Abstract
Purpose:
To report outcomes and assess the risk factors for failure of trabeculectomy (trab), trabeculectomy with mitomycin-C (trabMMC), and combined trabeculectomy with cataract extraction (CT) in vernal keratoconjunctivitis (VKC) eyes with steroid-induced glaucoma (SIG).
Methods:
Trab was performed in 45 eyes of 30 subjects, trabMMC in 36 eyes of 25 subjects, and CT in 34 eyes of 27 subjects. Success was complete when intraocular pressure (IOP) was between 6 and 21 mm Hg without anti-glaucoma medications (AGM) and qualified with AGM.
Results:
Median age (14 vs. 16.3 and 17.4 years) was significantly less in the trab cohort (P = 0.007). Majority (88%–93%) were male (P = 0.78). Preoperatively, median duration of steroid usage was >2 years (P = 0.52), mean IOP (32, 29.4, and 28.4; P = 0.26) and median cup:disc ratio (CDR) (0.9; P = 0.27) were similar in the three groups. Complete success (76%, 71%, and 66% at 5 years; P = 0.91), and qualified success (88%, 97%, and 94% at 5 years; P = 1.0) with trab, trabMMC, and CT, respectively, were similar. Preoperative factors significantly associated with qualified failure (multiple logistic regression) were older children, longer duration of VKC, longer duration and mixed type of steroid use (all P < 0.001) and larger CDR (P < 0.02). At the last follow-up, 38% in trab, 33% in trabMMC, and 50% eyes in CT were blind (visual acuity ≤20/400 and/or visual field ≤10°) due to glaucoma (P = 0.33).
Conclusion:
The surgical success for all three types of surgery was similar at 5-years. Chronic VKC and long-term steroid use were associated with surgical failure. The majority had advanced disease and a significant proportion were blind due to glaucoma.
Keywords: Allergic eye disease, steroid-induced glaucoma, trabeculectomy, VKC
Vernal keratoconjunctivitis (VKC) is a severe form of ocular allergy that affects mostly children and young adults living in tropical and warm climates.[1,2,3,4,5] The milder forms of allergy is managed with topical anti-allergic medications and mast cell stabilizers, and acute or severe ocular allergy is treated with topical steroids.[6] Chronic use of topical steroids in VKC results in steroid-induced glaucoma (SIG) in approximately 2%–4% of the subjects.[1,2,3,4,7,8,9,10,11,12] The other serious side effect of long-term steroid use is steroid-induced cataract.[8,13,14,15]
SIG is difficult to control medically and about a quarter to a third of them require glaucoma surgery for IOP control.[7,16,17,18] In India, the SIG in eyes with VKC is very severe, with a third of them blind at presentation.[7,17] Despite VKC being the commonest ocular allergic disease in children with a significantly high incidence of steroid-induced glaucoma and a large proportion needing glaucoma surgery,[7] the literature on this topic is sparse. Two small case series reported outcomes of trabeculectomy in VKC with SIG: 14 eyes in one study (with trabeculectomy; 84% success with follow-up at 15–120 months),[19] and a second study with 8 eyes (trabeculectomy with MMC; success was 87%, follow-up not specified).[20]
Our study is a large series with 115 eyes with VKC and SIG that underwent either trabeculectomy, trabeculectomy with MMC, or combined trabeculectomy with cataract surgery as a primary procedure. We evaluated the success of glaucoma filtering surgery and the risk factors for failure for the same in the three homogenous cohorts with VKC and SIG.
Methods
We retrospectively reviewed the charts of patients with VKC and glaucoma who underwent either glaucoma surgery or combined cataract and glaucoma procedure at our institute between 1998 and 2013. The institutional review board approval was obtained and the study adhered to the tenets of the Declaration of Helsinki.
VKC eyes on topical steroids with open angles on gonioscopy with two or more of the following were considered as SIG: a) IOP >21 mm Hg or IOP <21 mm Hg on anti-glaucoma medications; b) glaucomatous optic disc damage (focal or diffuse neuroretinal rim thinning, localized notching, or nerve fiber layer defects); c) corresponding visual field (VF) defects. Among these, eyes that underwent trabeculectomy, trabeculectomy with MMC, or combined cataract with trabeculectomy as a primary surgery at our institute were included. Those with juvenile open-angle glaucoma or eyes with SIG other than VKC, and VKC eyes with prior ocular surgeries, were excluded.
Preoperative details included demographic characteristics, medical history, type and duration of steroid use, anti-glaucoma medications (AGM), visual acuity, severity and activity of VKC, type of VKC, IOP, lens status, gonioscopy, disc evaluation, and VF examination. Betamethasone, prednisolone, and dexamethasone were considered strong steroids; loteprednol and fluorometholone were considered weak steroids. The details of the surgery, operative, and postoperative complications if any were noted. Postoperative details included visual acuity, IOP, AGM, activity of VKC, need for topical steroids for VKC recurrence, disc changes, and VF details wherever available.
The primary outcome measure was success, which was defined as complete if IOP was between 6 and 21 mm Hg without any AGM and as qualified if IOP was between 6 and 21 mm Hg with AGM. IOP >21 mm Hg on AGM, resurgery for IOP control, and loss of light perception due to a surgical complication were labeled as failure.
Blindness was defined as per the WHO criteria: eye with visual acuity less than 3/60 (20/400 or BCVA worse than 1.3 LogMAR units) and or a visual field of 10°.
Statistical analysis
Descriptive statistics included mean and standard deviation (SD) for normally distributed continuous variables and median and interquartile range (IQR) for nonnormal continuous variables. Kaplan–Meier survival curves were used to assess the cumulative probability of success. Associations between failure and baseline factors such as age, gender, duration of VKC, duration and type of steroid use, preoperative IOP and optic disc damage, number of preoperative medications, age at surgery, and duration of follow-up were assessed using the multivariate logistic regression model. Association between the postoperative activity of VKC and need for pulse steroids were also assessed. As both eyes of few patients were included for the analysis, the cluster of data for the study subject was considered as a unit and standard error was adjusted during survival analysis. P < 0.05 was considered statistically significant. Statistical analysis was performed using commercial software (Stata ver. 10.0; StataCorp, College Station, TX).
Results
During the study period, there were 302 VKC subjects with glaucoma. Among these, 115 eyes of 82 subjects fit the inclusion criteria. Trabeculectomy (trab) was performed in 45 eyes of 30 subjects, trabeculectomy with mitomycin-C (trabMMC) in 36 eyes of 25 subjects, and combined cataract surgery with trabeculectomy (CT) in 34 eyes of 27 patients.
Demographic and preoperative clinical features: The demographic and clinical characteristics of subjects in all three groups are provided in Table 1. Median age at presentation ranged between 14.8 and 18.5 years in the three groups; mean age was significantly lesser in the trab group (P < 0.007). Between 88% and 93% of the subjects were male in all three groups (P = 0.78). The median duration of VKC was 24–36 months in the three groups and the majority (87%–97%) of eyes had limbal and tarsal VKC. Median duration of steroid usage was more than 2 years in all three groups (P = 0.52), 32 months in the trab group, 25.5 months in the trabMMC group, and 24 months in the CT group. Median LogMAR visual acuity (VA) at presentation ranged between 0.5 and 0.9 in the three groups, and median preoperative LogMAR VA was significantly less in the CT group (P = 0.02) due to cataract. The IOP at presentation was similar (P = 0.71); however, the preoperative highest IOP was significantly more in the trabMMC group (P = 0.03). The median number of preoperative AGM was similar in all three groups (P = 0.21). There was advanced disc damage in all three groups with a median cup:disc ratio of 0.9 in all three groups (P = 0.27).
Table 1.
Demographic and clinical features of subjects before surgery
| Trabeculectomy (n=45 eyes, 30 subjects) G1 | Trabeculectomy with MMC (n=36 eyes, 25 subjects) G2 | Combined surgery (n=34 eyes of 27 subjects) G3 | Test stat. | P | |
|---|---|---|---|---|---|
| Number of eyes | 45 | 36 | 34 | ||
| Median (IQR) age at presentation | 14 (8.3,17) | 16.3 (11.4,20.1) | 17.4 (12.3,21.8) | Kruskal-Wallis test | 0.03 |
| G1-G2 | Pairwise Wilcoxon rank-sum tests with Bonferroni correction | 0.20 | |||
| G1-G3 | 0.03 | ||||
| G2-G3 | 1 | ||||
| Median (IQR) age at surgery | 14.8 (8.4,17.6) | 17.4 (15,22.2) | 18.5 (13.7,22.6) | Kruskal-Wallis test | 0.007 |
| G1-G2 | Pairwise Wilcoxon rank-sum tests with Bonferroni correction | 0.03 | |||
| G1-G3 | 0.02 | ||||
| G2-G3 | 1 | ||||
| Gender, F:M | 3 (10%): 27 (90%) | 3 (12%): 22 (88%) | 2 (7%): 25 (93%) | Fisher’s exact test | 0.78 |
| Median (IQR) duration of VKC in months | 36 (24,48) | 34 (24,73.5) | 24 (12,48) | Kruskal-Wallis test | 0.18 |
| Preoperative steroid type | Fisher’s exact test | 0.06 | |||
| Low potent | 6 (13.3) | 1 (2.8) | 0 (0) | ||
| Mixed types | 26 (57.8) | 27 (75) | 28 (82.4) | ||
| Strong steroid | 13 (28.9) | 8 (22.2) | 6 (17.6) | ||
| Median (IQR) duration of steroid (in months) | 32 (12,48) | 25.5 (12,48) | 24 (12,47.5) | Kruskal-Wallis test | 0.52 |
| Median (IQR) LogMAR VA at first visit | 0.5 (0.4,1.3) | 0.7 (0.2,1.4) | 0.9 (0.4,1.3) | Kruskal-Wallis test | 0.59 |
| Median (IQR) LogMAR VA preoperative | 0.5 (0.3,1.2) | 0.4 (0,0.8) | 0.8 (0.5,1.3) | Kruskal-Wallis test | 0.02 |
| G1-G2 | Pairwise Wilcoxon rank-sum tests with Bonferroni correction | 0.60 | |||
| G1-G3 | 0.23 | ||||
| G2-G3 | 0.03 | ||||
| Mean (SD) IOP at first visit | 29.7 (13) | 32 (12.1) | 30.1 (12.9) | ANOVA F-test (2, 112 df) = 0.35 | 0.71 |
| Mean (SD) Preoperative IOP | 32 (8.9) | 29.4 (9.1) | 28.4 (12) | ANOVA F-test (2, 111 df) = 1.38 | 0.26 |
| Mean (SD) highest IOP | 37.6 (7.6) | 42.2 (8.1) | 37.6 (10.6) | ANOVA F-test (2, 112 df) = 3.39 | 0.04 |
| G1-G2 | Pairwise t tests with Bonferroni correction | 0.03 | |||
| G1-G3 | 1 | ||||
| G2-G3 | 0.20 | ||||
| Median (IQR) Number of preoperative AGM | 2 (2,3) | 3 (2,3) | 3 (2,3) | Kruskal-Wallis test | 0.21 |
| Preoperative median (IQR) CDR | 0.9 (0.8,0.9) | 0.9 (0.8,0.9) | 0.9 (0.8,1) | Kruskal-Wallis test | 0.27 |
| Type of VKC | Fisher’s exact test | 0.14 | |||
| Limbal + Tarsal | 39 (87) | 35 (97) | 33 (97) | ||
| Limbal | 5 (11) | 0 (0) | 1 (3) | ||
| Tarsal | 1 (2) | 1 (3) | 0 (0) | ||
| Cataract (PSC) | 25 (55.6) | 20 (55.6) | 34 (100) | ANOVA F-test | <0.001 |
| FU duration years | 4.18 (2,8) | 1.6 (.4,4) | 3.4 (.6,5.4) | Kruskal-Wallis test | 0.01* |
IQR: Interquartile range, SD: Standard deviation, MMC: Mitomycin C, IOP: Intraocular pressure, CDR: Cup-to-disc ratio, AGM: Anti-glaucoma medications, PSC: Posterior subcapsular cataract, FU: Follow-up, VKC: Vernal keratoconjunctivitis, F: Female, M: Male, LogMAR VA: Logarithm of the minimum angle of resolution. Follow-up duration was significantly less (P<0.01) in the trab MMC group compared to that in the trabeculectomy group
Postoperative clinical features: There was good IOP control in all three groups, with median IOP of 11 mm Hg in the trab group, 11 mm Hg in the trabMMC group, and 12 mm Hg in the combined cataract and trab group at last follow-up [Table 2]. The difference in IOP reduction was similar in all three groups at all time points except at 30 months. Pairwise Wilcoxon rank-sum test with Bonferroni correction showed the difference between trab and CT to be statistically significant (P = 0.04). Median number of AGMs decreased significantly (P < 0.001) and were similar in all the three groups (P = 0.31). Two eyes in the trab group and one eye in the trabMMC group required cataract surgery during the follow-up period. Repeat glaucoma surgery was required in 2 eyes in the trab group (1 Ahmed glaucoma valve (AGV), 1 repeat trabMMC; 4%), 1 eye in the trabMMC group (repeat trab MMC; 3%), and 1 eye in the CT group (1 AGV; 3%) for IOP control. There were no intraoperative or postoperative complications in the 3 cohorts. The median postoperative follow-up was 4.18 (2, 8) years in the trab cohort, 1.6 (0.4, 4) years in the trabMMC cohort, and 3.4 (0.6, 5.4) years in the CT cohort. The follow-up was significantly less in the trabMMC cohort compared to that in the trab cohort (P = 0.01).
Table 2.
The postoperative characteristics and IOP control in the three groups
| Clinical Parameter | G1 Trabeculectomy (n=45) Median (IQR) | G2 Trabeculectomy with MMC (n=36) Median (IQR) | G3 Combined surgery (n=34) Median (IQR) | P |
|---|---|---|---|---|
| IOP at 3 months | 12 (10,16) | 12 (11,14) | 12 (10,14) | 0.90* |
| IOP at 6 months | 12 (10,16) | 12 (10,14) | 11 (10.5,15) | 0.84* |
| IOP at 12 months | 12 (10,14) | 11 (10,14) | 12 (11,16) | 0.93* |
| IOP at 18 months | 12.5 (10,18) | 11 (10,12.5) | 12 (10.5,14) | 0.30* |
| IOP at 24 months | 12 (11,16) | 11 (10,12) | 12 (10,16) | 0.21* |
| IOP at 30 months | 11 (10,12) | 12 (10,12) | 12 (12,20.5) | 0.03* |
| IOP at 48 months | 11.7 (2.1) | 12.4 (2.2) | 16 (5.8) | 0.10# |
| IOP at 60 months | 12.9 (3.3) | 10.8 (1.9) | 16.2 (9.9) | 0.37# |
| IOP at last follow up | 11 (10,16) | 12 (10,13.5) | 12 (10,14) | 0.62* |
| AGM at last follow up | 0 (0,0) | 0 (0,1) | 0 (0,1) | 0.31* |
| CDR at last follow up | 0.9 (0.8,0.9) | 0.9 (0.9,0.9) | 0.9 (0.9,1) | 0.15* |
| BCVA at last follow up in LogMAR | 0.2 (0.1,0.6) | 0.2 (0,0.5) | 0.2 (0.1,2.1) | 0.39* |
| Number of eyes blind at last follow-up | 17 (VA: 7, VF10) | 12 (VA: 3, VF: 9) | 17 (VA: 9, VF: 8) | 0.33* |
*Kruskal-Wallis test, #ANOVA F-test. The difference was significant at 30 months. Pairwise Wilcoxon Rank Sum Tests with Bonferroni correction showed the difference between G1 and G3 to be statistically significant (P=0.038). G1-G2 (P=1.0), G2-G3 (P=0.38). IOP: Intraocular pressure; FU: Follow-up, CDR: Cup to disc ratio; AGM: Anti-glaucoma medications; LogMAR: Logarithm of minimal angle of resolution, BCVA: Best-corrected visual acuity, VKC: Vernal keratoconjunctivitis; LFU: Last follow-up; MMC: Mitomycin C, AGV: Ahmed glaucoma valve; IOL: Intraocular lens, ECCE: Extracapsular cataract extraction; VA: Visual acuity, VF: Visual field
Success probability: Figs. 1 and 2 are Kaplan–Meier survival curves showing the cumulative probability of complete success and qualified success after trab, trabMMC, and CT. The complete success at 1 year and 5 years were 84.4% (95% confidence interval: 70 and 92) and 75.6% (59, 86) in the trab group, 80% (60, 90) and 71% (45, 87) in the trab MMC group, and 74.7% (54, 87) and 66% (45, 81) in the CT group. The complete success probabilities were similar in all three groups (P = 0.91). Qualified success at 1 year and 5 years were 97.7% (85, 99) and 88% (72, 95) in the trab group, 96.6% (79, 99) (1–5 years) in the trabMMC group, and 100% and 94% (66, 99) in the CT group. The qualified success probabilities were similar in all three groups, (P = 1.0).
Figure 1.
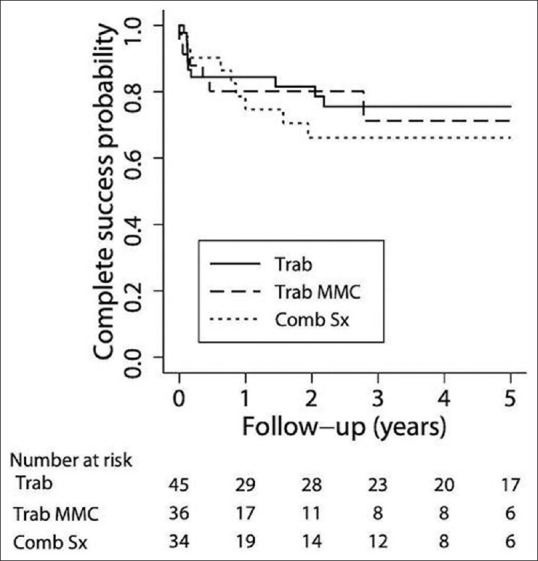
Kaplan–Meier survival curve showing the cumulative probability of complete success following trabeculectomy (group 1), trabeculectomy with MMC (group 2), and combined cataract and glaucoma surgery (group 3)
Figure 2.
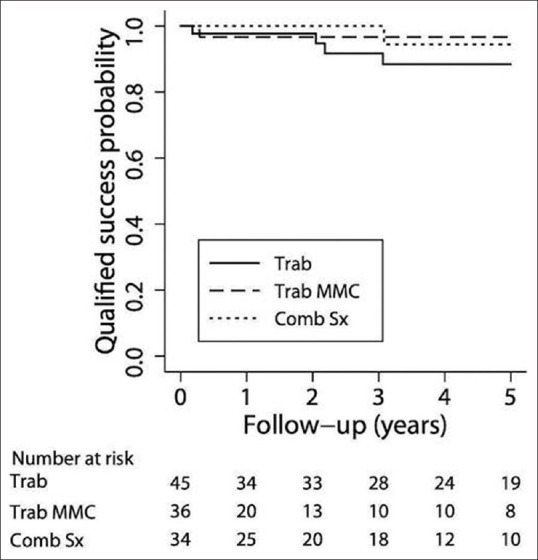
Kaplan–Meier survival curve showing the cumulative probability of qualified success after trabeculectomy (group 1), trabeculectomy with MMC (group 2), and combined cataract and glaucoma surgery (group 3)
Recurrence of VKC and need for postoperative steroids: The activity of VKC during postoperative follow-up was noted in 37% eyes in the trab group, in 39% eyes in the trabMMC group, and in 62% eyes in the CT group. However, severe inflammation needing a short course of topical steroids was noted in 17 eyes (38%) in the trab group, in 7 eyes (19%) in the trabMMC group, and in 8 eyes (23%) in the CT group (P = 0.17). Among those that had postoperative recurrence of active VKC, 6/21 in CT, 7/16 in trab, and 3/14 in trabMMC failed. Among the eyes that needed topical pulse steroid (2–3 weeks of soft steroid) for recurrence of VKC, 3/7 in CT, 4/17 in trab, and 3/8 in trabMMC failed.
Blindness due to glaucoma: Preoperatively, 39 eyes were noted to be blind (both by VA and VF); however, this estimation was a possible underestimation as several of them had high IOP preoperatively and had cataract that affected the visual field and visual acuity estimation. At the last follow-up, there were 46 eyes (40%) blind due to glaucoma. Nineteen eyes were blind by VA criteria, and 27 eyes were blind by visual field criteria. The details are presented in Table 2. Among the groups, 17 eyes (38%) in the trab group, 12 eyes (33%) in the trabMMC group, and 17 eyes (50%) in the CT group were blind due to glaucoma (P = 0.33).
Risk factors for failure of glaucoma surgery: Associations between failure and baseline factors such as age, gender, duration of VKC, duration of steroid use, preoperative IOP, number of preoperative medications, age at surgery, type of steroid, baseline disc damage, type of surgery, and duration of follow-up were assessed using the multivariate logistic regression model. Among the risk factors assessed, older children (P < 0.001), longer duration of VKC (P < 0.001), longer duration of steroid use (P < 0.001), larger CDR (P < 0.02), and mixed type of steroid use (P < 0.001) were significantly associated with qualified failure of trabeculectomy. None of these factors had significant association to predict complete failure of glaucoma surgery in the three cohorts.
Discussion
Our study highlights the outcomes of glaucoma filtering surgery and risk factors for failure in young patients with VKC and steroid-induced glaucoma. In a clinical condition with severe ocular allergy and inflammation, conjunctival procedures have a high risk of failure.[21] Our study showed encouraging results with all three types of surgeries: trabeculectomy, trabeculectomy with MMC, and combined trabeculectomy with cataract surgery. The complete success rate was moderate at 1 year (84%, 80%, and 75%) and at 5 years (76%, 71%, and 66%) following trab, trabMMC, and CT, respectively. The qualified success was good at 1 year (98%, 97%, and 100%) and at 5 years (88%, 97%, and 94%) with trab, trabMMC, and CT, respectively. Hence, when the clinical situation warrants glaucoma filtering surgery alone or cataract surgery to be combined with trabeculectomy, the surgical outcomes are safe and effective; the results of our study support it.
Longer duration of VKC and long-term steroid usage were found to be risk factors for failure of trabeculectomy in the current cohort. Chronic VKC with long-term steroid usage is possibly the reason for advanced glaucoma and high prevalence of blindness in this cohort. Use of mixed type of steroid eye drops (strong and weak steroid in combination or successively) and advanced glaucomatous disc damage were also associated with higher risk of failure. Both these factors probably indicate chronic and severe VKC, which could predispose these eyes to scarring and trabeculectomy failure. Trabeculectomy in young is associated with a higher rate of failure due to increased healing response and subconjunctival scarring;[22,23,24] the risk further increases in young patients with VKC.[21] The two small case series that reported outcomes of trabeculectomy in VKC eyes with SIG did not evaluate risk factors for failure. We compared the risk factors for failure of trabeculectomy in young patients with JOAG eyes from the same population.[25] The younger age (age less than 20 years) was the only factor significantly associated with trabeculectomy failure (P = 0.03); the probability of success increased with increasing age (decade wise). Age was not a significant risk factor in our series as the majority were in the 1–2 decade and probably had a similar risk for failure.
In India, steroid-induced glaucoma is the most prevalent secondary acquired glaucoma in children and the majority is contributed by VKC.[17,18,26] Tropical climate, chronic course of VKC with seasonal exacerbations of ocular allergy, symptomatic relief with steroid eye drops, and easy access to over-the-counter steroid eye drops predispose these eyes to long-term steroid use and their side effects such as glaucoma and posterior subcapsular cataract. The prevalence of steroid-induced glaucoma in VKC eyes was 2.24% as reported from a large south Indian cohort.[7] Among the SIGs, more than 1/3 (34% eyes) needed glaucoma surgery for IOP control. The risk factors that predicted the need for glaucoma surgery were high IOP at presentation and longer duration of steroid use,[7] which seem to concur with severity and chronicity of VKC as well. The need for glaucoma surgery in eyes with SIG from mixed cohorts varied from 5% to 26.5%.[16,27]
Majority of the eyes in the VKC cohort from same population had advanced glaucoma, with close to 32% eyes bilaterally blind at presentation.[7] The asymptomatic nature of glaucoma and lack of access or delay in seeking eye care contributes to advanced glaucoma in the majority of these young patients, as is also seen in the current study.
There is limited literature on outcomes of glaucoma surgery in eyes with steroid-induced glaucoma and further sparse in VKC eyes with SIG. The studies that evaluated outcomes of glaucoma surgery in VKC eyes are small case series with short-term outcomes. Honjo et al.[19] evaluated outcomes of external trabeculotomy in 14 eyes of seven patients with SIG and reported good outcome (83.6%, with follow-up period ranging 15–120 months). Although a small series, Ang et al.[20] reported good success following trabeculectomy with MMC in eight eyes of six patients with VKC. Success was reported in seven out of eight eyes, and one eye needed repeat trabeculectomy after 18 months of primary surgery. The authors reported postoperative improvement in the ocular surface, decrease in VKC activity, and corneal epitheliopathy with improvement in visual acuity. The authors postulated that MMC at 0.02% concentration, when applied directly, inhibited the fibroblasts on the ocular surface sufficiently to reduce the signs and symptoms of VKC. There was no difference in the recurrence of VKC activity post trabeculectomy with or without the use of MMC (37% vs. 39%) in our study.
Recurrent episodes of active inflammation in the postoperative period may predispose these eyes to scarring and failure of trabeculectomy; this, along with the need for topical steroids to control the inflammation, may result in elevated IOP. In our study, 20%–40% of the eyes needed pulse topical steroids in the postoperative period. We evaluated if the VKC activity or pulse steroid use were risk factors for failure; they did not seem to affect the success of trabeculectomy in this cohort. Close monitoring of IOP and VKC activity are important to help with early recognition of disease activity and appropriate treatment. Pulse treatment with topical steroids helps to control the inflammation and may prevent conjunctival scarring and bleb failure.
Limitations of our study include its retrospective nature, lack of availability of bleb morphology details, and a nonrandomized design with the choice of surgery being surgeon-dependent. In children, we do not use MMC routinely with primary trabeculectomy. We cannot rule out selection bias that MMC was used in eyes with more severe ocular allergy. This could explain why there was no significant difference in survival in the MMC group compared to that in the no-MMC group.
The fact that SIG is severe, with a significant proportion of them irreversibly blind due to glaucoma from a preventable cause, emphasizes the need for patient and parent education, advocacy to avoid over-the-counter sale, and unmonitored use of topical steroids.
Conclusion
In eyes with refractory steroid-induced glaucoma, glaucoma surgery can help control the IOP and prevent blindness. Glaucoma filtering surgery seems to have reasonable long-term success in these eyes even when combined with cataract surgery. Long-term follow-up to identify and treat the acute exacerbations of VKC and monitor the IOP is recommended.
Ethics approval
(Ethics Ref No LEC-BHR-R-07-21-705).
Financial support and sponsorship
Hyderabad Eye Research Foundation.
Conflicts of interest
There are no conflicts of interest.
References
- 1. Buckley RJ. Allergic eye disease--A clinical challenge. Clin Exp Allergy. 1998;28((Suppl 6)):39–43. doi: 10.1046/j.1365-2222.1998.0280s6039.x. [DOI] [PubMed] [Google Scholar]
- 2. Bonini S, Bonini S, Lambiase A, Marchi S, Pasqualetti P, Zuccaro O, et al. Vernal keratoconjunctivitis revisited:A case series of 195 patients with long-term followup. Ophthalmology. 2000;107:1157–63. doi: 10.1016/s0161-6420(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 3. Ang M, Ti SE, Loh R, Farzavandi S, Zhang R, Tan D, et al. Steroid-induced ocular hypertension in Asian children with severe vernal keratoconjunctivitis. Clin Ophthalmol. 2012;6:1253–8. doi: 10.2147/OPTH.S32936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberti G, Oddone F, Agnifili L, Katsanos A, Michelessi M, Mastropasqua L, et al. Steroid-induced glaucoma:Epidemiology, pathophysiology, and clinical management. Surv Ophthalmol. 2020;65:458–72. doi: 10.1016/j.survophthal.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 5. Leonardi A, Busca F, Motterle L, Cavarzeran F, Fregona IA, Plebani M, et al. Case series of 406 vernal keratoconjunctivitis patients:A demographic and epidemiological study. Acta Ophthalmol Scand. 2006;84:406–10. doi: 10.1111/j.1600-0420.2005.00622.x. [DOI] [PubMed] [Google Scholar]
- 6. Leonardi A. Management of vernal keratoconjunctivitis. Ophthalmol Ther. 2013;2:73–88. doi: 10.1007/s40123-013-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Senthil S, Thakur M, Rao HL, Mohamed A, Jonnadula GB, Sangwan V, et al. Steroid-induced glaucoma and blindness in vernal keratoconjunctivitis. Br J Ophthalmol. 2020;104:265–9. doi: 10.1136/bjophthalmol-2019-313988. [DOI] [PubMed] [Google Scholar]
- 8. Saboo US, Jain M, Reddy JC, Sangwan VS. Demographic and clinical profile of vernal keratoconjunctivitis at a tertiary eye care center in India. Indian J Ophthalmol. 2013;61:486–9. doi: 10.4103/0301-4738.119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones R, 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma:A brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163–7. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 10. Gilbert C, Foster A. Causes of blindness in children attending four schools for the blind in Thailand and the Philippines. A comparison between urban and rural blind school populations. Int Ophthalmol. 1993;17:229–34. doi: 10.1007/BF01007745. [DOI] [PubMed] [Google Scholar]
- 11. Kersey JP, Broadway DC. Corticosteroid-induced glaucoma:A review of the literature. Eye. 2006;20:407–16. doi: 10.1038/sj.eye.6701895. [DOI] [PubMed] [Google Scholar]
- 12. Sen P, Jain S, Mohan A, Shah C, Sen A, Jain E. Pattern of steroid misuse in vernal keratoconjunctivitis resulting in steroid induced glaucoma and visual disability in Indian rural population:An important public health problem in pediatric age group. Indian J Ophthalmol. 2019;67:1650–5. doi: 10.4103/ijo.IJO_2143_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohan R, Muralidharan AR. Steroid induced glaucoma and cataract. Indian J Ophthalmol. 1989;37:13–6. [PubMed] [Google Scholar]
- 14. Tabbara KF. Ocular complications of vernal keratoconjunctivitis. Can J Ophthalmol. 1999;34:88–92. [PubMed] [Google Scholar]
- 15. Dikshit SK, Avasthi PN. Posterior lenticular opacities in children on corticosteroid therapy. Indian J Pediatr. 1965;32:93–6. doi: 10.1007/BF02756568. [DOI] [PubMed] [Google Scholar]
- 16. Sihota R, Konkal VL, Dada T, Agarwal HC, Singh R. Prospective, long-term evaluation of steroid-induced glaucoma. Eye. 2008;22:26–30. doi: 10.1038/sj.eye.6702474. [DOI] [PubMed] [Google Scholar]
- 17. Gupta S, Shah P, Grewal S, Chaurasia AK, Gupta V. Steroid-induced glaucoma and childhood blindness. Br J Ophthalmol. 2015;99:1454–6. doi: 10.1136/bjophthalmol-2014-306557. [DOI] [PubMed] [Google Scholar]
- 18. Kaur S, Dhiman I, Kaushik S, Raj S, Pandav SS. Outcome of ocular steroid hypertensive response in children. J Glaucoma. 2016;25:343–7. doi: 10.1097/IJG.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 19. Honjo M, Tanihara H, Inatani M, Honda Y. External trabeculotomy for the treatment of steroid-induced glaucoma. J Glaucoma. 2000;9:483–5. doi: 10.1097/00061198-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 20. Ang M, Ho CL, Tan D, Chan C. Severe vernal keratoconjunctivitis requiring trabeculectomy with mitomycin C for corticosteroid-induced glaucoma. Clin Exp Ophthalmol. 2012;40:e149–55. doi: 10.1111/j.1442-9071.2011.02591.x. [DOI] [PubMed] [Google Scholar]
- 21. Broadway DC, Chang LP. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma. 2001;10:237–49. doi: 10.1097/00061198-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 22. D'Ermo F, Bonomi L, Doro D. A critical analysis of the long-term results of trabeculectomy. Am J Ophthalmol. 1979;88:829–35. doi: 10.1016/0002-9394(79)90559-2. [DOI] [PubMed] [Google Scholar]
- 23. Levene RZ. Glaucoma filtering surgery factors that determine pressure control. Trans Am Ophthalmol Soc. 1984;82:282–301. [PMC free article] [PubMed] [Google Scholar]
- 24. Borisuth NS, Phillips B, Krupin T. The risk profile of glaucoma filtration surgery. Curr Opin Ophthalmol. 1999;10:112–6. doi: 10.1097/00055735-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 25. Pathania D, Senthil S, Rao HL, Mandal AK, Garudadari CS. Outcomes of trabeculectomy in juvenile open angle glaucoma. Indian J Ophthalmol. 2014;62:224–8. doi: 10.4103/0301-4738.101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Senthil S, Badakere S, Ganesh J, Krishnamurthy R, Dikshit S, Choudhari N, et al. Profile of childhood glaucoma at a tertiary center in South India. Indian J Ophthalmol. 2019;67:358–65. doi: 10.4103/ijo.IJO_786_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inatani M, Iwao K, Kawaji T, Hirano Y, Ogura Y, Hirooka K, et al. Intraocular pressure elevation after injection of triamcinolone acetonide:A multicenter retrospective case-control study. Am J Ophthalmol. 2008;145:676–81. doi: 10.1016/j.ajo.2007.12.010. [DOI] [PubMed] [Google Scholar]


