liu, Xiao, & Cao, 2016), the methods of nutritional intervention can partly explain these differences.
Prealbumin, albumin, and retinol-binding protein are important components of plasma total protein and are important indicators for nutritional evaluation. Patients in stress and critical conditions after surgery basically have a decrease in plasma protein (Wu, Ho, Lai, Chen, & Lin, 2021). Studies have shown that n-3 PUFAs can improve the quality of life, functional status and nutritional status of patients (van der Meij et al., 2010, van der Meij et al., 2012). n-3 PUFAs are also recommended as fatty acid supplements in the nutritional treatment of many diseases (Lemoine et al., 2019, Pappalardo and AlmeidaA, 2015). However, a study of 137 patients with advanced non-small cell lung showed no effect of taking n-3 PUFAs on patients' nutritional status (Lu, Chen, Wei, Hu, & Sun, 2018). The results of our meta-analysis showed that taking n-3 PUFAs did not increase the levels of prealbumin, albumin and retinol binding protein. Regarding the analysis results of the three proteins in the nutritional indicators, because the number of trials we included is small and heterogeneous, there may be potential bias in the interpretation of the results.
Our meta-analysis has several limitations. First of all, Although all the included studies were randomized controlled trials, most of them were single-center trials with small sample sizes. Second, there was moderate heterogeneity in the pooled outcome of the level of prealbumin and significant heterogeneity in the pooled outcome of the level of retinol-binding protein. Patients characteristics and the type, duration, timing of n-3 PUFAs interventions, as well as patient nutritional status across the trials, could be the source of heterogeneity. Finally, due to the limitation of the number of existing studies, our research results should be confirmed by long-term follow-up randomized controlled trials, which require sufficient sample size and fixed-dose data.
7. Conclusions
As a basic nutritional supplement, n-3 PUFAs can effectively enhance the immune function of patients with gastrointestinal cancer and reduce the level of inflammatory cytokines, and shortening the length of hospital stay, but it has no significant impact on the incidence of infectious-related complications and the level of nutrient protein. The results of this study can provide a basis for the clinical application of n-3 PUFAs. However, due to the limitations of the included studies and the potential risk of bias, it is necessary to conduct a large-scale, randomized, prospective trial to further evaluate the impact of n-3 PUFAs supplementation on patients with gastrointestinal tumors after surgery.
Funding
This work was supported by National Natural Science Foundations of China (Grant No. 81660484), Major special projects of the ministry of science and technology of China (Grant No.2017YFC309200) and Project of Beijing Key Laboratory(Grant No. 2020KF01).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Penghui Yang, Email: ypenghuiamms@hotmail.com.
Benqiang Rao, Email: raobenqiang@bjsjth.cn.
References
- Ancrile B., Lim K.-H., Counter C.M. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes & Development. 2007;21(14):1714–1719. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker N., van den Helder R.S., Stoutjesdijk E., van Pelt J., Houdijk A.P.J. Effects of perioperative intravenous ω-3 fatty acids in colon cancer patients: A randomized, double-blind, placebo-controlled clinical trial. American Journal of Clinical Nutrition. 2020 Feb 1;111(2):385–395. doi: 10.1093/ajcn/nqz281. [DOI] [PubMed] [Google Scholar]
- Cheng M., Zhang S., Ning C., Huo Q. Omega-3 Fatty Acids Supplementation Improve Nutritional Status and Inflammatory Response in Patients With Lung Cancer: A Randomized Clinical Trial. Frontiers Nutrition. 2021 Jul;30(8) doi: 10.3389/fnut.2021.686752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Jen M.a., Jin-Ming,, et al. Prospective double-blind randomized study on the efficacy and safety of an n-3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutrition Journal. 2015 doi: 10.1186/1475-2891-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don B.R., Kaysen G. Serum albumin: Relationship to inflammation and nutrition. Seminars in Dialysis. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- Ghavami S., Eshraghi M., Kadkhoda K., Mutawe M.M., Maddika S., Bay G.H., et al. Role of BNIP3 in TNF-induced cell death—TNF upregulates BNIP3 expression. Biochimica et Biophysica Acta (BBA)-Molecular. Cell Research. 2009;1793(3):546–560. doi: 10.1016/j.bbamcr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Giger-Pabst U., Lange J., Maurer C., et al. Short-term preoperative supplementation of an immunoenriched diet does not improve clinical outcome in well-nourished patients undergoing abdominal cancersurgery. Nutrition. 2013;29:724–729. doi: 10.1016/j.nut.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Horgan A. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Techniques in Coloproctology. 2014;18:1137–1138. doi: 10.1007/s10151-014-1205-1. [DOI] [PubMed] [Google Scholar]
- Kew S., Mesa M.D., Tricon S., et al. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. American Journal of Clinical Nutrition. 2004;79(4):674–681. doi: 10.1093/ajcn/79.4.674. [DOI] [PubMed] [Google Scholar]
- Kłek S, Kulig J, Szczepanik AM, et al. (2005 Apr) The clinical value of parenteral immuno nutrition in surgical patients. Acta Chirurgica Belgica. 105(2):175 -9. [PubMed]
- Knüpfer H., Preiss R. Serum interleukin-6 levels in colorectal cancer patients—a summary of published results. The International Journal of Colorectal Disease. 2010;25(2):135–140. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- Koch T., Heller A.R. Benefits of ω-3 fatty acids in parenteral nutrition. Clinical Nutrition Supplements. 2005;1(3):17–24. [Google Scholar]
- Lemoine S.C.M., Brigham E.P., Woo H., Hanson C.K., McCormack M.C., Koch A., et al. Omega-3 fatty acid intake and prevalent respiratory symptoms among U.S. adults with COPD. BMC Pulmonary Medicine. 2019 doi: 10.1186/s12890-019-0852-4. May 21;19(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B., Shan W., Ye Y.J., et al. Impact of postoperative omega-3 fatty acid-supplemented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World Journal of Gastroenterology. 2008 doi: 10.3748/wjg.14.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B., Wang S., Ye Y.J., et al. Impact of postoperative omega-3 fatty acid supplented parenteral nutrition on clinical outcomes and immunomodulations in colorectal cancer patients. World Journal of Gastroenterology. 2008 Apr 21;14(15):2434–2439. doi: 10.3748/wjg.14.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang X., Liu Z., et al. Effects of enteral nutrition rich in ω-3 polyunsa- turated fatty acids on the nutritional status and quality of life of patients with gastric cancer. Chinese Journal of Clinical Nutrition. 2020;28(3):151–157. [Google Scholar]
- Lu Y., Chen R.-g., Wei S.-z., Hu H.-g., Sun F. Yu C-h. Effect of omega 3 fatty acids on C-reactive protein and interleukin-6 in patients with advanced nonsmall cell lung cancer. Medicine. 2018:97:e11971. doi: 10.1097/MD.0000000000011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.J., Liu L., Xiao J., Cao B.W. Perioperative omega-3 polyunsaturated fatty acid nutritional support in gastrointestinal cancer surgical patients: A systematic evaluation. Nutrition and Cancer. 2016;68:568–576. doi: 10.1080/01635581.2016.1158291. [DOI] [PubMed] [Google Scholar]
- Marano L., Porfidia R., Pezzella M., et al. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: A prospective randomized study. Annals of Surgical Oncology. 2013;20(12):3912–3918. doi: 10.1245/s10434-013-3088-1. [DOI] [PubMed] [Google Scholar]
- Matos D., Santana R., Garcia T., et al. Parenteral fish oil as a pharmacological agent to modulate post-operative immune response: A randomized, double-blind, and controlled clinical trial in patients with gastrointestinal cancer. Clinical Nutrition. 2013;32(4):503–510. doi: 10.1016/j.clnu.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Pappalardo G., AlmeidaA R.P. Eicosapentaenoic acid in cancer improves body composition and modulates metabolism. Nutrition. 2015;31(4):549–555. doi: 10.1016/j.nut.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Pisaniello A.D., Psaltis P.J., King P.M., et al. Omega-3 fatty acids ameliorate vascular inflammation: A rationale for their atheroprotective effects. Athero sclerosis. 2021;324 doi: 10.1016/j.atherosclerosis.2021.03.003. [DOI] [PubMed] [Google Scholar]
- Qu B.G., Bi W.M., Qu B.T., et al. PRISMA-compliant article: Clinical characteristics and factors influencing prognosis of patients with Hepatoid Adenocarcinoma of the stomach in China. Medicine. 2016;95(15) doi: 10.1097/MD.0000000000003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M.R., Hoeflich A., Fischer J.R., Wolf E., Sordat B., Lahm H. Interleukin-6 stimulates clonogenic growth of primary and metastatic human colon carcinoma cells. Cancer Letters. 2000;151(1):31–38. doi: 10.1016/s0304-3835(99)00401-2. [DOI] [PubMed] [Google Scholar]
- Song H, Zhu J, Lu D. (2016). Molecular-targeted first-line therapy for advanced gastric cancer. Cochrane Database System Review 7:Cd011461. [DOI] [PMC free article] [PubMed]
- Sorensen L.S., Thorlacius-Ussing O., Schmidt E.B.R., et al. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. British Journal of Surgery. 2014 Jan;101(2):33–42. doi: 10.1002/bjs.9361. [DOI] [PubMed] [Google Scholar]
- Suzuki D., Furukawa K., Kimura F., et al. Effects of perioperative immuno nutrition on cell-mediated immunity, T helper type 1 (Th1)/Th2 differentiation, and Th17 response after pancreaticoduodenectomy. Surgery. 2010;148(3):573–581. doi: 10.1016/j.surg.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Tsekos E., Reuter C., Stehle P., et al. Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clinical Nutrition. 2004;23(3):325–330. doi: 10.1016/j.clnu.2003.07.008. [DOI] [PubMed] [Google Scholar]
- Turbitt W.J., Black A.J., Collins S.D., et al. Fish oil enhances T cell function and tumor infiltration and is correlated with a cancer prevention effect in HER-2/neu but not PyMT transgenic mice. Nutrition and Cancer. 2015;67(6):965–975. doi: 10.1080/01635581.2015.1060351. [DOI] [PubMed] [Google Scholar]
- van der Meij B.S., Langius J.A., Smit E.F., Spreeuwenberg M.D., von Blomberg B.M., Heijboer A.C., et al. Oral nutritional supplements containing (n-3) polyunsaturated fatty acids affect the nutritional status of patients with stage III non-small cell lung cancer during multimodality treatment. Journal of Nutrition. 2010;140:1774–1780. doi: 10.3945/jn.110.121202. [DOI] [PubMed] [Google Scholar]
- van der Meij B.S., Langius J.A., Spreeuwenberg M.D., Slootmaker S.M., Paul M.A., Smit E.F., et al. Oral nutritional supplements containing n-3 polyunsaturated fatty acids affect quality of life and functional status in lung cancer patients during multimodality treatment: An RCT. European Journal of Clinical Nutrition. 2012 Mar;66(3):399–404. doi: 10.1038/ejcn.2011.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace F.A., Miles E.A., Evans C., et al. Dietary fatty acids influence the production of Th1- but not Th2-type cytokines. Journal of Leukocyte Biology. 2001;69(3):449–457. [PubMed] [Google Scholar]
- Wei Z., Wang W., Chen J., Yang D., Yan R., Cai Q. A prospective, randomized, controlled study of ω-3 fish oil fat emulsion-based parenteral nutrition for patients following surgical resection of gastric tumors. Nutrition Journal. 2014 Mar;24(13):25. doi: 10.1186/1475-2891-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.M., Ho T.W., Lai I.R., Chen C.N., Lin M.T. Parenteral glutamine supplementation improves serum albumin values in surgical cancer patients. Clinical Nutrition. 2021 Feb;40(2):645–650. doi: 10.1016/j.clnu.2020.06.015. [DOI] [PubMed] [Google Scholar]
- Yaqoob P., Calder P.C. The effects of dietary lipid manipulation on the production of murine T cell-derived cytokines. Cytokine. 1995;7(6):548–553. doi: 10.1006/cyto.1995.0074. [DOI] [PubMed] [Google Scholar]
- Zhang C.H., Li N., Wang X.Y., et al. Influence of Lipoplus fat emulsion on postoperative nutritional status and early inflammatory response in patients with gastrointestinal malignancies. Zhonghua wei chang wai ke za zhi = Chinese Journal of Gastrointestinal Surgery. 2012;15(5):448–451. [PubMed] [Google Scholar]
- Zhang Y., Ma Z., Rui Q., et al. Impact of postoperative fish oil fat emulsion supplementation on monocytes function in gastrointestinal cancer patients. Chinese Journal of Clinical Nutrition. 2014;22(3):158–161. [Google Scholar]
- Zhu M.W., Tang D.N., Hou J., et al. Impact of fish oil enriched total parenteral nutrition on elderly patients after colorectal cancer surgery. Chinese Medical Journal (England) 2012 Jan;125(2):178–181. [PubMed] [Google Scholar]