Summary
Accumulation of immunoglobulin and complement components within the kidneys is a hallmark of glomerulonephritis. Staining and detection of IgG, IgA, IgM, and C3 deposits can assist in diagnosing the underlying causes of nephritis and has implications for the pathological processes underpinning glomerulonephritis. Here, we describe a protocol to detect immune deposits within biological specimens such as mouse kidneys. We detail tissue isolation and processing, immunostaining, and fluorescence microscopy to characterize and quantify the extent of immunological deposits contributing to kidney injury.
For complete details on the use and execution of this protocol, please refer to Jiang et al. (2021).
Subject areas: Immunology, Microscopy, Model Organisms
Graphical abstract
Highlights
-
•
Isolation and processing of murine kidneys for cryopreservation
-
•
Sectioning of cryomolds for tissue staining
-
•
Antibody staining of tissue sections for immunoglobulins
-
•
Analysis of fluorescent images for immunoglobulin deposition in the kidneys
Accumulation of immunoglobulin and complement components within the kidneys is a hallmark of glomerulonephritis. Staining and detection of IgG, IgA, IgM, and C3 deposits can assist in diagnosing the underlying causes of nephritis and has implications for the pathological processes underpinning glomerulonephritis. Here, we describe a protocol to detect immune deposits within biological specimens such as mouse kidneys. We detail tissue isolation and processing, immunostaining, and fluorescence microscopy to characterize and quantify the extent of immunological deposits contributing to kidney injury.
Before you begin
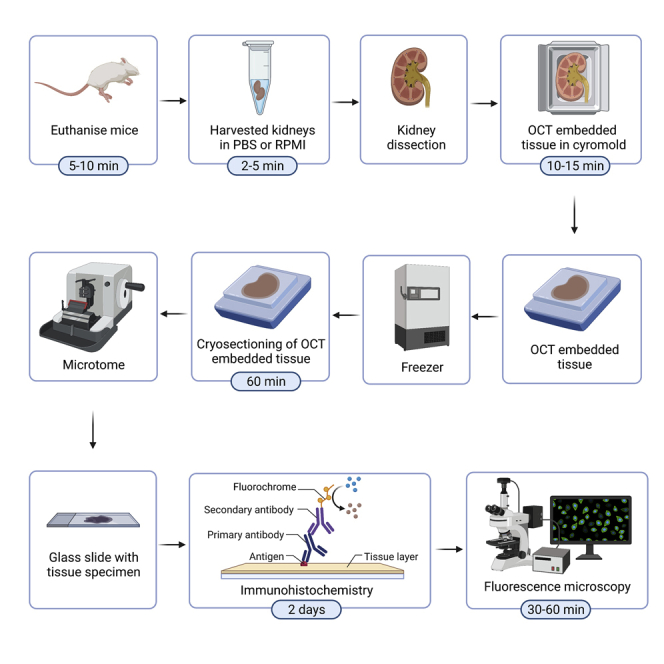
The current protocol outlines the major parts for staining immunoglobulin deposits within kidney samples obtained from mice. This protocol can also be used for staining sectioned human kidney samples. Parts 1–3 outline the process of isolation and freezing of kidney specimens from mice. Where samples have already been cryopreserved, parts 4–6 of the protocol can be followed to complete the sectioning and immunostaining of the biological samples.
Institutional permissions
The Australian National University Animal Experimentation Ethics Committee approved all the experiments, and these experiments conform to the relevant regulatory standards.Please note that scientists should acquire permissions from the relevant institutions before undertaking this protocol.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| IgG (1:150) | Invitrogen | Cat# A28175 |
| IgA (1:150) | Southern Biotech | Cat# 1040-02 |
| IgM (1:150) | Invitrogen | Cat# SA5-10152 |
| C3 (1:400) | MP Biomedicals | Cat# SKU 0855500 |
| Biological samples | ||
| Mouse kidney samples | This study | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Tissue-Tek O.C.T. | Sakura | Cat# 4583 |
| Triton X | Merck | Cat# X100-100ML |
| PBS | Thermo Scientific | Cat# 20012027 |
| RPMI 1640 medium | Invitrogen | Cat# 21870076 |
| Positively charged microscope slides | Thermo Fisher Scientific | Cat# 4951PLUS |
| Bovine Serum Albumin Fraction V | Bovogen Biologicals Pty Ltd | Cat# BSAS 0.50 |
| ProLong™ Gold Antifade Mountant | Invitrogen | Cat# P36930 |
| Immersion oil Type-F | Olympus | Cat# IMMOIL-F30CC |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6 mice, age 12 weeks, either gender | Charles River | C57BL/6 |
| Mouse: Vangl1+/− mice age 12 weeks, either gender | KOMP | EPD0164_3_G07 |
| Other | ||
| ImmEdge hydrophobic barrier pen | Vector Labs | Cat# H-4000 |
| Tissue-Tek Cryomold | ProSciTech | Cat# Y566 |
| Microtome | Epredia | HM525 NX |
| Olympus IX 71 inverted microscope | Olympus | Cat# IX71 |
| Olympus DP70 microscope digital camera | Olympus | Cat# DP70 |
| X-Cite series 120-Q | Excelitas | X-Cite 120Q |
| Euthanasia chamber | Able Scientific | Cat# ASEC1 |
| Mechanical restraint device | Kent Scientific | Cat# RSTR541 |
| Heat lamp | Morganville Scientific | Cat# HL0100 |
| Hypodermic needle (27 Gauge) | Terumo | Cat# DS-N028 |
Materials and equipment
Blocking Buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Bovine Serum Albumin | 3% | 3 g |
| PBS (10×) | 1× | 10 mL |
| Total | n/a | 100 mL |
Note: Can store 4–7 days at 4°C.
Antibody buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Bovine Serum Albumin | 5% | 5 g |
| PBS (10×) | 1× | 10 mL |
| Total | n/a | 100 mL |
Note: Can store 4–7 days at 4°C.
Step-by-step method details
Part 1: Euthanasia by carbon dioxide asphyxiation
Timing: 5–10 min (for each cage of mice)
Note: Cervical dislocation could also be used as an acceptable alternative to asphyxiation.
This section describes ethical euthanasia of subject mice.
-
1.
The mice are placed in the euthanasia chamber.
-
2.
The air inside the chamber is slowly displaced by 100% CO2.
-
3.Asphyxiation is performed in three sequential phases:
-
a.Phase I- Induction: There is a gradual displacement of air with CO2 for about a minute. As a result, the mice lose consciousness.
-
b.Phase II- Euthanasia: The flow rate of CO2 is increased to 100% for two minutes during which respiration ceases.
-
c.Phase III- Emptying: This lasts 2 min and is characterized by CO2 absorption.
-
a.
-
4.
It is advised to maintain the CO2 flow for at least 1–2 min post-respiratory arrest (Grosjean et al., 2021).
Part 2: Isolation of mouse kidneys for processing
Timing: 2–5 min
This section describes the process of isolating mouse kidneys for preservation.
-
5.
Perform the following steps in a sterile hood (if part of the tissue has to be used for culture) at room temperature (21°C).
-
6.
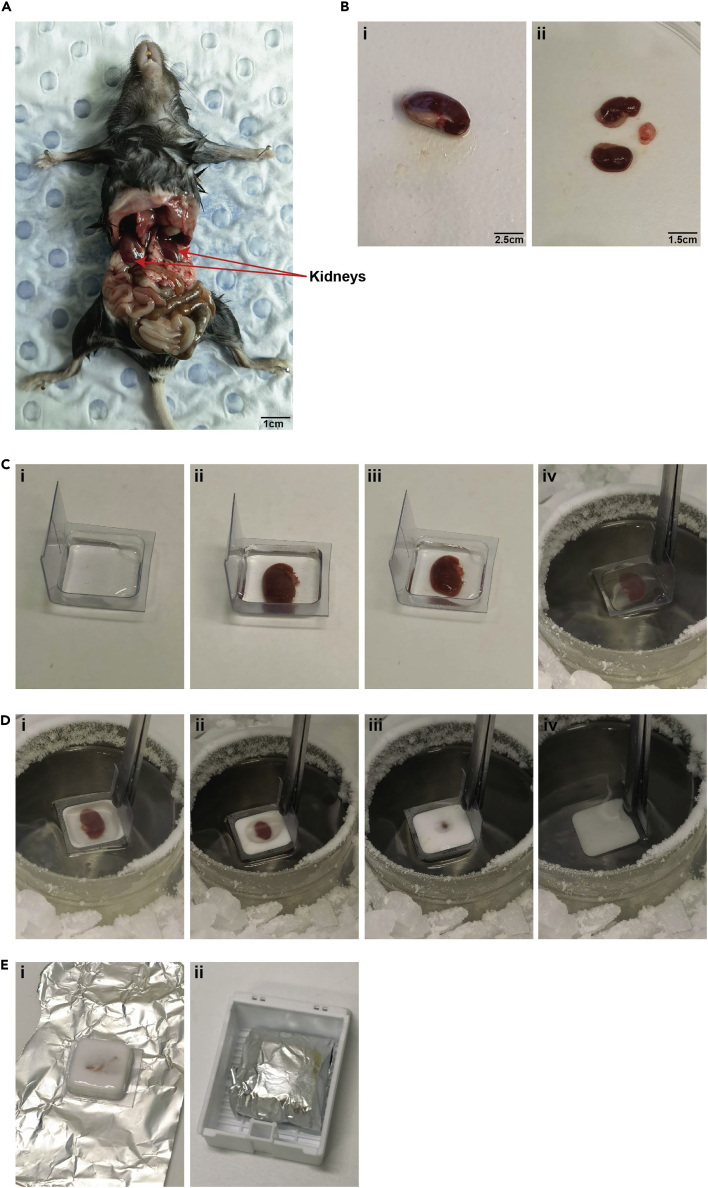
Place the euthanized mouse in a supine position on a dissection platform that has been disinfected with 70% ethanol (see Figure 1).
-
7.
Pin the legs of the mouse at a 90° angle to the body on the dissection board.
-
8.
Spray ethanol on the ventral side of the mouse.
-
9.
Using dissection scissors, make a midline incision pointing towards the tail to help cut open the skin.
-
10.
Make lateral incisions at the shoulder joints and the pelvic girdles so that it is easy to peel open the skin.
-
11.
Use forceps and fingers to carefully separate the skin layers while keeping the internal organs intact (Bajpai et al., 2016).
-
12.
Using dissection scissors, cut open the abdominal membrane to expose the internal organs.
-
13.
Locate the kidneys, gently separate them from associated tissue, and using clean tweezers and dissection scissors, cut the renal artery, vein and ureter to liberate the kidneys.
-
14.
Transfer the kidneys to a 1.5 mL eppendorf tube containing 1× PBS (Phosphate Buffered Saline) or RPMI-1640 (Rosewell Park Memorial Institute 1640) media.
Figure 1.
Isolation and OCT cryopreservation of mouse kidneys
(A) Using dissection scissors, the abdominal membrane is cut to expose the internal organs of the mouse.
(B) Sectioning of the kidney. i - Kidneys isolated in 1× PBS or RPMI media are placed in a petri dish for sectioning. ii - One of the kidneys is cut in half using a scalpel blade making sure to remove the capsule.
(C) Preparation of the cryomold with the kidney sample for freezing. i- Cryomold is then partially filled with OCT compound. ii - One half of the mouse kidney is placed on the OCT compound. iii- The cryomold is then filled with OCT compound such that it covers the kidney completely. iv- Cryomold with the OCT compound and kidney is dipped in chilled acetone.
(D) Freezing of the kidney. i – iv - Depicts the progressive rapid freezing of the kidney sample in the cryomold.
(E) Storage of the frozen kidney. i-ii - The completely frozen cryomold with kidney embedded in OCT compound is wrapped in aluminum foil, placed in a tissue cassette and frozen at −80°C.
Part 3: OCT (optimal cutting temperature) cryopreservation of kidneys
Timing: 10–15 min
This section describes cryopreservation of mouse kidneys.
-
15.
The amount of OCT required for proper infiltration of the tissue is about 25-30-fold relative to the tissue size. Ensure that the size of the cryomold or the tissue is adequate to allow for this.
-
16.
Gently fill the corners of the cryomold (Tissue-Tek) with the OCT compound (tissue embedding medium) making sure it is present evenly throughout the mold. Ensure no introduction of bubbles as this may lead to artifact formation while sectioning using a microtome.
-
17.
Fill a steel can halfway with 100% acetone and rest it on dry ice for a minute. Add one or two small chips of dry ice at a time to the acetone to lower the temperature. Caution: adding excess dry ice may cause the acetone to overflow.
-
18.
Take the isolated kidney and cut lengthwise with the help of a scalpel or dissection scissors. (Please note that the following steps are to be performed on ice).
-
19.
With clean forceps, carefully place the desired section of the tissue in the center of the OCT compound on the cryomold with the incision surface face down. Note: the section that is facing the bottom of the cryomold, will be the side sectioned first.
-
20.
Carefully dispense more OCT onto the tissue specimen till it is completely covered and allow it to settle for 15–30 s. Care should be taken to avoid air bubbles. If air bubbles form, remove them before proceeding.
-
21.
Dip the cryomold containing the tissue and OCT compound into the chilled acetone using long forceps. This will freeze the contents of the cryomold turning it completely white (opaque). Brittleness may result from slow freezing of the specimen and hence care should be taken to ensure the rapid OCT freezing is complete within 2 min.
-
22.
Remove the tissue block from the cryomold and trim the edges with a razor blade.
-
23.
Place the OCT embedded tissue in cryovial/tissue cassettes (pre-cooled) and store it at −80°C (Wiles, 2021).
Part 4: Cryosectioning of a cryopreserved kidney specimen
Timing: 60 min
This section describes cryosectioning of cryopreserved kidneys. Also see Methods video S1.
-
24.
The OCT embedded tissue block is placed on dry ice and transported to the cryostat.
-
25.
Transfer the specimen tissue block to the cryostat chamber and let it equilibrate to the set temperature of the cryostat.
CRITICAL: The above step of acclimatization will help in yielding high quality tissue slices by preventing the curling of the specimen ribbons. It is critical to avoid sample thawing (Li et al., 2021).
-
26.
Mount the tissue block onto the tissue block holder of the cryostat with OCT.
-
27.
Align the tissue block with the correct orientation of the specimen.
-
28.
Choose the desired thickness (we use 7 μm) of the sections.
-
29.
Turn the cryostat handwheel to form a strip or ribbon of sections of the desired thickness.
-
30.
Transfer the sections onto a clean pre-chilled microscope slide and rub the back of the slide with the hand to warm it to ensure the sections adhere well.
(To adhere the tissue section, coated or positively charged slides are used. Poly-L-Lysine coated slides have greater retainability of tissues).
-
31.
Air-dry the slides at room temperature (21°C) for 20–30 min to ensure the tissue section adheres more strongly to the slide.
CRITICAL: Air drying time is critical to ensure tissue samples adhere to the microscope slides.
-
32.
Slides can be placed in a slide box wrapped in foil and placed inside a zip-top bag. Store at −80°C (Can be stored viably for at least 6 months).
-
33.
The remaining OCT embedded tissue can be stored at −80°C in cryomold with a sufficient amount of OCT compound to prevent freeze-drying during storage (Fra-Bido et al., 2021) (Can be stored viably for at least 5 years).
Part 5: Immunohistochemistry
Timing: 2 days
This section describes antibody staining to detect immunoreactants in mouse kidney.
-
34.
Remove slides stored at −80°C and thaw at room temperature (21°C).
-
35.
Dry the sides of the slides with a lint-free tissue.
-
36.
To rehydrate the slides, arrange them in an appropriate slide staining jar.
-
37.
Wash the slides in Phosphate Buffered Saline (PBS) (wash buffer) for 5 min (3 times).
-
38.
Change the solution between each wash.
Note: From this step on, care should be taken to avoid slides from dehydrating which results in increased background staining and problems with imaging (Fra-Bido et al., 2021).
-
39.Using a mini PAP pen, mark out a boundary encircling but a few millimeters away from the tissue.Note: The hydrophobic wax residue will help localize the antibody solution within the marked boundary throughout the various stages of slide preparation.
-
a.Leave to rest for 1–2 min for the wax to completely dry.
-
a.
-
40.To prevent non-specific binding of the antibodies, add (∼300 μL) blocking buffer (3% Bovine Serum Albumin (BSA)/0.1% Triton X-100 in PBS) to the slides.
-
a.Incubate at room temperature (21°C) for 1–2 h on a rocker in a humidified chamber (Kang et al., 2021).
-
a.
-
41.
Remove the blocking buffer, add the appropriate dilution of the primary antibody in PBS and incubate the slides at 4°C overnight (18–20 h).
-
42.
Wash the slides in PBS for 5 min (thrice) at room temperature (21°C).
-
43.
(Optional step to be done when primary antibody is unconjugated) Add appropriate dilutions of secondary antibody labeled with the fluorochrome in 5% BSA/PBS and incubate at room temperature (21°C) for 1–2 h (Kang et al., 2021).
-
44.
(Optional step to be done when primary antibody is unconjugated) Rinse the slides in PBS for 5 min (thrice) at room temperature (21°C).
-
45.
Without disrupting the tissue, wipe off the excess PBS on the slide.
-
46.
Apply mounting medium (ProLong™ Gold Antifade Mountant) directly to the tissue and using forceps carefully place a coverslip on top of this.
-
47.
Remove any air bubbles and allow the mounting medium to set at room temperature (21°C) for 15–30 min.
-
48.
For imaging, store dried slides at 4°C (Fra-Bido et al., 2021).
Part 6: Image acquisition on fluorescence microscope
Timing: 30–60 min
This section describes acquisition of fluorescent images from mouse sections.
Stained and mounted slides can be imaged on any epifluorescence microscope with appropriate 20×, 40× or 60× oil objectives. Details given below apply to an Olympus IX 71 inverted microscope with an Olympus DP70 microscope digital camera.
-
49.
Switch mercury lamp on at least 10 min earlier to warm up (X-Cite Series 120-Q by Excelitas Technologies).
-
50.
Apply a small drop of immersion oil (Immersion oil Type-F IMMOIL-F30CC) onto slide or objective and place slide face down onto objective.
-
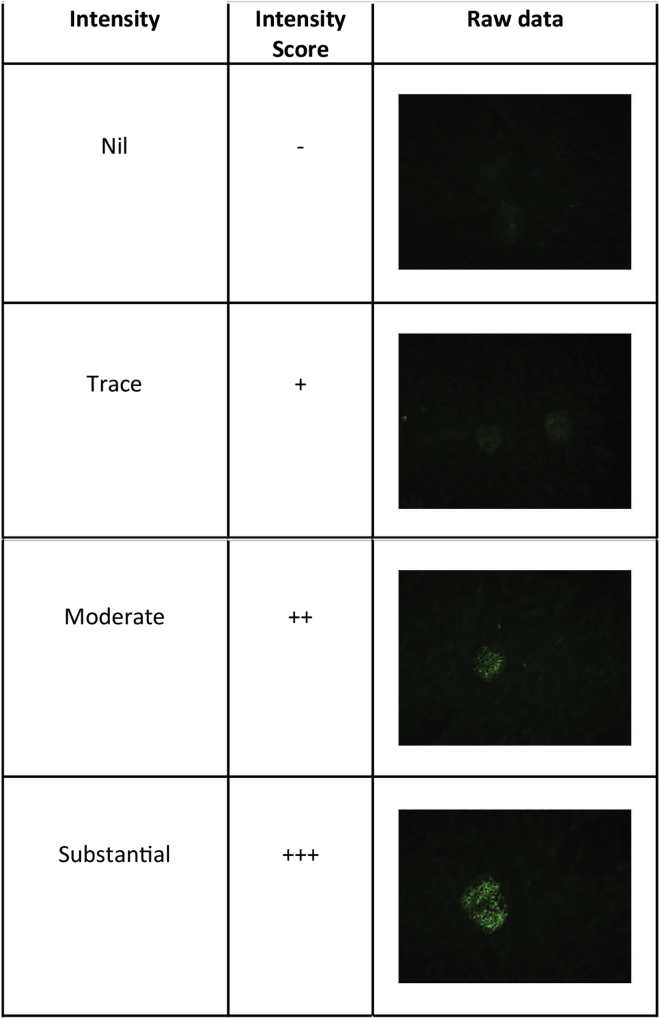
51.Focus on several glomeruli per slide section and capture images. Images are saved as high-resolution TIFF files.
-
a.Examples of fluorescence intensity in Figure 3.
-
a.
Figure 3.
Example of different immunofluorescent staining intensity
Optional step: Tail vein injection of mice with commercial immunoglobulin A
Timing: 7 days of tail vein injection with commercial IgA
This section describes the optional tail vein injection of immunoglobulin A.
-
52.
Weigh the mouse.
-
53.
Calculate the amount of immunoglobulin dose according to the weight of the mouse.
-
54.
Use a mechanical restraint device to restrain the mouse.
-
55.
Make sure the pores present in the front of the restraint device are free to ensure the mouse is breathing.
-
56.
Use the hole at the rear end of the device to stabilize the mouse tail.
-
57.
Place the tail of the mouse under the heat lamp for a minute for easy visualization and to facilitate vasodilation of the lateral vein of the mouse.
-
58.
Disinfect the tail with 75% ethanol.
-
59.
Adjust the position of the tail’s lateral side and secure it with the left hand (Wrobel et al., 2021).
-
60.
Insert the catheter needle at 30° angle into the vein (2 cm from the tip of the tail) and inject the immunoglobulin solution slowly and steadily.
-
61.
Once the contents have been injected, carefully remove the needle, and apply gentle pressure to the injection site till the bleeding stops.
-
62.
Release the mouse out of the restraint device and check for vital signs.
-
63.
Place the mouse back in the original cage immediately after injection (He et al., 2021).
Expected outcomes
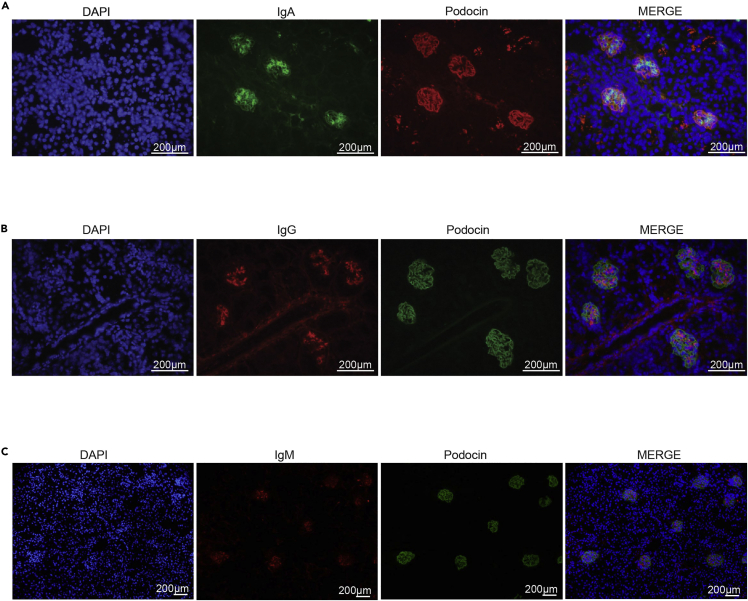
Immunofluorescent staining is expected to identify the presence of immunoglobulin within glomerular structures. This can be carried out by staining tissues using DAPI to mark nuclei, glomeruli with markers such as podocin and the relevant immunoglobulin subtype of interest (See Figures 2A and 2B for examples of IgG and IgA staining respectively). Normal mouse glomeruli should have no detectable immunoglobulin. The presence of immunoglobulin deposition should be correlated with Haematoxylin and Eosin (H&E) or Periodic Acid Schiff (PAS) staining on light microscopy to determine any pathological injury in glomeruli.
Figure 2.
Immunofluorescence of immunoglobulins in mouse kidney cryosections from 8-week-old Vangl1+/− mice
(A) Immunofluorescence of immunoglobulin A (green), podocin (red) and DAPI (blue).
(B) Immunofluorescence of immunoglobulin G (red), podocin (green) and DAPI (blue).
(C) Immunofluorescence of immunoglobulin M (red), podocin (green) and DAPI (blue).
Quantification and statistical analysis
Immunofluorescence is scored using an ordinal scaling system. A trained histopathologist should score the intensity of immunoglobulin deposition on a relative scale of 0, 1+, 2+, 3+. Use of an appropriate positive control mouse serum from autoimmune mouse models such as Lyn−/− mice can control for antibody staining and normalize intensity of immunoglobulin staining. Once scored, immunofluorescence intensity scores can be compared using the Mann-Whitney-U test. See Figure 3 for the example of scoring.
Limitations
In general, mice that have recently undergone sublethal irradiation (such as creating a bone marrow chimera) will have mild glomerular inflammation likely arising from inflammation. This may limit interpretation of antibody deposition arising from genetic/pathologic causes.
The most significant limitation is obtaining an ideal dilution of the primary staining antibody. Excess concentrations lead to oversaturation (high background staining) whilst low concentrations may lead to inability to detect immunoreactants in the sections making interpretation of results difficult.
Suboptimal freezing may make the OCT blocks containing kidney tissue difficult to subsequently section and mount onto slides and can also create artifacts affecting kidney structure.
Troubleshooting
Problem 1
Air bubbles in OCT-embedded kidney sections. Please refer to corresponding protocol step 16.
Potential solution
Remove any air bubbles inside the OCT with the help of a pipette tip. The air bubbles can interfere with cryosectioning and need to be removed.
Problem 2
The frozen tissue blocks are difficult to cryosection. Please refer to corresponding protocol step 29.
Potential solution
Keep OCT embedded kidneys on dry ice before sectioning to help with sectioning onto slides.
Problem 3
The tissue slices cut from the cryomold curls or does not adhere to the slide. Please refer to corresponding protocol step 30.
Potential solution
Given that the sections being cut are very thin, the cut slices may curl on itself. In such cases, gently flatten the corner of the section that curls with the help of a paintbrush or make use of an anti-roll plate for this purpose. If the cut sections do not adhere to the slide, it may indicate that the slide is too cold. Slightly warm up the back of the slide by rubbing against your hand and try again.
Problem 4
Tearing and introduction of strips on the cut section. Please refer to corresponding protocol step 30.
Potential solution
Use a different part of the blade by moving it along since it may be chipped or damaged.
Problem 5
High background staining. Please refer to corresponding protocol step 40.
Potential solution
It is recommended to first perform serial dilutions of antibodies on slides to identify an ideal concentration. With high background staining we suggest reducing antibody dilution, consider performing antibody titration and consider increasing BSA % in blocking buffer. In addition, do not allow the tissue section to dry after rehydrating with PBS as this might also contribute to high background.
Problem 6
Antibody cross reactivity with blocking buffer. Please refer to corresponding protocol step 40.
Potential solution
Antibodies listed in the key resources table were used in Jiang et al. (2021), but can be interchanged for other antibodies. Note that if swapping antibodies, check that fluorophore conjugated secondary detection antibodies are targeted against the appropriate host species of the primary-antibody (i.e., anti-goat-FITC if an anti-podocin goat antibody is used). In addition, ensure that the secondary antibody does not cross react with any of the components of the blocking buffer. For example, avoid using goat serum in the blocking buffer if antibodies raised in goat are used for primary and secondary antibody staining.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to, and will be fulfilled by the lead contact, Simon Jiang (simon.jiang@anu.edu.au).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
RACP Jacquot NHMRC Award for Excellence, Jacquot Research Entry Scholarship, Canberra Hospital Private Practice Fund, Capital Kidney Research Fund, and NHMRC project grants to S.H.J. The graphical abstract was created with BioRender.com.
Author contributions
S.B.N. and S.H.J. wrote the paper. V.A. wrote Part 6. M.S. and Y.Z. performed the experiments. All authors read and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xpro.2022.101375.
Data and code availability
This study did not generate or analyze datasets and use code.
References
- Bajpai V.K., Rather I.A., Kim K. Isolation of mouse internal organs for molecular and histopathological studies. Bangladesh J. Pharmacol. 2016;11:485. doi: 10.3329/bjp.v11i2.26957. [DOI] [Google Scholar]
- Fra-Bido S., Walker S.A., Innocentin S., Linterman M.A. Optimized immunofluorescence staining protocol for imaging germinal centers in secondary lymphoid tissues of vaccinated mice. STAR Protoc. 2021;2:100499. doi: 10.1016/j.xpro.2021.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean C., Quessada J., Nozais M., Loosveld M., Payet-Bornet D., Mionnet C. Isolation and enrichment of mouse splenic T cells for ex vivo and in vivo T cell receptor stimulation assays. STAR Protoc. 2021;2:100961. doi: 10.1016/j.xpro.2021.100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Yu W., Lu Y., Zhang W., Xu J., Zhang L. A protocol for in vivo analysis of liver tumorigenesis in mice using sleeping beauty transposon system. STAR Protoc. 2021;2:100445. doi: 10.1016/j.xpro.2021.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S.H., Mercan S., Papa I., Moldovan M., Walters G.D., Koina M., Fadia M., Stanley M., Lea-Henry T., Cook A., et al. Deletions in VANGL1 are a risk factor for antibody-mediated kidney disease. Cell Rep. Med. 2021;2:100475. doi: 10.1016/j.xcrm.2021.100475. https://www.sciencedirect.com/science/article/pii/S2666379121003475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Watanabe H., Shen J. Protocols for assessing neurodegenerative phenotypes in Alzheimer’s mouse models. STAR Protoc. 2021;2:100654. doi: 10.1016/j.xpro.2021.100654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Eadara S., Jeon S., Liu Y., Muwanga G., Qu L., Caterina M.J., Meffert M.K. Combined single-molecule fluorescence in situ hybridization and immunohistochemistry analysis in intact murine dorsal root ganglia and sciatic nerve. STAR Protoc. 2021;2:100555. doi: 10.1016/j.xpro.2021.100555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles, K. (2021). Cooperative HTNWD at VUMC 2021, ‘Tissue Procurement: Cryopreservation with OCT Compound’, protocols.io. https://www.protocols.io/view/tissue-procurement-cryopreservation-with-oct-compo-4r3l25xyql1y/v1
- Wrobel L., Siddiqi F.H., Rubinsztein D.C. Transient siRNA-mediated protein knockdown in mouse followed by feeding/starving cycle and liver tissue analysis. STAR Protoc. 2021;2:100500. doi: 10.1016/j.xpro.2021.100500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate or analyze datasets and use code.