Abstract
Purpose:
To investigate the retinal and choroidal microcirculation changes in celiac disease (CD) patients via optical coherence tomography angiography (OCT-A).
Methods:
This cross-sectional study included 44 pediatric patients with newly diagnosed CD and 44 healthy pediatric subjects. The vascular densities (VD) of the superficial, deep, and choriocapillar plexuses (VDs, VDd, and VDcc, respectively) (%), the superficial and deep foveal avascular zones (FAZs and FAZd) (%), the central macular thickness (CMT) (mm), and the subfoveal choroidal thickness (SFCT) (mm) were measured with swept-source OCT-A in addition to a complete ophthalmological examination.
Results:
Mean ages of the CD patients and the healthy participants were 12.02 ± 2.9 and 13.6 ± 2.3 years, respectively. The central sectors of the VDs and VDd measurements were found to be significantly higher in the study group compared to the control group (p = 0.006; P = 0.001, respectively), and the temporal and nasal values of the VDcc measurements were significantly lower in the study group than in the control group (p < 0.05 for both values). CMT and FAZ metrics did not differ between the groups (p > 0.05). SFCT was significantly reduced (p = 0.001), and choroidal thinning was more considerable in female CD patients (p = 0.045).
Conclusion:
CD seems to affect macular and choroidal microcirculation. The reduced choriocapillaris plexus parameters and choroidal thickness may provide disease activity information.
Keywords: Celiac disease, choroidal thickness, macular perfusion, retinal vasculature, swept-source optical coherence tomography angiography, vessel density
Celiac disease (CD) is a chronic, genetic, immune-mediated small intestine enteropathy that occurs in gluten-sensitive individuals.[1] Malabsorption symptoms, such as steatorrhea and weight loss, are among the typical hallmarks of CD. However, as most CD patients may experience atypical disease presentations, it is important for healthcare professionals to raise awareness of the prevalence of CD and resolve its various gastrointestinal and extraintestinal symptoms.
Up to 50% of newly diagnosed CD patients may initially present with symptoms that are not gastrointestinal.[2] Extraintestinal findings in the neurological, musculoskeletal, integumentary, or reproductive systems may be seen. As duodenal villous atrophy and antibodies to tissue transglutaminase (tTG) are found in most CD patients, their presence is required for a CD diagnosis. European guidelines make diagnosis possible without duodenal biopsy, provided that the patient meets certain symptomatic and serological criteria.[3] CD is treated by lifelong adherence to the challenging process of a gluten-free diet (GFD).
In recent years, ophthalmologists have researched ocular changes in CD patients, which are caused by the extraintestinal involvement characteristic of this autoimmune disease.[4,5,6,7,8,9,10] Vascular changes and endothelial dysfunction have recently been demonstrated in CD patients.[11] However, a comprehensive literature review has found no study investigating macular perfusion parameters in CD patients.
Swept-source optical coherence tomography angiography (OCT-A) has been developed for imaging the retinal vascular system without contrast material.[12] It allows quantitative measurement of both superficial and deep retinal vessels to assess macular perfusion and evaluate diseases affecting vascular structures,[13] which has increased its popularity and its usefulness for research purposes.
As far as known from the literature review, this is the first study to use OCT-A to examine retinal microcirculation in CD patients. Due to the systemic inflammation and vascular changes previously reported in CD, the project’s aim was to evaluate changes in OCT-A parameters indicating macular perfusion in the eyes of CD patients compared to healthy subjects.
Methods
Subjects
This study was conducted in a tertiary hospital’s Department of Ophthalmology and Paediatric Gastroenterology. Between March and October 2020, the right eyes of 44 newly diagnosed CD patients were examined. These patients had been diagnosed with CD at the same tertiary hospital’s department of paediatric gastroenterology and subsequently referred for a routine eye exam. The study was approved by the Institutional Review Board of the local ethics committee (Protocol No: 2020/37). Informed consent was received from all participants before enrolment, and the research adhered to the tenets of the Declaration of Helsinki.
Patients included in this study were newly diagnosed with CD and had been referred for a routine eye screening. Therefore, a routine, comprehensive ophthalmologic examination was carried out for each patient. In addition to gathering the patients’ histories, their best corrected visual acuity (BCVA) values were measured according to the standard Snellen chart, and their intraocular pressures were measured via Goldmann applanation tonometry. The patients’ anterior eye segments were examined with a slit lamp, and fundus evaluations were performed with a +90D lens after pharmacological dilatation with 1% Tropicamide. OCT-A measurements were taken after full pharmacological dilatation. Patients were excluded from the study if they had already begun a GFD, if their BCVA values were below 20/20, if they required high refractive vision correction (spherical equivalent higher than +3 or -3 diopter), if they had any intraocular disease or had undergone surgery, or if their examinations returned poor image quality due to unstable fixation in OCT-A measurements. OCT-A measurements were also taken from the control group: 44 ophthalmologically healthy age- and sex-matched individuals, without systemic disease, who applied to the ophthalmology department of the tertiary hospital.
Image acquisition and processing
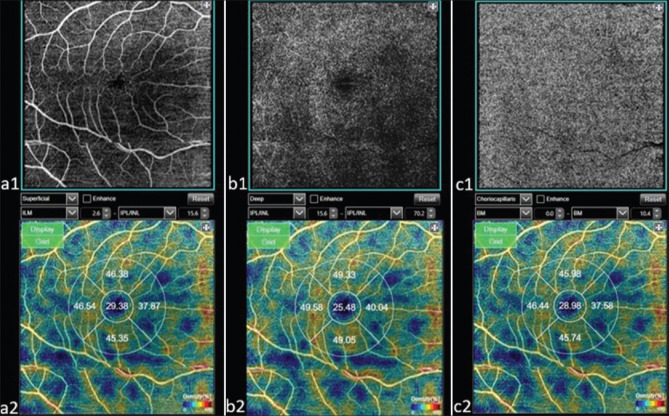
All subjects were examined via a swept-source DRI OCT Triton (Topcon, Tokyo, Japan) device using a 6 mm * 6 mm protocol. An OCT-A software system (IMAGEnet 6 V.1.14.8538) was applied, which provides non-invasive macular segmentation consisting of four ‘en face’ OCT slabs. (1) The superficial vascular complex (SVC) is defined as stretching from 2.6 mm below the internal limiting membrane (ILM) to 15.6 mm below the inner plexiform layer (IPL) [Fig. 1. a1 and a2]; (2) the deep vascular complex (DVC) is defined as stretching from the IPL offset of 15.6 mm to the IPL offset of 70.2 mm [Fig. 1. b1 and b2]; (3) the outer retinal slab is defined as stretching from 70.2 mm under the IPL to the Bruch’s membrane (BM); and (4) the choriocapillaris vascular complex (CC) is defined as stretching from the BM to 10.4 mm under the BM [Fig. 1. c1 and c2].[14]
Figure 1.
A 6 × 6 mm2 area OCT-A image (a1) and density (a2) in the superficial capillary plexus and a 6 × 6 mm2 area OCT-A image (b1) and vessel density (b2) in the deep capillary plexus. The choriocapillaris’ image (c1) and vessel density (c2) from Bruch’s membrane to 10.4 μm beneath Bruch’s membrane
The OCT-A device operates a 1.050-nm wavelength light source and completes 100,000 A-scans per second.[15] The GNU Image Manipulation Program (GIMP) 2.8.14 was used to quantitatively analyze the vascular densities (VD) for the SVC (VDs), the DVC (VDd), and the CC (VDcc). It was also used to quantitatively analyze the superficial foveal avascular zone (FAZs) and the deep foveal avascular zone (FAZd). Each VD measurement was calculated as the percentage of the vascularized tissue in the circumscribed area. Enhanced HD line scans provided the central macular and subfoveal choroidal thickness measurements (CMT and SFCT, respectively). An experienced operator (Y.K.) completed all measurements of the subjects. The right eye of each participant was included in the study. The measurements were performed from the subjects during the same time of day. The two experienced controllers examined two consecutive scans of a subject to determine reproducibility at different times. The value of two means, measured independently, was used in the study. Any pathological situations found in the structural OCT scans were excluded, as were images whose Image Quality Index (IQI) values were under 70.
Statistical analysis
The mean and standard deviations were measured for the quantitative OCT-A variables. The Kolmogorov–Smirnov test was applied to analyze the departures from normal distribution for each parameter. Categorical variables were compared between groups using the Chi-squared test. The independent samples test was used for parameters with normal distribution, and the Mann–Whitney U test was used for parameters with abnormal distribution to compare the means between groups. All statistical analyses were completed using the Statistical Package for the Social Sciences (SPSS) version 22.0 for Windows (IBM Corporation, Chicago, IL, USA). In all statistical tests, a P value of < 0.05 was considered statistically significant.
Results
A total of 44 patients with CD (right eye for each patient; 44 eyes) and 44 healthy subjects (right eye for each subject; 44 eyes) were included in this study. All subjects were under the age of 18, and the mean ages in the patient and control groups were 12.02 ± 2.9 and 13.6 ± 2.3 years, respectively. The ages and gender distributions of the participants in both groups showed no significant difference (p > 0.05). The subjects’ demographic data and the OCT-A parameter details are presented in Table 1.
Table 1.
The demographic characteristics, OCT-A parameters, and choroidal thickness of CD patients and healthy participants
| Celiac Disease Group | Control Group | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Mean± n-% | Median | Mean± n-% | Median | ||||||||
| Age | 12.02 | ± | 2.9 | 12.5 | 13.6 | ± | 2.396 | 14 | m | ||
|
| |||||||||||
| Gender | Female | 24 | 45.5% | 27 | 61.4% | X2 | |||||
| Male | 20 | 54.5% | 17 | 38.6% | |||||||
|
| |||||||||||
| FAZs | 238.3 | ± | 91.72 | 230.98 | 238,4 | ± | 97.41 | 227.8 | 0.997 | t | |
| FAZd | 317.9 | ± | 110.7 | 299,004 | 336,2 | ± | 116.5 | 305.7 | 0.428 | m | |
| VDs Central | 26.05 | ± | 6.03 | 24.75 | 22.9 | ± | 4.294 | 22.94 | 0.006 | t | |
| VDs Superior | 51.77 | ± | 5.906 | 50.305 | 53.2 | ± | 7.285 | 51.53 | 0.234 | m | |
| VDs Temporal | 47.36 | ± | 4.611 | 47.675 | 48.1 | ± | 4.241 | 48.43 | 0.443 | t | |
| VDs Inferior | 48.68 | ± | 5.63 | 48.185 | 48,95 | ± | 4.552 | 49.62 | 0.805 | t | |
| VDs Nasal | 45.07 | ± | 5.243 | 45.755 | 45.3 | ± | 4.633 | 46.07 | 0.730 | t | |
| VDd Central | 24.98 | ± | 6.622 | 23.16 | 20.8 | ± | 4.305 | 19.96 | 0.001 | t | |
| VDd Superior | 54.51 | ± | 6.021 | 53.715 | 54.3 | ± | 2.771 | 54.63 | 0.421 | m | |
| VDd Temporal | 49.28 | ± | 5.113 | 49.81 | 50.8 | ± | 4.091 | 51.03 | 0.125 | t | |
| VDd Inferior | 50.38 | ± | 6.012 | 50.03 | 51.7 | ± | 4.246 | 52.17 | 0.254 | t | |
| VDd Nasal | 49 | ± | 5.583 | 48.8 | 49.4 | ± | 4.408 | 49.62 | 0.284 | m | |
| VDc Central | 25.63 | ± | 6.288 | 24.005 | 31.5 | ± | 15.14 | 24.45 | 0.582 | m | |
| VDc Superior | 51.87 | ± | 5.782 | 50.485 | 52.3 | ± | 2.54 | 52.13 | 0.642 | t | |
| VDc Temporal | 47.34 | ± | 4.6 | 47.675 | 60.6 | ± | 76.17 | 49.95 | 0.035 | ||
| VDc Inferior | 48.86 | ± | 5.629 | 48.255 | 50.1 | ± | 4.61 | 50.83 | 0.256 | t | |
| VDc Nasal | 45.68 | ± | 5.212 | 45.385 | 47.2 | ± | 5.627 | 47.08 | 0.185 | ||
| CMT | 183 | ± | 14.68 | 180 | 183.6 | ± | 13.41 | 183 | 0.825 | m | |
| SFCT | 267 | ± | 74.25 | 264 | 313.4 | ± | 53.88 | 309 | 0.001 | t | |
mMann-Whitney u test / ’independent sample t test/x Chi-squa red test. Sd: standart deviation; FAZssuperficial foveal avascular zone; FAZd: deep foveal avascular zone; VDs: superficial vascular density; VDd: deep vascular density; VDcc: choriocapi I laris vascular density;CMT: central macular thickness; SFCT: subfovea I choroidal thickness. Italicized bold values represent p<0.05.
Test results for anti-endomysial antibodies and tissue transglutaminase (tTG) immunoglobulin A [IgA] and immunoglobulin G [IgG]) were positive in all CD patients. HLA-DQ2 and HLA-DQ8 were also found in the CD patients at positivity rates of 34% and 17%, respectively.
FAZs and FAZd values were not significantly altered in patients compared to the control group (p > 0.05). However, the central values of the VDs and VDd were significantly increased in CD cases compared to the control group (p = 0.006; P = 0.001). Other sectors of the VDs and VDd showed no significant differences (p > 0.05).
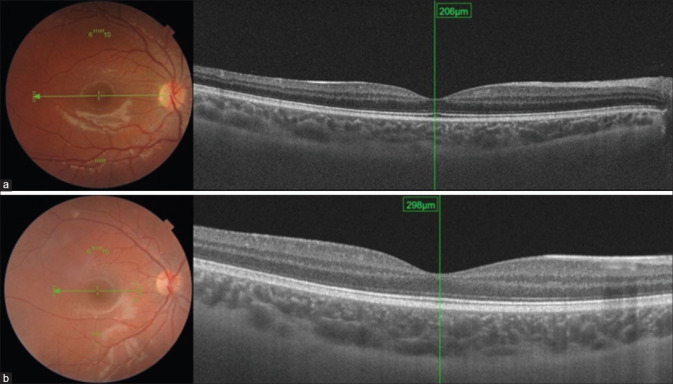
In CD patients, the VDcc temporal and VDcc nasal values were significantly lower than those in the control group (p < 0.05 for both types of values). Other VDcc sectors had not changed significantly. While CMT measurements did not differ between the groups (p > 0.05), SFCT values were significantly decreased (p = 0.001) [Fig. 2]. Reduced choroidal thickness was more prominent in females than in males (p = 0.045).
Figure 2.
The subfoveal choroidal thickness of a patient with celiac disease (a) and a healthy subject (b) quantitatively measured by swept-source OCT using a built-in software
Discussion
CD is among the most common autoimmune diseases, affecting approximately 1% of the world population.[16,17] While CD prevalence is gradually increasing, the management of intestinal and extraintestinal findings has become an important issue.[3] Ocular involvement in CD patients may be explained by genetic factors, circulating immune complexes, autoantibodies, or vitamin deficiencies caused by malnourishment. Pseudotumor cerebri, malnutrition retinopathy, uveitis, and cataracts are ocular manifestations that can be seen as a result of autoantibody-related systemic inflammation or malnutrition in CD patients.[4] None of these diseases were found in the current study. In recent years, changes in choroidal thickness,[9,10] ocular surface and tear film,[5] anterior chamber features,[6] and retinal nerve fiber layer thickness[6] have been the ocular parameter differences reported in CD patients. Changes in choroidal thickness may be associated with possible vascular involvement. Accordingly, vascular angiogenesis and endothelial dysfunction have been discussed in untreated CD patients.[11] Therefore, the current study aimed to evaluate the macular microcirculation in eyes of newly diagnosed CD patients via OCT-A.
Patients with CD in this study showed similar FAZ metrics (FAZs and FAZd) compared to the healthy controls. Inner retinal thickness and FAZ area were in accordance with each other.[18] Yener et al.[19] (2020), who have also used swept-source OCT among patients with CD, do not report changes in the inner retinal layer. The current authors believe that their finding of similar CMT measurements in CD patients compared to healthy subjects may be related to the absence of change in the FAZ area. This situation may also be explained by the fact that the patients’ CD was newly diagnosed, and thus they lacked the significant damage to vascular structures associated with longer, untreated disease courses.
According to the current data, the central VDs and VDd sector values were significantly higher in the CD group than in the healthy group. An increased VDd central value may be influenced by an increased VDs central value because the deep vascular plexus is anastomosed with the superficial vascular plexus.[20] However, no statistical differences were found for VDs and VDd in sectors other than the central sector. Therefore, it may be inferred that CD causes little change to the inner retinal circulation.
The posterior and anterior ciliary arteries, which originate from the ophthalmic artery, supply the choriocapillaris plexus, the retinal pigment epithelium, and the photoreceptors through the blood–retina barrier.[20] The choroidal circulation accounts for 85% of the total blood flow in the eye.[21] It is likely to be affected by systemic diseases or inflammatory conditions due to high vascular flow. For instance, alteration in choroidal flow and thickness has been seen in cardiovascular diseases and neurodegenerative diseases related to vascular flow disturbances.[22,23] In the current study, the VD measurements of the temporal and nasal sectors were significantly decreased in patients compared to healthy subjects at the level of the choriocapillaris plexus. Immune activation and circulating autoantibodies can affect the choroidal vasculature.[21] Gluten can trigger structural and inflammatory changes in CD,[24] and CD autoantibodies can reduce endothelial branching, increase endothelial permeability, and increase lymphocyte adhesion to the endothelium.[11,25] Therefore, it is possible to say that CD may affect vascular structures. As the patient population consists of newly diagnosed celiac patients, the local compensatory mechanisms seem to be sufficient to maintain retinal VD in the early period of CD, which supports the hypothesis that retinal vascularization is auto-regulated by the local factors released from endothelial cells.[26,27] SFCT was also found to be significantly lower among the CD patients. However, Bolukbasi et al.[10] (2019), using the enhanced depth imaging (EDI) mode of spectral-domain OCT, report enlargement of the choroidal vessels and increased choroidal thickness following adherence to a GFD in adult patients. They believe that it is caused by systemic inflammation due to CD, and they state that patients following GFDs experience high levels of choroidal thickness, reduced disease activity, or the disease healing process.[10] However, Doğan et al.[9] (2019), using SD-OCT, show that a longer GFD duration is associated with adherence difficulty and lower choroidal thickness in pediatric patients. In the present study, the authors thought that the decrease in SFCT could be associated with disease activity, since the sample was made up of newly diagnosed patients, who had not yet begun a GFD. Circulating autoantibodies stimulated by gluten exposure are considered to be the cause of choroidal thinning following the inflammatory process. Since the current authors obtained SFCT values quantitatively, using the choroidal thickness measurement mode of the swept-source OCT, they believed that they obtained more reliable results.
Although CD is more common in females, disease presence did not differ according to gender in this study.[28] However, this research may support the idea that the clinical presentation of CD is not the same in males and females. Choroidal thickness was found to be much lower in the female CD patients than in the male CD patients. Therefore, CD activity may vary by gender. The authors believed that the lower average SFCT value in the sampled females was caused by greater autoimmune-related damage.
The findings in this report have some limitations. First, these data apply only to newly diagnosed CD patients. Second, subgroups that were positive for specific autoantibodies and HLA-DQ2 and HLA-DQ8 haplotypes could not be created and examined here because the sample size was not large enough. Another study may address this issue. Third, changes in the OCT-A parameters and choroidal thickness after GFD initiation could not be evaluated because this was a cross-sectional study, and there was no follow-up period. In addition, indocyanine green angiography, a useful test to evaluate choroidal inflammation and ischemia, could not be performed in the hospital study conducted. In this study, iron-deficiency parameters, Vitamin A and Vitamin B12, which may affect the OCT-A parameters, could not measured. The current study may guide a new study to evaluate these parameters as a subgroup analyses, which may show the specific results. Vascular parameters and/or choroidal thickness may be follow-up marker(s) in terms of disease activity and compatibility with GFD. Further studies should be performed concerning ocular parameters before and after GFD initiation. In the long term, the authors will continue to follow these sampled patients by recommending GFDs and, subsequently, examining and presenting the results for the effect of those GFDs on the patients’ OCT-A parameters.
Conclusion
Ocular involvement may be asymptomatic in CD patients. Among the examined OCT-A parameters, an increase was observed in the central sector of the superficial and deep vascular plexuses, whereas a decrease was observed in the temporal and nasal sector VD measurements for the choriocapillaris plexus. The authors, therefore, determined that there might be a relationship between choroidal thinning and CD activity with gluten exposure. Since choroidal thinning is more evident in females, it may also be concluded that the course of CD may have gendered differences and be worse in females.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from each participant included in the study. Patients signed informed consent regarding the publishing of their data.
Financial support and sponsorship
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
References
- 1. Glissen Brown JR, Singh P. Coeliac disease. Paediatr Int Child Health. 2019;39:23–31. doi: 10.1080/20469047.2018.1504431. [DOI] [PubMed] [Google Scholar]
- 2. Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet (London, England) 2018;391:70–81. doi: 10.1016/S0140-6736(17)31796-8. [DOI] [PubMed] [Google Scholar]
- 4. Martins T, Costa A, Oyamada M, Schor P, Sipahi A. Ophthalmologic manifestations of celiac disease. Int J Ophthalmol. 2016;9:159–62. doi: 10.18240/ijo.2016.01.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uzel MM, Citirik M, Kekilli M, Cicek P. Local ocular surface parameters in patients with systemic celiac disease. Eye. 2017;31:1093–8. doi: 10.1038/eye.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karatepe Hashas AS, Altunel O, Sevınc E, Duru N, Alabay B, Torun YA. The eyes of children with celiac disease. J Pediatr Ophthalmol Strabismus. 2017;21:48–51. doi: 10.1016/j.jaapos.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 7. Hazar L, Oyur G, Atay K. Evaluation of ocular parameters in adult patients with celiac disease. Curr Eye Res. 2020;46:122–6. doi: 10.1080/02713683.2020.1780266. [DOI] [PubMed] [Google Scholar]
- 8. Fousekis FS, Katsanos A, Katsanos KH, Christodoulou DK. Ocular manifestations in celiac disease:An overview. Int Ophthalmol. 2020;40:1049–54. doi: 10.1007/s10792-019-01254-x. [DOI] [PubMed] [Google Scholar]
- 9. Doğan G, Şen S, Çavdar E, Mayalı H, Cengiz Özyurt B, Kurt E, et al. Should we worry about the eyes of celiac patients? Eur J Ophthalmol. 2019;30:886–90. doi: 10.1177/1120672119850071. [DOI] [PubMed] [Google Scholar]
- 10. Bolukbasi S, Erden B, Cakir A, Bayat AH, Elçip lu M, Ocak SY, et al. Pachychoroid pigment epitheliopathy and choroidal thickness changes in coeliac disease. J Ophthalmol. 2019;2019:6924191. doi: 10.1155/2019/6924191. doi:10.1155/2019/6924191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ciaccio EJ, Lewis SK, Biviano AB, Iyer V, Garan H, Green PH. Cardiovascular involvement in celiac disease. World J Cardiol. 2017;9:652–66. doi: 10.4330/wjc.v9.i8.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55. doi: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang J, Yuan M, Wang E, Chen Y. Comparison of the repeatability of macular vascular density measurements using four optical coherence tomography angiography systems. J Ophthalmol. 2019;2019:4372580. doi: 10.1155/2019/4372580. doi:10.1155/2019/4372580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al-Sheikh M, Falavarjani KG, Akil H, Sadda SR. Impact of image quality on OCT angiography based quantitative measurements. Int J Retina Vitreous. 2017;3:13. doi: 10.1186/s40942-017-0068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, et al. Optical coherence tomography angiography:A comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100. doi: 10.1016/j.preteyeres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Biagi F, Klersy C, Balduzzi D, Corazza GR. Are we not over-estimating the prevalence of coeliac disease in the general population? Ann Med. 2010;42:557–61. doi: 10.3109/07853890.2010.523229. [DOI] [PubMed] [Google Scholar]
- 17. Yu XB, Uhde M, Green PH, Alaedini A. Autoantibodies in the extraintestinal manifestations of celiac disease. Nutrients. 2018;10:1123. doi: 10.3390/nu10081123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chui TYP, VanNasdale DA, Elsner AE, Burns SA. The association between the foveal avascular zone and retinal thickness. Invest Ophthalmol Vis Sci. 2014;55:6870–7. doi: 10.1167/iovs.14-15446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yener AÜ, Akpinar MY, Kahramanoğlu E, Sapmaz F, Nazligul Y. Ocular parameters quantified by the swept-source optical coherence tomography in celiac disease. Cumhuriyet Med J. 2020;42:327–33. [Google Scholar]
- 20. Campbell JP, Zhang M, Hwang TS, Bailey ST, Wilson DJ, Jia Y, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201. doi: 10.1038/srep42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steiner M, Esteban-Ortega MDM, Muñoz-Fernández S. Choroidal and retinal thickness in systemic autoimmune and inflammatory diseases:A review. Surv Ophthalmol. 2019;64:757–69. doi: 10.1016/j.survophthal.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 22. Yeung SC, You Y, Howe KL, Yan P. Choroidal thickness in patients with cardiovascular disease:A review. Surv Ophthalmol. 2020;65:473–86. doi: 10.1016/j.survophthal.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 23. Pellegrini M, Vagge A, Ferro Desideri L, Bernabei F, Triolo G, Mastropasqua R, et al. Optical coherence tomography angiography in neurodegenerative disorders. J Clin Med. 2020;9:1706. doi: 10.3390/jcm9061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leffler D, Schuppan D, Pallav K, Najarian R, Goldsmith JD, Hansen J, et al. Kinetics of the histological, serological and symptomatic responses to gluten challenge in adults with coeliac disease. Gut. 2013;62:996–1004. doi: 10.1136/gutjnl-2012-302196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myrsky E, Caja S, Simon-Vecsei Z, Korponay-Szabo I, Nadalutti C, Collighan R, et al. Celiac disease IgA modulates vascular permeability in vitro through the activity of transglutaminase 2 and RhoA. Cell Mol Life Sci. 2009;66:3375–85. doi: 10.1007/s00018-009-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delaey C, van de Voorde J. Regulatory mechanisms in the retinal and choroidal circulation. Ophthalmic Res. 2000;32:249–56. doi: 10.1159/000055622. [DOI] [PubMed] [Google Scholar]
- 27. Pournaras CJ, Rungger-Brändle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 28. Bardella MT, Fredella C, Saladino V, Trovato C, Cesana BM, Quatrini M, et al. Gluten intolerance:Gender- and age-related differences in symptoms. Scand J Gastroenterol. 2005;40:15–9. doi: 10.1080/00365520410008169. [DOI] [PubMed] [Google Scholar]