Abstract
The influence of pH on the adhesion of two Lactobacillus strains to Caco-2 human intestinal cells was investigated. One strain, Lactobacillus johnsonii La1, was adherent at any pH between 4 and 7. The other one, L. acidophilus La10, did not attach to this cell line under the same experimental conditions. On the basis of these results, we used the monoclonal antibody technique as a tool to determine differences on the surface of these bacteria and to identify a factor for adhesion. Mice were immunized with live La1, and the hybridomas produced by fusion of spleen cells with ONS1 cells were screened for the production of antibodies specific for L. johnsonii La1. A set of these monoclonal antibodies was directed against a nonproteinaceous component of the L. johnsonii La1 surface. It was identified as lipoteichoic acid (LTA). This molecule was isolated, chemically characterized, and tested in adhesion experiments in the same system. The adhesion of L. johnsonii La1 to Caco-2 cells was inhibited in a concentration-dependent way by purified LTA as well as by L. johnsonii La1 culture supernatant that contained LTA. These results showed that the mechanism of adhesion of L. johnsonii La1 to human Caco-2 cells involves LTA.
Lactobacilli are normal inhabitants of the human gastrointestinal tract (48). Lactobacillus is a subdominant genus in the colon, where it is largely outnumbered by other genera, such as Bacteroides and Bifidobacterium. In contrast, lactobacilli are among the dominant bacteria in the small bowel, although at much lower levels than in the colon (45, 48). In addition, they are of industrial interest in the manufacture of fermented milk. Some specially selected strains recently have been introduced in products for which health claims have been made (36). The beneficial biological effects attributed to these strains are the promotion of colonization, metabolic activities beneficial to host health, and immune stimulation of the host (25). Of particular importance is the potential of probiotics to reinforce mucosal defense, especially at the gastric and small bowel levels (9). However, little is known about the precise mechanisms by which lactobacilli exert their biological effects in vivo.
Among the properties exerted by lactobacilli, adhesion to enterocytes is a key feature of probiotic bacteria. In the small bowel, this property may contribute to the creation of a transient barrier effect. It is currently not known by which mechanism(s) lactobacilli colonize the gastrointestinal tract. The capacity of lactobacilli to adhere to the intestinal epithelium remains controversial. This property is important, since it prevents their elimination by peristalsis and thus represents an ecological competitive advantage in the gastrointestinal tract ecosystem. Evidence that adhesion to intestinal epithelial cells is biologically relevant and important for the establishment of the bacteria in the gut ecosystem has been obtained in recent studies (2, 32). Despite recent in vitro experiments with cultured human intestinal cell lines as models of mature enterocytes of the small intestine (7, 12, 17, 22), bacterial cell surface-associated factors of lactobacilli potentially acting as adhesins remain to be characterized. Different mechanisms have been proposed, and it seems that the adhesion of lactobacilli and bifidobacteria does not depend on a unique and ubiquitous mechanism. Some Lactobacillus strains have the abilities to hemagglutinate human erythrocytes (47) and to bind to mannose (1), to rat colonic mucins (40, 50), or to glycolipids isolated from rat intestinal mucosa (59).
Mechanisms involving proteins or proteinaceous components as mediators of adhesion have been described for some lactobacillus strains, including Lactobacillus johnsonii La1 (7), L. acidophilus BG2F04 (17) and LB12 (12), L. fermentum 104 (28), and L. crispatus JCM 5810 (53). The importance of lipoteichoic acid (LTA) as a mediator of the adhesion of Lactobacillus spp. or other bacteria to human epithelial cells also has been demonstrated (5, 11, 41, 42, 47, 51, 52). The adhesion of Lactobacillus spp. to epithelial cells also depends on bacterial physiology and physicochemical parameters, which can be modulated by growth conditions (19, 39, 43). Finally, it appears that in vitro the pH of the adherence assay may be of crucial importance, as reported recently (27, 28).
In this study, we examined at a molecular level the mechanism by which L. johnsonii La1, which has antipathogenic effects in vitro and in vivo, adheres to cultured human intestinal cells (7, 8). We report data showing that LTA present at the bacterial cell surface is involved in the adhesion of L. johnsonii La1 to Caco-2 intestinal cells. In addition, we show that La1 adhesion, although influenced by pH, occurs at any pH between 4 and 7.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. johnsonii La1, Lj1, and La16 and L. acidophilus La10 and La27 were grown under anaerobic conditions in De Man-Rogosa-Sharpe (MRS) broth (Difco) overnight at 37°C when used for the preparation of antigenic material or for 48 h when used for the adhesion assay. All lactobacilli were from the Nestlé Culture Collection (Lausanne, Switzerland).
Preparation of bacterial surface proteins by guanidinium hydrochloride extraction.
An overnight culture of 40 ml of bacteria was prepared and centrifuged for 10 min at 4,000 × g and 4°C. The pellet was washed once with cold phosphate-buffered saline (PBS) (pH 7.2) and then resuspended in 5 M guanidinium hydrochloride (pH 7) (1 ml/10 mg [wet weight]). The supernatant fluid was dialyzed overnight against running water at 4°C in dialysis tubes with a cutoff of 12,000 to 14,000 daltons and lyophilized.
Preparation of bacterial surface antigens by Triton X-100 extraction.
An overnight culture of 40 ml of bacteria was prepared, centrifuged at room temperature for 10 min at 4,000 × g, and resuspended in PBS (1 ml/100 mg of bacterial pellet). The suspension was placed in ice and sonicated six times for 30 s each time at 75 W with a Microson ultrasonic cell disruptor (Heat Systems Ultrasonics). Phenylmethylsulfonyl fluoride and Triton X-100 (Sigma) were then added to final concentrations of 5 mM and 1%, respectively. The lysate was strongly vortexed, kept overnight at 4°C, centrifuged, and stored at −20°C.
Preparation of monoclonal antibodies against living La1 bacteria and/or La1 surface proteins.
BALB/c mice were injected subcutaneously in the lymph nodes with either 0.1 mg of La1 surface proteins prepared by guanidinium HCl extraction or 0.1 ml of packed living La1 cells two times at a 1-week interval. Three days after the second injection, the spleens were removed and squeezed to a suspension, which was mixed with NS1 cells (American Type Culture Collection). Fusion with polyethylene glycol was performed as described previously (26).
Screening for antibodies against bacterial surface proteins.
Maxisorb polyvinyl wells (Nunc) were coated with 100 μl of a bacterial surface protein solution (10 μg/ml) overnight at 4°C. After saturation with 250 μl of a solution (10 mg/ml) of gelatin in 0.1 M Tris-HCl buffer (pH 8), the wells were incubated for 2 h with 100 μl of hybridoma cell culture supernatant fluid (SN). After three washings with PBS (pH 7.2) containing 0.05% Tween 20 (PBS-Tween), 100 μl of goat anti-mouse total immunoglobulin (Ig) coupled to biotin (Dako Diagnostic) and diluted in PBS-Tween containing 10 mg of bovine serum albumin (BSA) per ml was added, followed by avidin peroxidase (Dako Diagnostic) as described by the manufacturer. Enzymatic activity bound to the wells was revealed with a TMB (tetramethyl benzidine) substrate kit (Pierce) and measured at 450 nm.
Screening for antibodies against living bacteria.
Bacteria (L. johnsonii and L. acidophilus strains) from overnight cultures were centrifuged, washed two times with PBS (pH 7.2), and adjusted to an optical density at 650 nm (OD650) of 10 in PBS. For coating, 0.3 ml of this suspension was diluted to a final volume of 10 ml with a carbonate-bicarbonate buffer (pH 9.6) (coating buffer). Maxisorb polyvinyl wells were coated overnight at 4°C with 100 μl of this suspension. After saturation with gelatin, bacteria on the wells were incubated for 2 h at room temperature on a rotating shaker with 100 μl of SN. After three washings with PBS-Tween, the wells were incubated for 1 h at room temperature with a 1/1,000 solution in PBS-Tween of goat anti-mouse total Ig coupled to alkaline phosphatase (Sigma). After three washings with the same buffer, the substrate p-nitrophenyl phosphate (Sigma) dissolved in the appropriate buffer was added to the wells. Readings of enzymatic activity were made at 405 nm after 2 h at 37°C with a Dynatech MR 5000 microtiter plate reader.
Immunoblotting experiments.
Each bacterial surface protein preparation or Triton X-100 extract (10 μg) was separated on sodium dodecyl sulfate–4 to 20% gradient polyacrylamide gels (Bio-Rad) under reducing and nonreducing conditions (35) and blotted onto nitrocellulose filters (Schleicher & Schüll). After saturation with 10 mg of BSA per ml in 0.1 M Tris-HCl (pH 7.5)–0.15 M NaCl–0.1% Tween 20 (buffer I), membranes were incubated with each of the SNs showing binding activity toward La1 surface proteins in an enzyme-linked immunosorbent assay (ELISA). They were then washed three times for 15 min each time with the same buffer and incubated for 1 h with a 1/2,000 dilution in buffer I of goat anti-mouse Ig coupled to peroxidase; the buffer also contained 10 mg of BSA per ml. After three more washings, membranes were incubated with a chemiluminescent substrate (ECL kit; Amersham). Chemiluminescent bands were revealed on film (667 iso 3000; Polaroid).
Electron microscopy.
For transmission electron microscopy, bacteria in the stationary phase were collected by centrifugation at 4,000 × g for 10 min at 4°C. They were fixed overnight with 0.1% glutaraldehyde–2% formaldehyde in 0.1 M phosphate buffer (pH 7.4) and then washed with phosphate buffer. The cells were embedded in 2% warm agar and, after solidification, small pieces were cut with a razor blade and dehydrated stepwise in ethanol (50 to 100%) at room temperature. The agar blocks were infiltrated with L. R. White resin (hard) and cut into ultrathin sections (60 to 80 nm) with a Reichert ultramicrotome, and the sections were floated on 1% bovine albumin–0.2% polyethylene glycol (Carbowax 20-M; Union Carbide) in 50 mM Tris-HCl (pH 8.0). The sections were incubated with SNs and controls (culture medium) overnight at 4°C. After a wash in Tris-HCl buffer, labeling was performed with goat anti-mouse Ig–gold conjugate (10 nm; Bio Cell) for 3 h at 4°C. The sections were washed with Tris-HCl buffer containing 0.2% polyethylene glycol and examined in a Philips CM 12 electron microscope at an acceleration voltage of 60 kV. For preembedding labeling, washed bacteria were incubated with antibodies for 1 h at 0°C, washed three times with PBS, and treated as described above.
Detection of lysate antigenic activity.
Bacterial lysates containing 0.14 mg of protein and 0.2% Triton X-100 were adjusted to a 1.1-ml volume with coating buffer and loaded onto a calibrated Superose HR 6 column (Pharmacia) equilibrated with the same buffer. Elution was done at a flow rate of 0.4 ml/min/fraction. The contents of each fraction were diluted four times in coating buffer and used to coat polyvinyl wells (100 μl/well). Detection of antigenic activity was done by incubating each fraction with different monoclonal antibodies followed by the secondary antibody as described above.
Purification of LTA from whole La1 bacteria.
LTA was purified as described by Fisher et al. (23). One liter of an overnight culture of La1 bacteria was centrifuged for 20 min at 4,000 × g and room temperature, and the pellet was resuspended in 0.1 M sodium acetate buffer (pH 4.5) at 800 mg (wet weight)/ml of buffer. Bacteria were defatted by being mixed with 2 volumes of methanol and 1 volume of chloroform overnight at room temperature. They were recovered by filtration, washed with 2 volumes of methanol, and resuspended in 20 ml of 0.1 M sodium acetate buffer (pH 5). The suspension was mixed with an equal volume of 80% aqueous phenol and stirred constantly for 45 min in a water bath at 65°C. The emulsion formed after cooling was broken by centrifugation at 5,000 × g and 4°C for 30 min. The upper phase was collected and extensively dialyzed against 0.1 M acetate (pH 5).
Nucleic acids were digested for 24 h at room temperature by the addition of 5 μg of DNase (Sigma) per ml and 50 μg of RNase A (Sigma) per ml. Toluene (1 ml) was added to the tube to prevent bacterial contamination. The preparation was then concentrated to 5 ml by rotary evaporation at room temperature. One-milliliter aliquots were passed through a Millipore filter (0.2-μm-pore size) and loaded onto a Superose HR 6 column equilibrated with 0.1 M acetate buffer (pH 4.7). Fractions were collected at a flow rate of 0.4 ml/min/fraction and assayed for antigenic activity as described for the Triton X-100 extract of La1 bacteria. The active fractions were concentrated by rotary evaporation, equilibrated in 0.1 M acetate buffer (pH 4.7) containing 0.05% Triton X-100, and applied to a DEAE-Sephacel column (Pharmacia). Elution was done with this buffer and a gradient of 0 to 0.5 M NaCl. Each fraction was analyzed for its content of total neutral sugars (20) and phosphorus (13). Removal of Triton X-100 from LTA was done by passage of purified LTA through an HR 6 column equilibrated with 0.1 M ammonium bicarbonate buffer (pH 5).
Detection of LTA in La1 culture supernatant fluids.
L. johnsonii La1 was cultivated to the stationary phase and centrifuged as described above. The spent culture supernatant fluid (SCS) was adjusted to pH 5 with 1 M NaOH, diluted 1/1 with 0.1 M ammonium bicarbonate buffer (pH 8.8), and loaded batchwise onto a Superose HR 6 column. Antigenic activity was detected as described before. Positive fractions were lyophilized for further studies.
Composition of the antigenic complex recognized by monoclonal antibodies.
Total neutral sugar content was measured as described by Dubois et al. (20). Phosphorus content was estimated as described by Chen et al. (13). For quantitative analysis of carbohydrates, 50 μg of total neutral sugars was hydrolyzed in 1.5 ml of 4 N trifluoroacetic acid (Fluka) for 1 h at 125°C. The solution was evaporated under a stream of nitrogen in a heating block at 45°C. The hydrolyzed material was dissolved in 0.5 ml of Milli-Q water and passed through a Millipore filter (0.2-μm-pore size). Twenty-five microliters was injected into a CarboPac PA1 column of a Dionex carbonate system with amperometric detection (carbohydrate potentials on ED40) and analyzed by the methods described by the manufacturer.
Ribitol and glycerol were detected on the same apparatus connected to a CarboPac MA1 column. Alcohol sugars were eluted under isocratic conditions with 0.48 M NaOH at a flow rate of 0.4 ml/min.
Qualitative analysis of hydrolysate carbohydrate content was done with thin-layer chromatography silica plates. One microliter of hydrolysate containing 1 μg of total neutral sugars was loaded together with known standards and chromatographed with CHCl3-AcOH acid-H2O buffer (30:35:5). Detection was done by immersion in lead tetraacetate-sodium fluorescein reagent (49).
For quantitation of fatty acids, pentadecanoic acid (1 μg) was added to the preparations as an internal standard. After hydrolysis with 2 M HCl for 2.5 h at 100°C, HCl was evaporated under nitrogen in a heating block at 45°C. The dried material was then treated as described by Lepage and Roy (37) and analyzed by gas chromatography-mass spectrometry with a Finnigan SSQ-7000 mass spectrometer connected to an HP-5890 gas chromatograph (Hewlett-Packard).
For amino acid analysis, an aliquot of the preparations was evaporated and hydrolyzed in 200 μl of a 0.5% phenol solution in 6 N HCl for 24 h at 110°C. Free α-amino acids were analyzed by precolumn derivatization with phenylisothiocyanate as described by Bindlingmeyer et al. (8a).
Cell culture.
Caco-2 intestinal cells (American Type Culture Collection) were used between passages 40 and 90. Cells were routinely grown in Dulbecco modified Eagle minimal essential medium (25 mM glucose) (Gibco BRL) supplemented with 20% heat-inactivated (30 min, 56°C) fetal calf serum (Boehringer) and 1% nonessential amino acids (Gibco BRL) as previously described (7, 8, 12, 15–17). Cells were cultured at 37°C in a 10% CO2–90% air atmosphere. The culture medium was changed every 2 days. Cells were used at postconfluence after 15 days of culturing (fully differentiated cells) (44). NS1 myeloma cells and hybridoma cells were cultivated as described by Granato and Piguet (26).
Adhesion of L. johnsonii La1 and L. acidophilus La10 to Caco-2 cells.
La1 and La10 bacteria were grown in MRS broth for 48 h in the presence of 10 μCi of tritiated adenine per ml (2.0 Ci/mmol; Amersham). For both bacteria, the specific radioactivities obtained were in the range of 10−2 to 10−3 cpm/CFU. The bacteria were washed three times with PBS (pH 7.2) and resuspended in different buffers at a concentration of 108 CFU/ml at pHs ranging from 3 to 7 (0.5 pH unit/step). For pH 5.5 to 7, 7.5 mM phosphate buffer–138 mM NaCl–3 mM KCl was used, as suggested by Greene and Klaenhammer (27). For pH 3.5 to 5, 10 mM sodium acetate–138 mM NaCl–3 mM KCl was used. One milliliter of bacterial suspension was incubated at 37°C for 1 h on Caco-2 cells (six-well plates) which had been previously washed with the buffers at the respective pHs. Afterward, supernatant fluids were removed and cells were washed three times with the buffers at the respective pHs. Cell monolayers were disrupted by the addition of 1 ml of 1 M NaOH. The lysate and a 0.5-ml wash sample were transferred to a counting vial containing 1 ml of benzethonium hydroxide (Sigma). The vial was placed in an incubator at 60°C for 1 h and cooled, and counts were determined after the addition of 10 ml of Ultima Gold (Packard). Statistical evaluation of adherence data was performed with a paired t test.
For adhesion inhibition assays (7, 15), Caco-2 cells were preincubated for 1 h at 37°C with 1 ml of inhibitor diluted in acetate buffer (pH 5). After one wash with the same buffer, radiolabeled La1 cells resuspended in pH 5 buffer were added as described above. MRS broth acidified with lactic acid to pH 5 was added as a control for La1 SCS. Statistical evaluation of adherence data was performed with a paired t test.
Measurement of cell integrity.
In each experiment, cell integrity was determined by measuring lactate dehydrogenase in postadhesion assay culture medium (Enzyline LDH kit; Biomérieux).
RESULTS
Monoclonal antibodies from mice immunized with L. johnsonii La1 surface proteins.
SNs were analyzed in an ELISA for binding to L. johnsonii and L. acidophilus surface proteins extracted with guanidinium hydrochloride. All the SNs binding to La1 surface proteins cross-reacted with the surface proteins of the other strains. This result was confirmed in blotting experiments in which a major protein with an apparent molecular mass of 46 kDa was recognized on all blots (data not shown). The positive SNs were also tested for binding to living La1 coating polyvinyl wells. Almost no activity was detected against the native determinants of the surface of these bacteria.
Monoclonal antibodies against living L. johnsonii La1.
Antibodies from 14 wells were reactive against living L. johnsonii strains but not against the two strains of L. acidophilus. The antibody activity could be divided into two categories. The first one recognized L. johnsonii surface proteins. The second one recognized the three strains of L. johnsonii in an ELISA or in suspension but did not bind to their surface proteins in Western blotting or ELISA experiments, suggesting that the antigenic determinant was not carried by a protein. We focused our attention on this latter category and selected hybridoma cells secreting IgM antibodies (clone 113D2b3) and IgG antibodies (clone 116A3b3). Both types of monoclonal antibodies were used in parallel to identify the antigenic moiety recognized on strains La1, La16, and Lj1.
Electron microscopy.
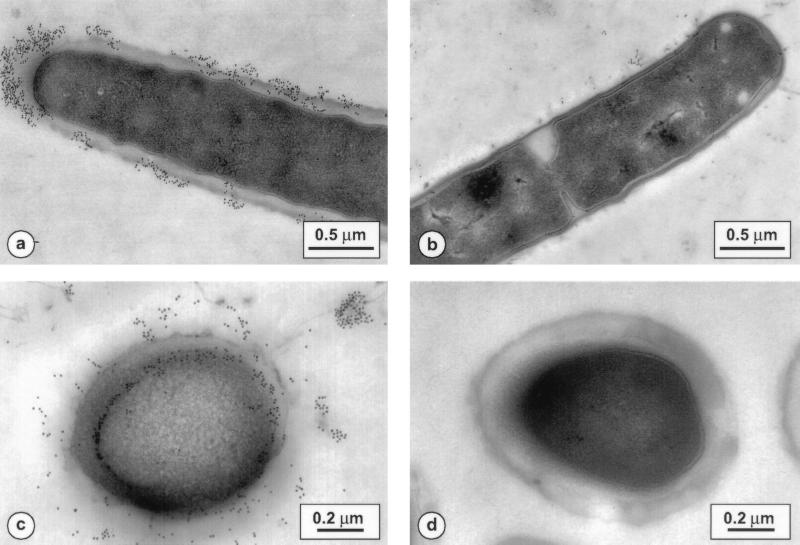
L. acidophilus and L. johnsonii fixed sections were incubated with SNs from 113D2b3 and 116A3b3 hybridoma cells, respectively. The control was hybridoma culture medium. Results were the same for both types of monoclonal antibodies. As shown in Fig. 1a, c, and e, the antibody from 113D2b3 was bound to antigenic moieties localized on the outer surface of L. johnsonii, in the wall, and inside the cytoplasmic membrane. In some cases, the molecules recognized by the antibody seemed to be dispersed in patches on the surface of L. johnsonii. There was no significant difference in staining between La1 and Lj1 bacteria. As shown in Fig. 1b for La10, there was no staining with the different L. acidophilus strains. The same staining pattern was observed with pre- or postembedding labeling of bacteria.
FIG. 1.
Immunogold staining of thin sections. (a) L. johnsonii La1 treated with monoclonal antibodies from the 113D2b3 clone supernatant. (b) L. acidophilus La10 incubated with the same antibodies. (c and e) L. johnsonii Lj1 incubated with the 113D2b3 clone supernatant before embedding. (d) L. johnsonii La1 incubated with hybridoma culture medium.
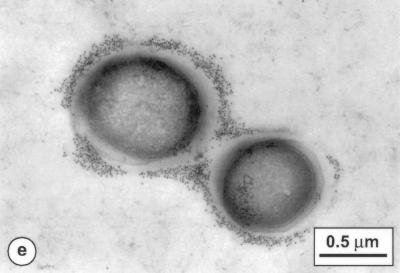
Detection of the antigenic activity recognized by monoclonal antibodies from clones 113D2b3 and 116A3b3.
Fractions from Superose HR 6 chromatography of an La1 surface protein Triton X-100 extract were analyzed for binding to the monoclonal antibodies described above. As shown in Fig. 2, two main peaks of antigenic activity were detected. They corresponded to molecular masses of approximately 600 and 1,000 to 2,000 kDa. An identical pattern was observed for the L. johnsonii Lj1 extract (data not shown). When eluted with 0.1 M ammonium bicarbonate buffer (pH 8.8), the same Triton X-100 extract gave a single peak reacting with both monoclonal antibodies and eluting with an apparent molecular mass of 106 Da.
FIG. 2.
Detection of antigenic activity of L. johnsonii La1 Triton X-100 extract by chromatography on Superose HR 6. Molecular mass markers: 2,000 kDa, fraction 20; 750 kDa, fraction 30; 150 kDa, fraction 38; 67 kDa, fraction 43. OD 450, optical density at 450 nm.
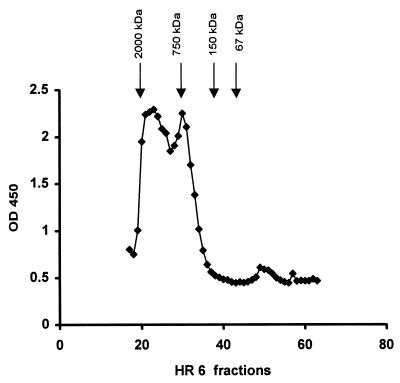
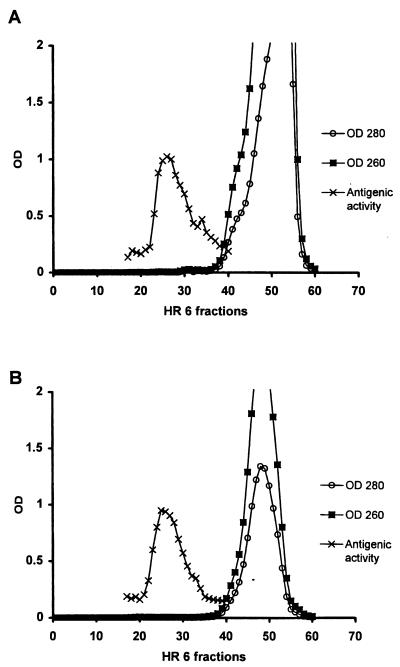
Purification of LTA from whole L. johnsonii La1.
LTA was purified as described in Materials and Methods. The peak of antigenic activity corresponded to the one from a Triton X-100 extract eluted with 0.1 M ammonium bicarbonate buffer but not to an absorption peak at 260 or 280 nm (Fig. 3A). This result excluded contamination from proteins and nucleic acids. The last purification step was done by anion-exchange chromatography with a linear NaCl gradient. The antigenic activity was eluted at 0.1 to 0.2 M NaCl. The active fractions were lyophilized for further analysis. The yield from 1 liter of culture was approximately 4 mg (2.4 mg of total neutral sugars).
FIG. 3.
Detection of antigenic activity of the aqueous fraction of delipidated L. johnsonii La1 after phenol extraction and nucleic acid digestion (A) or La1 culture supernatant (B) by chromatography on Superose HR 6. OD, optical density (numbers with OD are nanometers).
Detection of LTA in La1 SCS.
La1 SCS (0.5 ml) taken at the stationary phase and adjusted to pH 5 was mixed with 0.6 ml of 0.1 M ammonium bicarbonate buffer and loaded onto a Superose HR 6 column under the same conditions as those described before. As shown in Fig. 3B, analysis of the HR 6 fractions by an ELISA showed that the antigenic activity recognized by the monoclonal antibodies eluted as described for LTA purified from defatted La1 bacteria (Fig. 3A) and the Triton X-100 extract (Fig. 2). Figure 3B also shows that the material bound by the antibodies again did not show an absorption peak at 260 or 280 nm. An estimation of the concentration of LTA in La1 SCS was made by comparison of its reactivity in the same ELISA. It seemed that the amount of LTA present in 1 liter of La1 supernatant taken at the stationary phase was at least 10 times higher than that found in pelleted bacteria.
LTA analysis.
Chemical analysis of the pure product showed the presence of phosphorus, glycerol, alanine, glucose, galactose, and fatty acids at proportions of 1, 0.35, 0.5, 0.28, 0.18, and 0.14, respectively, which corresponded to data obtained for LTA from other lactobacilli (23). d-Glucose and d-galactose were present at a ratio of 3:2. Phosphorus, glycerol, hexoses, alanine, and fatty acids were also detected in the La1 SCS LTA fraction. In contrast to the results for pure LTA, N-acetylglucosamine, N-acetylgalactosamine, and mannose were also found in the hydrolysate of the peak of antigenic activity from La1 SCS. They probably originated from the culture medium and from the presence of an La1 polysaccharide. Analysis of amino acids revealed only alanine, which excluded contamination of the micelles of LTA with peptidoglycan or proteins. Analysis of fatty acids showed the presence of C12:0, C14:0, C16:0, C18:1, and C18:0 at proportions of 0.6, 1, 5.8, 6.6, and 5, respectively.
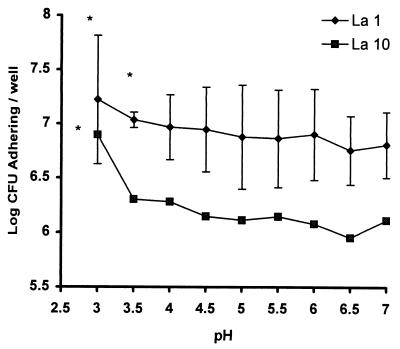
Influence of pH on the adhesion of La1 and La10 to Caco-2 cells.
It was recently reported that pH influences the binding of lactobacilli to cultured human intestinal Caco-2 cells (27). In order to improve our understanding of Lactobacillus adhesion, a set of experiments under different pH conditions was conducted. As shown in Fig. 4, there was a significant difference in adhesion between L. acidophilus La10 and L. johnsonii La1. The adhesion of L. johnsonii La1 was not pH specific, since L. acidophilus La10 remained nonadherent at any pH of >4. On the other hand, L. johnsonii La1 was adherent over the entire pH range tested. As predicted, an acidic pH seemed to favor the adhesion of La1, although the difference in adhesion between pHs 4 and 7 was not statistically significant. Cell viability controls were performed with lactate dehydrogenase cell release. No significant loss of viability at a pH of >4 could be demonstrated under the conditions used in these experiments (data not shown).
FIG. 4.
Influence of pH on the adhesion of L. johnsonii La1 and L. acidophilus La10 to Caco-2 cells. Results are the mean ± standard deviations for five experiments done in triplicate. The asterisk indicates a P value of <0.05 when data were compared to pH 7 data for each strain by a t test. For La10, standard deviations were always <0.0437.
La1 adhesion inhibition assays.
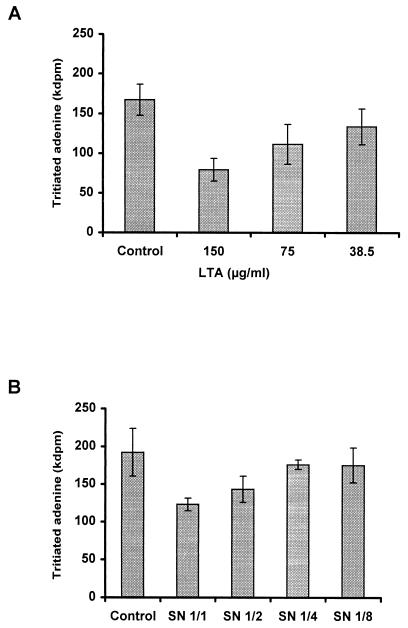
Pure LTA was tested in adhesion experiments with L. johnsonii La1 and enterocyte-like Caco-2 cells. Preincubation of the cells with increasing amounts of LTA in 50 mM acetate buffer (pH 5) resulted in adhesion inhibition in the range of 60% for a concentration of 150 μg/ml or 0.09 mg of total neutral sugars per ml (Fig. 5A). Since L. johnsonii La1 secreted LTA into the SCS, adhesion inhibition experiments were also performed with La1 SCS harvested at the stationary phase. Figure 5B shows concentration-dependent inhibition of La1 adhesion to Caco-2 cells. Significant statistical inhibition was obtained in experiments with four different La1 SCS samples and Caco-2 cells taken at four different passages (P, 0.01).
FIG. 5.
Inhibition of La1 adhesion to Caco-2 cells at pH 5. Caco-2 cells were preincubated with increasing amounts of purified LTA (A) or La1 culture supernatant (B). Controls were acetate buffer (pH 5) (A) or MRS broth acidified with lactic acid (B). Each assay was done in triplicate. Error bars are standard deviations.
DISCUSSION
The use of exogenous probiotics (e.g., Lactobacillus spp. and Bifidobacterium spp.) has been proposed as a way to reinforce mucosal defenses, especially in the small intestine, an organ relatively poorly colonized in comparison with the colon (9). In this context, L. johnsonii La1 and L. casei GG were recently shown to have antipathogenic effects either in cellular or in animal models (7, 8, 31). However, the adhesion mechanism is not understood, and its specificity was recently questioned (27). In the case of lactobacilli, it seems that the pH of in vitro adhesion assays is a crucial parameter (27, 28). The pH of the luminal small bowel is about 7. However, it is likely that in vivo, due to metabolic activities of intestinal cells and bacteria and to the presence of a mucus overlay, the pH in the vicinity of the brush border of the enterocytes is acidic (21). In this study, we demonstrated that although pH may play an important role in enhancing the adhesion of lactobacilli, it is not the sole factor involved. Indeed, a nonadherent bacterium, L. acidophilus La10, remained nonadherent at any pH. In contrast, L. johnsonii La1 was adherent at any pH of >4, and this adhesion was not significantly different from that observed at pH 7.
These results prompted us to look for a difference in the molecules exposed on the surfaces of L. johnsonii and L. acidophilus strains. First, monoclonal antibodies were produced against La1 surface proteins obtained after guanidinium HCl extraction. The antibodies cross-reacted with the surface proteins of both L. johnsonii and L. acidophilus strains but did not bind to La1 native bacteria. This result implied that surface proteins obtained by guanidinium HCl extraction (L. johnsonii does not have S layers, contrary to L. acidophilus) (4) are denatured and should perhaps not be used as representative models of the surfaces of bacteria for adhesion experiments.
This observation led us to immunize animals with living La1. This mode of immunization allowed us to detect on L. johnsonii the presence of an antigenic determinant that was absent from or masked on the surface of L. acidophilus. This result was confirmed by ELISAs with living bacteria and by immunoelectron microscopy studies. Several observations suggested that this antigenic determinant was carried by LTA: (i) its chromatographic behavior, which was characteristic of amphiphiles (14), and (ii) its detection in Triton X-100 lysates, bacterial extracts, and culture supernatants, which has already been described for many oral streptococci and lactobacilli (38). Finally, results obtained by chemical analysis of the purified material were well in agreement with the chemical nature of LTA (46, 56, 58). Localization of the reactive determinant in the membrane, in the cell wall, and on the surface of La1 was in accordance with the model proposed by Wicken and Knox (57), who proposed a wall-membrane model in which LTA molecules were embedded in the plasma membrane at their hydrophobic glycolipid ends, while the long polar glycerol phosphate chains extended by intercalation into the polysaccharide-peptidoglycan network of the cell wall. It was suggested that, in some cases, these chains came close enough to the surface of the bacteria to act as surface antigens. Immunogold labeling of La1 or Lj1 bacteria was similar to the pattern observed for L. casei and L. fermentum (54).
LTA has been found in many lactobacilli and plays a major role in the serological classification of lactobacilli (33, 34). Moreover, numerous cross-reactions due to the common glycerophosphate backbone of the different LTAs have been demonstrated (29, 34, 47). As our monoclonal antibodies are specific for L. johnsonii, it is conceivable that the antigenic determinant is carried on the carbohydrate moiety of LTA.
A strong inhibition of adhesion was observed with purified LTA as well as with La1 SCS, indicating that LTA plays a role in the mechanism of adhesion of L. johnsonii. Indeed, studies with staphylococci (51), streptococci (3, 5, 41, 52), Bifidobacterium bifidus (42), and lactobacilli (11, 47) have shown that LTA can inhibit their adhesion to epithelial cells. Binding of LTA to mammalian membranes has already been described for group A streptococci (6), in which LTA is anchored to a protein on the surface of the bacterial cells and interacts through its lipid moiety with fibronectin molecules deposited on and bound to the epithelial cells. Sherman and Savage (47) found a positive correlation between the presence of LTA on Lactobacillus strains and their adherence properties. This correlation could have been due to the negative charge distributed on the surface of the bacteria (43), probably as a result of the low isoelectric point of LTA molecules. Electrostatic binding to molecules at the surface of epithelial cells is possible as a general mechanism of adhesion and does not exclude the presence of other proteinaceous adhesins (15, 17) or lectin-like molecules of adhesion (1, 40). The lack of adhesion observed with La10 bacteria also could have been due to the fact that they may not have LTA exposed on their surface.
In conclusion, using a set of monoclonal antibodies specific for a nonproteinaceous component of the La1 bacterial surface, we were able to detect and isolate LTA, a molecule which participates in adhesion of these bacteria to Caco-2 intestinal cells. Future studies with other antibodies specifically recognizing La1 cell wall proteins will aid in an understanding of whether LTA is part of a complex system of adhesion molecules, as suggested for other lactobacilli (10, 18, 24, 27, 30, 45, 55), or can be solely responsible for the binding of certain bacteria to epithelial cells. This conclusion constitutes a revised opinion of our previous reports (7, 12, 17).
ACKNOWLEDGMENTS
We thank P. Nicolas, S. Reymond, and N. Kusy for carbohydrate analyses, K. Gartenman for amino acid analyses, and S. Metairon and L. Fay for fatty acid analyses. We are also much indebted to M.-F. Clerc for the artwork, to F. Stingele for reviewing the manuscript, and to J.-R. Neeser for helpful discussions.
REFERENCES
- 1.Adlerbeth I, Arhné S, Johansson M-L, Molin G, Hanson L A, Wold A. A mannose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29. Appl Environ Microbiol. 1996;62:2244–2251. doi: 10.1128/aem.62.7.2244-2251.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alander M, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, von Wright A. Recovery of Lactobacillus rhamnosus GG from human colonic biopsies. Lett Appl Microbiol. 1997;24:361–364. doi: 10.1046/j.1472-765x.1997.00140.x. [DOI] [PubMed] [Google Scholar]
- 3.Alkan M, Ofek I E, Beachey E H. Adherence of pharyngeal and skin strains of group A streptococci to human skin and oral epithelial cells. Infect Immun. 1977;18:555–557. doi: 10.1128/iai.18.2.555-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahl H, Scholz H, Bayan N, Chami M, Leblon G, Gulik-Krzywicki T, Schechter E, Fouet A, Mesnage S, Tosi-Couture E, Gounon P, Mock M, Conway de Macario E, de Macario A J L, Fernandez-Herrero L A, Olabarria G, Berenguer J, Blaser M J, Kuen B, Lubitz W, Sara M, Pouwels P H, Kolen C P A M, Boot H J, Lechleitner S, Resch S. Molecular biology of S-layers. FEMS Microbiol Rev. 1997;20:47–98. doi: 10.1111/j.1574-6976.1997.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 5.Beachey E, Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded M protein. J Exp Med. 1976;143:759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beachey E H, Giampapa C S, Abraham S N. Bacterial adherence: adhesin receptor-mediated attachment of pathogenic bacteria to mucosal surfaces. Am Rev Respir Dis. 1988;138:6–8. doi: 10.1164/ajrccm/138.6_Pt_2.S45. [DOI] [PubMed] [Google Scholar]
- 7.Bernet M-F, Brassart D, Neeser J-R, Servin A L. Lactobacillus acidophilus La1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–484. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernet-Camard M-F, Liévin V, Brassart D, Neeser J-R, Servin A L, Hudault S. The human Lactobacillus acidophilus strain La1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol. 1997;63:2747–2753. doi: 10.1128/aem.63.7.2747-2753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Bindlingmeyer B A, Cohen S A, Tarvin T L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984;336:93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- 9.Brassart D, Schiffrin E J. The use of probiotics to reinforce mucosal defense mechanisms. Trends Food Sci Technol. 1997;9:321–326. [Google Scholar]
- 10.Brooker B E, Fuller R. Adhesion of lactobacilli to the crop epithelium. J Ultrastruct Res. 1975;52:21–31. doi: 10.1016/s0022-5320(75)80019-0. [DOI] [PubMed] [Google Scholar]
- 11.Chan R C Y, Reid G, Irvin R T, Bruce A W, Costerton J W. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect Immun. 1985;47:84–89. doi: 10.1128/iai.47.1.84-89.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauvière G, Coconnier M-H, Kerneis S, Fourniat J, Servin A L. Adherence of Lactobacillus acidophilus onto human enterocyte-like cells Caco-2 and HT-29 in culture. J Gen Microbiol. 1992;138:1689–1696. doi: 10.1099/00221287-138-8-1689. [DOI] [PubMed] [Google Scholar]
- 13.Chen P S, Toribara T Y, Warner H. Microdetermination of phosphorus. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 14.Chiu T H, Emdur I, Platt D. Lipoteichoic acids from Streptococcus sanguis. J Bacteriol. 1974;118:471–479. doi: 10.1128/jb.118.2.471-479.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coconnier M-H, Bernet M-F, Kernéis S, Chauvière G, Fourniat J, Servin A L. Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol Lett. 1993;110:299–306. doi: 10.1111/j.1574-6968.1993.tb06339.x. [DOI] [PubMed] [Google Scholar]
- 16.Coconnier M-H, Liévin V, Bernet-Camard M-F, Hudault S, Servin A L. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob Agents Chemother. 1997;41:1046–1052. doi: 10.1128/aac.41.5.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coconnier M-H, Klaenhammer T R, Kerneis S, Bernet M-F, Servin A L. Protein-mediated adhesion of Lactobacillus acidophilus BG2FO4 on human enterocyte and mucus-secreting cell lines in culture. Appl Environ Microbiol. 1992;58:2034–2039. doi: 10.1128/aem.58.6.2034-2039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway P, Kjelleberg S. Protein-mediated adhesion of Lactobacillus fermentum strain 737 to mouse stomach squamous epithelium. J Gen Microbiol. 1989;135:1175–1186. doi: 10.1099/00221287-135-5-1175. [DOI] [PubMed] [Google Scholar]
- 19.Cook R L, Harris R J, Reid G. Effect of culture media and growth phase on the morphology of lactobacilli and on their ability to adhere to epithelial cells. Curr Microbiol. 1988;17:159–166. [Google Scholar]
- 20.Dubois M A, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 21.Duncan H E, Edberg S C. Host microbial interactions in the gastro-intestinal tract. Crit Rev Microbiol. 1995;21:85–100. doi: 10.3109/10408419509113535. [DOI] [PubMed] [Google Scholar]
- 22.Elo S, Salminen S. Attachment of Lactobacillus casei strain GG to human colon carcinoma cell line Caco-2: comparison with other dairy strains. Lett Appl Microbiol. 1991;13:154–156. [Google Scholar]
- 23.Fisher W, Koch H U, Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983;133:523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 24.Fuller R. Nature of the determinant responsible for the adhesion of lactobacilli to chicken crop epithelial cells. J Gen Microbiol. 1975;87:245–250. doi: 10.1099/00221287-87-2-245. [DOI] [PubMed] [Google Scholar]
- 25.Gibson G R, Saavedra J M, McFarlane S, McFarlane G T. Probiotics and intestinal infections. In: Fuller R, editor. Probiotics 2: applications and practical aspects. London, England: Chapman & Hall, Ltd.; 1997. pp. 10–31. [Google Scholar]
- 26.Granato D, Piguet P-F. A mouse monoclonal IgE antibody anti-bovine milk β-lactoglobulin allows studies of allergy in the gastrointestinal tract. Clin Exp Immunol. 1986;63:703–710. [PMC free article] [PubMed] [Google Scholar]
- 27.Greene J D, Klaenhammer T R. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl Environ Microbiol. 1994;60:4487–4494. doi: 10.1128/aem.60.12.4487-4494.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henriksson A, Szewyk R, Conway P L. Characteristics of the adhesive determinants of Lactobacillus fermentum 104. Appl Environ Microbiol. 1991;57:499–502. doi: 10.1128/aem.57.2.499-502.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewett M J, Knox K W. Studies on the group F antigen of lactobacilli: detection of antibodies by haemagglutination. J Gen Microbiol. 1970;60:315–322. doi: 10.1099/00221287-60-3-315. [DOI] [PubMed] [Google Scholar]
- 30.Hood S K, Zottola E A. An electron microscopic study of the adherence of Lactobacillus acidophilus to human intestinal cells. Food Microstruct. 1989;8:91–97. [Google Scholar]
- 31.Hudault S, Liévin V, Bernet-Camard M-F, Servin A L. Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection. Appl Environ Microbiol. 1997;63:513–518. doi: 10.1128/aem.63.2.513-518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson M-L, Molin G, Jeppsson B, Nobaek S, Ahrné S, Bengmark S. Administration of different Lactobacillus strains in fermented oatmeal soup: in vivo colonization of human intestinal mucosa and effects on the indigenous flora. Appl Environ Microbiol. 1993;59:15–20. doi: 10.1128/aem.59.1.15-20.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knox K W, Wicken A J. Immunological properties of teichoic acids. Bacteriol Rev. 1973;37:215–257. doi: 10.1128/br.37.2.215-257.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knox K W, Hewett M J. Studies on the group F antigen of lactobacilli: antigenicity and serological specificity of teichoic acid preparations. J Gen Microbiol. 1970;60:303–313. doi: 10.1099/00221287-60-3-303. [DOI] [PubMed] [Google Scholar]
- 35.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y K, Salminen S. The coming of age of probiotics. Trends Food Sci Technol. 1995;6:241–245. [Google Scholar]
- 37.Lepage G, Roy C C. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 38.Markham J L, Knox K W, Wicken A J, Hewett M J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975;12:378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGrady J A, Butcher W G, Breighton D, Switalski L M. Specific and charge interactions mediate collagen recognition by oral lactobacilli. J Dent Res. 1995;74:649–657. doi: 10.1177/00220345950740020501. [DOI] [PubMed] [Google Scholar]
- 40.Mukai T, Arihara K, Itoh H. Lectin-like activity of Lactobacillus acidophilus strain JCM 1026. FEMS Microbiol Lett. 1992;98:71–74. doi: 10.1016/0378-1097(92)90134-a. [DOI] [PubMed] [Google Scholar]
- 41.Ofek I, Beachey E H, Jefferson W, Campbell G L. Cell membrane binding properties of group A streptococcal lipoteichoic acid. J Exp Med. 1975;141:990–1003. doi: 10.1084/jem.141.5.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Op den Camp H J M, Oosterhof A, Veerkamp J H. Interaction of bifidobacterial lipoteichoic acid with human intestinal epithelial cells. Infect Immun. 1985;47:332–334. doi: 10.1128/iai.47.1.332-334.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelletier C, Bouley C, Cayuela C, Bouttier S, Bourlioux P, Bellon-Fontaine M-N. Cell surface characteristics of Lactobacillus casei subsp. casei, Lactobacillus paracasei subsp. paracasei, and Lactobacillus rhamnosus strains. Appl Environ Microbiol. 1997;63:1725–1731. doi: 10.1128/aem.63.5.1725-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 45.Savage D C. Association and physiological interaction of indigenous microorganisms and gastrointestinal epithelia. Am J Clin Med. 1972;25:1372–1379. doi: 10.1093/ajcn/25.12.1372. [DOI] [PubMed] [Google Scholar]
- 46.Sharpe E, Brock J H. Glycerol teichoic acid as a common antigenic factor in lactobacilli and some other gram-positive organisms. J Gen Microbiol. 1973;74:119–126. doi: 10.1099/00221287-74-1-119. [DOI] [PubMed] [Google Scholar]
- 47.Sherman L A, Savage D C. Lipoteichoic acids in Lactobacillus strains that colonize the mouse gastric epithelium. Appl Environ Microbiol. 1986;52:302–304. doi: 10.1128/aem.52.2.302-304.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simon G L, Gorbach S H. Normal alimentary tract microflora. In: Blaser M J, Smith P D, Ravdin J I, Greenberg H B, Guerrant R L, editors. Infections of the gastrointestinal tract. New York, N.Y: Raven Press, Ltd.; 1995. pp. 53–69. [Google Scholar]
- 49.Stepanek J. Quantitative determination of myo-inositol in pharmaceutical preparations and organic extracts by high-performance thin-layer chromatography using fluorescence. J Chromatogr. 1983;257:405–410. doi: 10.1016/s0021-9673(01)88200-5. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi N, Saito T, Ohwada S, Ota H, Hashiba H, Itoh T. A new screening method for the selection of Lactobacillus acidophilus group lactic acid bacteria with high adhesion to human colonic mucosa. Biosci Biotech Biochem. 1996;60:1434–1438. doi: 10.1271/bbb.60.1434. [DOI] [PubMed] [Google Scholar]
- 51.Teti G, Chiofalo M S, Tomasello F, Fava C, Mastroeni P. Mediation of Staphylococcus saprophytus adherence to uroepithelial cells by lipoteichoic acid. Infect Immun. 1987;55:839–842. doi: 10.1128/iai.55.3.839-842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teti G, Tomasello F, Chiofalo M S, Orefici G, Mastroeni P. Adherence of group B streptococci to adult and neonatal epithelial cells mediated by lipoteichoic acid. Infect Immun. 1987;55:3057–3064. doi: 10.1128/iai.55.12.3057-3064.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toba T, Virkola R, Westerlund B, Bjökman Y, Sillanpää J-C, Vartio T, Kalkkinen N, Korhonen T K. A collagen-binding S-layer protein in Lactobacillus crispatus. Appl Environ Microbiol. 1995;61:2467–2471. doi: 10.1128/aem.61.7.2467-2471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van Driel D, Wicken A J, Dickson M R, Knox K W. Cellular location of the lipoteichoic acids of Lactobacillus fermenti NCTC 6991 and Lactobacillus casei NCTC 6375. J Ultrastruct Res. 1973;43:483–497. doi: 10.1016/s0022-5320(73)90025-7. [DOI] [PubMed] [Google Scholar]
- 55.Wadström T, Andersson K, Sydow M, Axelsson L, Lindgren S, Gullmar B. Surface properties of lactobacilli isolated from the small intestine of pigs. J Appl Bacteriol. 1987;62:513–520. doi: 10.1111/j.1365-2672.1987.tb02683.x. [DOI] [PubMed] [Google Scholar]
- 56.Wicken A J. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970;60:293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]
- 57.Wicken A J, Knox K W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975;187:1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- 58.Wicken A J, Gibbens J W, Knox K W. Comparative studies on the isolation of membrane lipoteichoic acid from Lactobacillus fermenti. J Bacteriol. 1973;113:365–372. doi: 10.1128/jb.113.1.365-372.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto K, Miwa T, Taniguchi H, Nagano T, Shimamura K, Tanaka T, Kumagai H. Binding specificity of Lactobacillus to glycolipids. Biochem Biophys Res Commun. 1996;228:148–152. doi: 10.1006/bbrc.1996.1630. [DOI] [PubMed] [Google Scholar]