Abstract
Colorectal cancer (CRC) is a common cancer of the digestive system that endangers human health. Immunotherapy is widely used in the treatment of patients with cancer. Some patients with dMMR/MSI-H CRC benefit from treatments that use immune checkpoint inhibitors, but most CRC patients are not sensitive to immunotherapy. Furthermore, internal resistance and immune escape lead to a reduced immunotherapy response. Therefore, the development of an effective combination therapy to improve the response rate to immunotherapy is a goal of cancer research. Natural products are potential candidates for comprehensive cancer treatments due to their wide range of immunomodulatory effects through multifactorial underlying mechanisms. In this review, we summarize the challenges in the treatment of CRC and assess the immunomodulatory effects of natural products and their active components. Our work suggests that natural products represent potential options for combined CRC immunotherapy.
Keywords: colorectal cancer, immunotherapy, immune checkpoint inhibitor, natural products, immunomodulation
Introduction
The incidence rate and mortality rate of colorectal cancer (CRC) are third and second among all diseases, respectively, and CRC is characterized by a lack of obvious symptoms in the early stage and poor prognosis in the advanced stage (1). The main treatments for CRC are surgery, chemotherapy, radiotherapy, and targeted therapy. The emergence of immunotherapy has provided a transformative new method for the comprehensive treatment of cancer. An important function of the human immune system is to recognize and eliminate tumor cells, a process known as tumor immune surveillance, which is mainly performed by antigen-presenting cells, T lymphocytes, B lymphocytes and natural killer (NK) cells (2, 3). Cancer cells inhibit the body’s immune system in various ways to avoid the surveillance of the immune system, resulting in tumor immune escape (4, 5). Tumor immunotherapy is a treatment method used to control and eliminate cancer cells by restarting and maintaining the tumor immune cycle and restoring the body’s normal antitumor immune response. Tumor immunotherapy mainly involves immune checkpoint inhibitors (ICIs), cellular immunotherapy and cancer vaccines. At present, the administration of ICIs is the most widely used tumor immunotherapy method. Among ICIs, the representative (PDCD1,PD-1) inhibitor, its (CD274,PD-L1) inhibitor, and cytotoxic T-lymphocyte associated protein 4 (CTLA4) restore the ability of immune cells to fight tumors by counteracting the inhibition of the immune system by tumor cells. At present, several ICIs targeting PDCD1(PD-1), CD274(PD-L1) and CTLA4 have been approved for the clinical treatment of various solid tumors, including MSI-H/dMMR CRC (4, 6, 7). However, there are still many challenges in the treatment of CRC with ICIs. MSI-H/dMMR tumors account for 5% of CRC cases, and some patients can benefit from ICIs, but most CRC tumors are still in a “cold” state. Therefore, it is necessary to find methods to transform “cold” tumors into “hot” tumors to make them more sensitive to ICIs.
Natural products, including plants, mushrooms, bacteria, animal metabolism products or organs and even mineral substances, characterized by various structure and activities, are well-known by the researchers gradually in recent years. Some evidence show that natural products have potential immunomodulatory effects and can play a synergistic role when combined with ICIs. So it is more important to explore the mechanisms of nature products for providing strongly clinical evidence. In this review, we summarized the effects of natural products on modulating macrophages, T cells, NK cells and a combination of ICIs.
The Advantages and Limitations of ICIs for CRC Treatment
Currently, the main treatment for resectable CRC is surgery combined with chemotherapy or targeted medicines. However, metastatic CRC treatments remain challenging. According to retrospective analyses, some patients benefit less from 5-FU adjuvant chemotherapy (8, 9) because the molecular mechanism of CRC is different, and it may lead to more heterogeneity.
In 1997, the National Cancer Institute first defined microsatellite instability (MSI), which is a form of genomic instability associated with defective DNA mismatch repair (dMMR) in tumors; two mononucleotide repeats (BAT26 and BAT25) and three dinucleotide repeats (D5S346, D2S123, D17S250) were validated in the detection panel (10). MSI can currently be assessed by immunohistochemistry (IHC), including the expression of MSH2, MSH6, PMS2, MLH1 and polymerase chain reaction (PCR); moreover, novel next-generation sequencing (NGS) has become a new testing option. Intriguingly, investigators found that MSI-high (MSI-H) CRC is associated with increased neoantigen load and immune infiltration (11–14), which means that immune checkpoint blockade may be an effective method of therapy.
Currently, MSI assessment can influence the selection of clinical medications and predict outcomes in colorectal cancer (15, 16). KEYNOTE-164, a phase II clinical trial, demonstrated that pembrolizumab was effective in MSI-H-dMMR CRC; it displayed a higher overall response rate (ORR) and improved progression-free survival (PFS) (17). KEYNOTE-177, which enrolled patients with stage IV MSI-H-dMMR CRC, demonstrated that the PFS in the pembrolizumab group was prolonged by 8.3 months, with an ORR of 43.8%, and there were fewer treatment-related adverse events in the pembrolizumab group than in the chemotherapy group (18, 19). The PDCD1(PD-1) inhibitor nivolumab showed durable responses and disease control, and 51 patients with metastatic MSI-H CRC had disease control for 12 weeks or longer; these results were similar to the findings of the CheckMate-142 study (20). Meanwhile, nivolumab plus the CTLA4 inhibitor ipilimumab displayed effective responses: 80% of the 119 patients had a disease control rate over 12%, and the investigator-assessed ORR was 55% (21). Considering the dose-dependent effect of ipilimumab, nivolumab combined with a low dose of ipilimumab showed robust and durable clinical benefit, with a 69% ORR and 84% disease control rate until the data cutoff (22). Based on the results of numerous clinical trials, the 2021 NCCN guidelines suggest that patients with advanced or metastatic CRC can use immunotherapy checkpoint blockade for subsequent therapy.
Although the results of clinical trials on dMMR/MSI-H are exciting, the response rates range between 30% and 50%, suggesting that resistance and immune escape still exist (23–25). More clinical trials have focused on the combination of VEGF inhibitors and chemotherapy (26–28); most of the studies are ongoing and the results are pending.
How to Make ICIs More Effective for CRC
The incidence of dMMR CRC is approximately 5%, which is far lower than that of proficient mismatch repair (pMMR) CRC (29). The conventional treatment of pMMR/microsatellite stable (MSS) CRC is systematic chemotherapy based on 5-fluorouracil and platinum, and most of patients have lower responses to immunotherapy due to intrinsic resistance. The mechanism may involve low TMB, a lack of tumor antigens and a suppressive microenvironment (30, 31). How to turn “cold” tumors into “hot” tumors is currently a popular topic in academic research.
Some studies have shown that chemotherapy can improve immunogenicity and enhance the efficacy of ICIs (32, 33). Currently, some ongoing clinical trials are exploring chemotherapy combined with angiogenesis medicine and ICIs. A protocol for unresectable metastatic CRC was described in the AtezoTRIBE study that enrolled patients receiving FOLFOXIRI plus bevacizumab; some patients received sequential atezolizumab (34). A study of chemorefractory MSS mCRC included two cohorts: one was pembrolizumab plus pemetrexed, and in the other oxaliplatin was added for the dose escalation portion of the study (35). Although the trials have not shown the endpoint and some did not consider the microsatellite status, they represent worthwhile attempts.
Meanwhile, antiangiogenic and multitarget drugs also display synergistic sensitivity to ICIs. Innate immunity and immune adoption can directly lead to tumor angiogenesis (36), and the most widely studied VEGF family also drives angiogenesis to promote immune escape and immune suppression (37). Bevacizumab is the first antiangiogenic drug approved for metastatic CRC, NSCLC, metastatic renal cell carcinoma, and recurrent/metastatic cervical cancer (38, 39). Combining bevacizumab with immunotherapy promoted T cell infiltration, enhanced local immune activation and inhibited the expansion of MDSCs in preclinical studies (40, 41). Likewise, the use of bevacizumab combined with ICIs has been studied in many clinical trials, such as those investigating NSCLC, recurrent glioblastoma (42) and ovarian cancer (6, 43, 44); clinical trials for CRC are still ongoing, especially for MSS/pMMR CRC (45–47). In addition, multitarget antiangiogenic drugs show better responses. An open-label, phase II trial that enrolled 25 CRC patients demonstrated that a dose of regorafenib 80 mg can increase sensitivity to nivolumab, and the median PFS was 7.9 months (48). Similarly, a case report showed that in an MSS patient who received fruquintinib plus sindilizumab for six cycles, the lymph nodes became fewer and smaller, and CA199 was decreased (49). In a phase II study of patients with advanced refractory CRC, the median OS was 6.6 months for patients who received durvalumab and tremelimumab compared with the cohort who received supportive care, and the patients accepted the continuation of treatment with TAS-102 or regorafenib after disease progression (50). Not only the clinical report but also the author-selected syngeneic MSS model demonstrated that the combination of the two drugs could inhibit proliferation, induce apoptosis and promote vascular normalization (49). Hence, ICIs combined with antiangiogenic drugs may be a promising method of MSS CRC treatment.
In addition, CRC has some mutations driven by RAS, BRAF, EGFR, HER-2 and POLE, and most of them can impact treatment and prognosis. For example, approximately 10% of patients diagnosed with CRC harbor the BRAF mutation, which is considered a poor prognostic factor, and one third of mutations are associated with MSI (51–54). As shown in the Checkmate-142 study, the ORR was 25% in the BRAF mutation group and similar to that in the combined nivolumab and ipilimumab group (21, 22). A case report suggested that a patient harboring MSS and BRAF V600E mutations responded well to nivolumab and bevacizumab, achieving more than 17 months of PFS (55). However, some studies have reported that the BRAF mutation does not influence the response to immunotherapy (56, 57), and combination therapy needs to be further explored in large samples. Apart from the BRAF V600E mutation, a common oncogenic mutation is RAS mutation, especially KRAS mutation, which accounts for approximately 40% of CRC cases, and is related to poor prognosis and metastasis (58, 59). In addition, the KRAS mutation in CRC is associated with immune suppression and immune infiltration (60, 61). Some current clinical trials are aimed at investigating these mutations. A phase I/II study enrolled CRC patients with RAS mutations regardless of MSS status to assess the safety and efficacy of the combination of durvalumab and tremelimumab (62). Likewise, a phase II study focused CRC with RAS or BRAF mutations and investigated the use of nivolumab combined with FOLFOXIRI/bevacizumab (63). These studies demonstrate that immunotherapy still has considerable potential for the treatment of CRC mutations.
Natural Products Exert Modulating Effects on the Immune System in CRC
In addition to using the above-mentioned methods, researchers have paid attention to natural products. Natural products include the active compounds in plants, mushrooms, bacteria, animal metabolism products or organs and even mineral substances. These products have been explored and used for a thousand years. Some active compounds have proven antitumor, antioxidant, and anti-inflammatory effects (64). However, there are still a number of natural products that have adverse or toxic effects; these products must be used properly or avoided. The specific mechanism of natural products is still unknown and requires further investigation. According to recent evidence, natural products can directly regulate innate immunity and adoptive immunity (65); they play a role in preventing tumor development and modulating immunity (66–68). Thus, natural products show promise as agents in immunotherapy.
First, natural products can influence the immune microenvironment of early CRC in multiple ways, affecting M2 macrophage polarization to M1 to exert an immunomodulation effect. Isoliquiritigenin, a flavonoid derived from licorice, blocks M2 polarization in colitis-related tumorigenesis and inhibits the development of colorectal cancer by downregulating PGE2/IL6 signaling (69). Apple polysaccharides not only prevent the carcinogenesis induced by AOM/DSS in mice but also modulate the M2 to M1 macrophage phenotype and upregulate TLR4/NF-κB signaling (70). Most basic experiments have adopted the AOM/DSS model or the CAC model to indicate the mechanism by which natural products affect macrophages (70–75). Taken together, these results show that there are many natural products that play important roles in inflammatory cancer transformation via different mechanisms, and natural products will intervene in CRC development in the near future.
Natural products can also influence T cells, NK cells and Treg cells. Black raspberries can significantly inhibit CRC progression and increase NK cells in tissues infiltrating the APC Min+/- DSS and AOM/DSS models, and the results were validated in human CRC tissue (76, 77). In addition, Ecklonia cava fucoidan (ECF) not only stimulates NK cell activation and proliferation but also induces NK cell activation through DCs (78). Moreover, rice hull polysaccharides (RHPS) can enhance NK cell activation and induce the secretion of INFG (INF-gamma) and TNF(TNF-alpha) in vitro; they also inhibit tumors in CT-26-bearing mice and enhance NK cell activation in vivo ( 79). It is clear that natural products demonstrate antitumor effects by influencing NK cell activity.
Similarly, natural products can cause T cells to exert immune modulating effects. Another well-known immunomodulatory natural product, curcumin, may suppress the expression of FOXP3 on Tregs and enhance the ability of T cells to kill tumor cells and modulate multiple immune cytokines (80–83). A control study revealed that the administration of curcumin can suppress the transcription of the FOXP3 gene and convert Tregs to Th1 cells by enhancing INFG (INF-gamma) production (84). In an in vivo experiment, researchers selected a CT-26 mouse model to compare curcumin and sildenafil combined with anti-PDCD-1(PD-1) and showed that the tumor volume was smaller in the combined treatment group (85). Based on preclinical research, curcumin is a potential natural product, especially when combined with immunotherapy. In addition, natural products can inhibit CRC metastasis by regulating the tumor microenvironment. The natural small molecule bigelovin may inhibit colorectal tumor growth by regulating the tumor immune microenvironment, increasing the T lymphocyte and macrophage populations, and inhibiting liver and lung metastasis of CRC through the IL6/STAT3 pathway (86). Furthermore, natural products can also upregulate IL-17 secretion to stimulate T cell proliferation or differentiation. Gan cao (Glycyrrhiza uralensis Fisch.) polysaccharides, especially those of low molecular weight, can upregulate IL-17 and enhance T lymphocyte proliferation (87). The author found that red wine extract could inhibit tumor progression and affect T lymphocyte cell differentiation into T helper 17 cells (88). Other studies have shown that natural products can alleviate tumor growth and modulate immunity by restoring intestinal barriers (89), inducing DC maturation (90) and reducing the accumulation of myeloid-derived suppressor cells (MDSCs) (91). It’s noteworthy that quercetin and alantolactone not only can induce immunogenic cell death and cell apoptosis for MSS CRC, but also can reduce immunosuppressive cell population like MDSCs, Treg and so on. This study adjusted nanoformulated codelivery, on the other hand provided a basis for multi-drug combination of nature products (92).
Natural products combined with ICIs demonstrate better responses in patients, and this strategy may be a prospective method for use in the clinic. Many studies have investigated natural products other than curcumin. Atractylenolide I significantly improves the cytotoxic effect of T lymphocytes on tumor cells and promotes the antigen presentation of tumor cells. Atractylenolide I has a synergistic effect in the treatment of CRC when combined with immune checkpoint inhibitors (93). Astragaloside IV can significantly induce M2 macrophages to M1 polarization, decrease the production of anti-inflammatory factors and increase proinflammatory INFG (INF-gamma) in colorectal tumors (94). Meanwhile, astragaloside IV combined with a PDCD1(PD-1) inhibitor exhibited a synergistic effect on inhibiting tumor growth and T cell infiltration. Inulin, which is derived from dietary fiber, can significantly improve the systemic antitumor efficacy of anti-PDCD-1(PD-1) therapy and effectively slow tumor growth by altering the gut microbiome. Compared with anti-PDCD-1(PD-1) alone, the synergistic use of inulin and anti-PDCD-1(PD-1) significantly increased CT-26 GP70-specific CD8+ T cells in mice. Interestingly, by transforming inulin into inulin gel before its use in combination with anti-PDCD-1(PD-1), the effect was improved (95). This suggests that natural products have potential regarding changes in the forms of administered medicines. Many experiments have simulated the combination of natural products and ICIs (96–98). A preclinical study was conducted that explored anti-PDCD-1(PD-1) alone and in combination with natural products and anti-CTLA4. High-dose vitamin C can decrease tumor volumes combined with anti-PDCD-1(PD-1) and anti-CTLA4 and enhance CD8+ T cell cytotoxic activity. This research was conducted not only in CRC but also in breast cancer, pancreatic cancer and melanoma with mismatch repair-deficient tumors with a high mutational burden (99) ( Table 1).
Table 1.
Natural products exert modulating effects on the immune system in CRC.
| Nature product | Model | Results | Reference |
|---|---|---|---|
| Isoliquiritigenin | AOM/DSS mice | PGE2 ↓ IL6 ↓ | (69) |
| Raw267.4 cell | M2 polarization ↓ | ||
| Mouse peritoneal macrophages | p-STAT3 ↓ iNOS ↑ | ||
| CLCX10 ↑ | |||
| Apple polysaccharide | AOM/DSS mice | TLR-4 Myd88 p65 ↑ | (70) |
| Raw267.4 cell | |||
| Vitexin | AOM/DSS mice | M1 ↑ | (71) |
| CAC model | TNF(TNF-alpha) IL-1β IL6 ↓ | ||
| NO in tumor tissue ↑ | |||
| β-Carotene | AOM/DSS mice | IL6 pSTAT3 ↓ | (72) |
| U937 cells | |||
| Berberine | AOM/DSS | EGFR-ERK signaling ↓ | (73) |
| APC Min/+ mice | |||
| Raw267.4 cell | |||
| Cardamonin | Raw267.4 cell | iNOS TNF(TNF-alpha)IL6 ↓ | (75) |
| HCT-116 | NF-κB ↓ | ||
| Black Raspberries | APC Min/+/DSS | NK cells infiltration ↑ | (76) |
| AOM/DSS | |||
| Human CRC tissues | |||
| Elkonia cava fucoidan | CT-26 | NK cells activation ↑ | (78) |
| NK cells and DCs from C57BL/6 spleen | INFG (INF-gamma) ↑ | ||
| Rice hull polysaccharides | NK-92 MI | NK cells activation ↑ | (79) |
| CT-26 | INFG (INF-gamma) ↑ | ||
| Curcumin | Advanced CRC patients PBMC | FOXP3+Treg ↓ | (84) |
| INFG (INF-gamma) ↑ | |||
| Curcumin | CT-26 | Tumor volume ↓ | (85) |
| Bigelovin | CT-26 | T lymphocyte | (86) |
| HCT-116 | Macrophage population ↑ | ||
| Glycyrrhiza uralensis Fisch. | CT-26 | IL-17 ↑ | (87) |
| T lymphocyte proliferation ↑ | |||
| Red wine extract | CT-26 | Th17 differentiation ↓ | (88) |
| HCT-116 | |||
| SW620 | |||
| MC38 | |||
| Dendrobium officinale polysaccharides | AOM/DSS | — | (89) |
| Maitake Z-fraction | Colon-26 mice | — | (90) |
| Juglone | CT-26 | INFG (INF-gamma) ↑ | (91) |
| MDSCs | MDSCs accumulation ↓ | ||
| Quercetin and Alantolactone | CT-26 | MDSCs Treg IL-10 IL-1β TGF-β CCL-2 ↓ | (92) |
| Orthotopic colorectal cancer model | |||
| Atractylenolide I | MC38 | CD8+ T cell ↑ | (93) |
| MC38-OVA | |||
| OT-1 mice | |||
| Astragaloside IV | CT-26 mice | TGF-β IL-10 VEGF-α ↓ | (94) |
| INFG (INF-gamma) IL-12 TNF(TNF-alpha) ↑ | |||
| Inulin | CT-26 mice | SCFAs (feces) ↑ | (95) |
| Induce stem-like Tcf+PD-1+T cell | |||
| Sanguisorbae Radix | Humanized PD-L1 MC38 mice | Infiltrated cytotoxic T cells ↑ | (96) |
| Recombinant Jurkat T cells | |||
| Pectin | MC38 mice | Infiltrated cytotoxic T cells ↑ | (97) |
| CRC patients fecal microbiota transplantation model | Butyrate (feces) ↑ | ||
| Andrographolide | CT-26 mice | CD8+ and CD4+ T cell function ↑ | (98) |
| Fasl perforin ↑Granzyme B ↑ | |||
| High-dose vitamin C | CT-26 | CD8+ T cell cytotoxic ↑ | (99) |
| MC38 |
Natural products combined with ICIs had better results in melanoma, lung cancer and breast cancer studies (100, 101), and they can be gradually extended to the study of pancancer in the future.
Conclusion and Perspectives
Epidemiologic evidence show that the CRC incidence is strongly related to interaction between the environment exposures and gene alternations (102). Colorectal carcinogenesis includes three major global genetic and epigenetic aberrations: chromosomal instability (CIN), CpG island methylator phenotype (CIMP) and MSI. Although gene factors may lead to the individual risk and increase hereditary susceptibility, CRC are largely affected by diet factors and lifestyle alterations (103), like smoking, alcohol, obesity and so on (104–106). A study demonstrated that high-fat-diet-induced obesity may impair CD8+T cell function in the murine tumor microenvironment through the metabolic pathway (107). Conversely lifestyle and diet factors can also affect gene alternation to contribute to the onset of CRC. Similarly smoking was associated with a 59% increased risk of CRC and strongly related to MSI-H and KRAS wild type CRC in a large case-control study which enrolled 4919 participants (108).
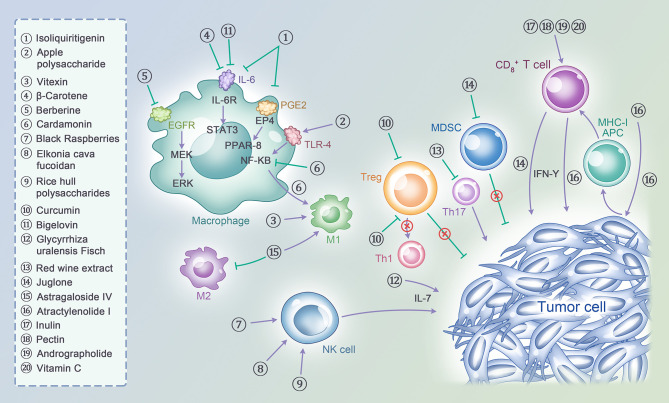
Base on the relationship between diet factors or lifestyle and immunity, some researchers addressed a concept of molecular pathological epidemiology (MPE) which could provide the better understanding of environment-tumor-immune interactions (109). Among them, the researches proposed several classes of substance with immunomodulatory effects in CRC, including aspirin, vitamin D, inflammatory diets and omega-3 polyunsaturated fatty acids. Take an example for omega-3 polyunsaturated, a higher intake of marine omega-3 polyunsaturated was associated with the risk of CRC with different density FOXP3+ cells (110). Since the introduction of immunotherapies, some patients have benefited, but there are still crucial problems to be solved. Preclinical studies have shown that natural products can exert antitumor effects and modulate immunity by affecting T cells, NK cells, and Tregs in CRC (Figure 1). If researches adopt the MPE model and integrate the immunotherapies to the model in the future, that will be a promising method which can provide more accurate strategies for the treatment, especially the field of nature products which link to the environment and immune. Natural products have some limitations; the ranges of safe doses remain undetermined and adverse effects such as hepatotoxicity and renal toxicity must be controlled. Natural products have the advantages of being easy to obtain and widely used, and they have multiple targets. Natural products have been proven effective in the early stage of CRC, especially on the transformation of adenoma to adenocarcinoma, and in advanced cancer stages, natural products can inhibit tumor progression. Meanwhile, combining natural products with ICIs can maximize the antitumor effects by acting on multiple targets.
Figure 1.
A variety of natural products act on act on multiple cell subtypes in the tumor immune microenvironment, including T cells, NK cells, Macrophages, MDSCs, Tregs and tumor cells themselves, to exert immunomodulatory effects and thus enhance the ability of anti-tumor immunity.
In summary, natural products can regulate the immune system and enhance immuno-oncological effects, especially when combined with ICIs, which will be a promising strategy in the future that is gradually accepted into clinical practice.
Author Contributions
HX and AZ proposed the topic and made the frame. JD and YQ contributed to original draft preparation. GZ, LL, and SZ participated in part of text arrangement and literature collection. LL and JD participated in the conception and drawing of the image. GJ, AZ, and HX revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Nature Science Foundation of China, No. 81874206, 82104466; Shanghai Frontiers Science Center of Disease and Syndrome Biology of Inflammatory Cancer Transformation (2021KJ03-12); Shanghai Rising-Star Program, No. 20QA1409300; and the Program for Young Eastern Scholar at Shanghai Institutions of Higher Learning, No. QD2019034.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRC, Colorectal cancer; dMMR, defective DNA mismatch repair; MSI, Microsatellite instability; MSI-H, Microsatellite instability High; NK cell, Nature killer cell; ICIs, Immune checkpoint inhibitors; PD-1, Programmed cell death 1; CD274(PD-L1), Programmed cell death ligand 1; CTLA4, Cytotoxic T-lymphocyte associated protein 4; IHC, Immunohistochemistry; PCR, Polymerase chain reaction; NGS, Novel next-generation sequencing; ORR, Overall response rate; PFS, Progression-free survival; NCCN, National Comprehensive Cancer Network; VEGF, Vascular endothelial growth factor; pMMR, proficient mismatch repair; MSS, Microsatellite stable; TMB, Tumor mutation burden; NSCLC, Non-small-cell lung cancers; MDSC, Myeloid-derived suppressor cells; OS, Overall survival; PGE2, Prostaglandin E2; IL6, Interleukin 6; AOM, Azoxymethane; DSS, Dextran sodium sulfate; TLR4, Toll-like receptor 4; NF-κB, Nuclear factor-kappa B; INFG (INF-gamma), Interferon-gamma; TNF(TNF-alpha), Tumor necrosis factor-alpha; STAT3, Signal transducer and activator of transcription 3.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Erratum: Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2020) 70(4):313. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Boulch M, Grandjean CL, Cazaux M, Bousso P. Tumor Immunosurveillance and Immunotherapies: A Fresh Look From Intravital Imaging. Trends Immunol (2019) 40(11):1022–34. doi: 10.1016/j.it.2019.09.002 [DOI] [PubMed] [Google Scholar]
- 3. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat Med (2018) 24(5):541–50. doi: 10.1038/s41591-018-0014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab Plus Dacarbazine for Previously Untreated Metastatic Melanoma. N Engl J Med (2011) 364(26):2517–26. doi: 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 5. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival With Ipilimumab in Patients With Metastatic Melanoma. N Engl J Med (2010) 363(8):711–23. doi: 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med (2018) 378(24):2288–301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 7. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors With Mismatch-Repair Deficiency. N Engl J Med (2015) 372(26):2509–20. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective Mismatch Repair as a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J Clin Oncol (2010) 28(20):3219–26. doi: 10.1200/JCO.2009.27.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, et al. Multicenter Retrospective Analysis of Metastatic Colorectal Cancer (CRC) With High-Level Microsatellite Instability (MSI-H). Ann Oncol (2014) 25(5):1032–8. doi: 10.1093/annonc/mdu100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for Cancer Detection and Familial Predisposition: Development of International Criteria for the Determination of Microsatellite Instability in Colorectal Cancer. Cancer Res (1998) 58(22):5248–57PMID: 9823339. [PubMed] [Google Scholar]
- 11. Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep (2016) 15(4):857–65. doi: 10.1016/j.celrep.2016.03.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The Vigorous Immune Microenvironment of Microsatellite Instable Colon Cancer is Balanced by Multiple Counter-Inhibitory Checkpoints. Cancer Discovery (2015) 5(1):43–51. doi: 10.1158/2159-8290.CD-14-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becht E, de Reyniès A, Giraldo NA, Pilati C, Buttard B, Lacroix L, et al. Immune and Stromal Classification of Colorectal Cancer Is Associated With Molecular Subtypes and Relevant for Precision Immunotherapy. Clin Cancer Res (2016) 22(16):4057–66. doi: 10.1158/1078-0432.CCR-15-2879 [DOI] [PubMed] [Google Scholar]
- 14. Lin A, Zhang J, Luo P. Crosstalk Between the MSI Status and Tumor Microenvironment in Colorectal Cancer. Front Immunol (2020) 11:2039. doi: 10.3389/fimmu.2020.02039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade. Science (2017) 357(6349):409–13. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, et al. ESMO Recommendations on Microsatellite Instability Testing for Immunotherapy in Cancer, and its Relationship With PD-1/PD-L1 Expression and Tumour Mutational Burden: A Systematic Review-Based Approach. Ann Oncol (2019) 30(8):1232–43. doi: 10.1093/annonc/mdz116 [DOI] [PubMed] [Google Scholar]
- 17. Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H, et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol (2020) 38(1):11–9. doi: 10.1200/JCO.19.02107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med (2020) 383(23):2207–18. doi: 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 19. Andre T, Amonkar M, Norquist JM, Shiu KK, Kim TW, Jensen BV, et al. Health-Related Quality of Life in Patients With Microsatellite Instability-High or Mismatch Repair Deficient Metastatic Colorectal Cancer Treated With First-Line Pembrolizumab Versus Chemotherapy (KEYNOTE-177): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol (2021) 22(5):665–77. doi: 10.1016/S1470-2045(21)00064-4 [DOI] [PubMed] [Google Scholar]
- 20. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in Patients With Metastatic DNA Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer (CheckMate 142): An Open-Label, Multicentre, Phase 2 Study. Lancet Oncol (2017) 18(9):1182–91. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol (2018) 36(8):773–9. doi: 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 22. Lenz HJ, Van Cutsem E, Luisa Limon M, Wong KYM, Hendlisz A, Aglietta M, et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J Clin Oncol (2021) 40(2):161–70. doi: 10.1200/JCO.21.01015. [DOI] [PubMed] [Google Scholar]
- 23. Vasaikar S, Huang C, Wang X, Petyuk VA, Savage SR, Wen B, et al. Proteogenomic Analysis of Human Colon Cancer Reveals New Therapeutic Opportunities. Cell (2019) 177(4):1035–1049.e19. doi: 10.1016/j.cell.2019.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gurjao C, Liu D, Hofree M, AlDubayan SH, Wakiro I, Su MJ, et al. Intrinsic Resistance to Immune Checkpoint Blockade in a Mismatch Repair-Deficient Colorectal Cancer. Cancer Immunol Res (2019) 7(8):1230–6. doi: 10.1158/2326-6066.CIR-18-0683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sahin IH, Akce M, Alese O, Shaib W, Lesinski GB, El-Rayes B, et al. Immune Checkpoint Inhibitors for the Treatment of MSI-H/MMR-D Colorectal Cancer and a Perspective on Resistance Mechanisms. Br J Cancer (2019) 121(10):809–18. doi: 10.1038/s41416-019-0599-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. PD-1 Inhibitors Combined With VEGF Inhibitors for Locally Advanced dMMR/MSI-H Colorectal Cancer. Nct04715633. Guangdong Province. Available at: https://clinicaltrials.gov/ct2/show/NCT04715633?cond=Nct04715633.&draw=2&rank=1#studydesc. [Google Scholar]
- 27. An Investigational Immuno-Therapy Study of Nivolumab, and Nivolumab in Combination With Other Anti-Cancer Drugs, in Colon Cancer That Has Come Back or Has Spread (Checkmate142). NCT02060188 https://clinicaltrials.gov/ct2/show/NCT02060188?cond=NCT02060188.&draw=2&rank=1start with February 11, 2014. [Google Scholar]
- 28. PD-1 Inhibitor Combined With Bevacizumab and FOLFIRI Regimen in the Second-Line Treatment of Advanced Colorectal Cancer. Nct05035381 Tianjin province: start with September 5, 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT05035381?cond=Nct05035381&draw=2&rank=1. [Google Scholar]
- 29. Koopman M, Kortman GA, Mekenkamp L, Ligtenberg MJ, Hoogerbrugge N, Antonini NF, et al. Deficient Mismatch Repair System in Patients With Sporadic Advanced Colorectal Cancer. Br J Cancer (2009) 100(2):266–73. doi: 10.1038/sj.bjc.6604867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim CW, Chon HJ, Kim C. Combination Immunotherapies to Overcome Intrinsic Resistance to Checkpoint Blockade in Microsatellite Stable Colorectal Cancer. Cancers (Basel) (2021) 13(19):4906. doi: 10.3390/cancers13194906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity (2016) 44(3):698–711. doi: 10.1016/j.immuni.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 32. Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JHM, et al. Enhancing Antitumor Response by Combining Immune Checkpoint Inhibitors With Chemotherapy in Solid Tumors. Ann Oncol (2019) 30(2):219–35. doi: 10.1093/annonc/mdy551 [DOI] [PubMed] [Google Scholar]
- 33. Guan Y, Kraus SG, Quaney MJ, Daniels MA, Mitchem JB, Teixeiro E, et al. FOLFOX Chemotherapy Ameliorates CD8 T Lymphocyte Exhaustion and Enhances Checkpoint Blockade Efficacy in Colorectal Cancer. Front Oncol (2020) 10:586. doi: 10.3389/fonc.2020.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antoniotti C, Borelli B, Rossini D, Pietrantonio F, Morano F, Salvatore L, et al. AtezoTRIBE: A Randomised Phase II Study of FOLFOXIRI Plus Bevacizumab Alone or in Combination With Atezolizumab as Initial Therapy for Patients With Unresectable Metastatic Colorectal Cancer. BMC Cancer (2020) 20(1):683. doi: 10.1186/s12885-020-07169-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Study of Pembrolizumab With Pemetrexed and Oxaliplatin in ChemoRefractory Metastatic Colorectal Cancer Patients. Nct03626922. Available at: https://clinicaltrials.gov/ct2/show/NCT03626922.
- 36. Albini A, Bruno A, Noonan DM, Mortara L. Contribution to Tumor Angiogenesis From Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front Immunol (2018) 9:527. doi: 10.3389/fimmu.2018.00527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rahma OE, Hodi FS. The Intersection Between Tumor Angiogenesis and Immune Suppression. Clin Cancer Res (2019) 25(18):5449–57. doi: 10.1158/1078-0432.CCR-18-1543 [DOI] [PubMed] [Google Scholar]
- 38. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat Rev (2020) 86:102017. doi: 10.1016/j.ctrv.2020.102017 [DOI] [PubMed] [Google Scholar]
- 39. Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. Continuation of Bevacizumab After First Progression in Metastatic Colorectal Cancer (ML18147): A Randomised Phase 3 Trial. Lancet Oncol (2013) 14(1):29–37. doi: 10.1016/S1470-2045(12)70477-1 [DOI] [PubMed] [Google Scholar]
- 40. Yasuda S, Sho M, Yamato I, Yoshiji H, Wakatsuki K, Nishiwada S, et al. Simultaneous Blockade of Programmed Death 1 and Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) Induces Synergistic Anti-Tumour Effect In Vivo. Clin Exp Immunol (2013) 172(3):500–6. doi: 10.1111/cei.12069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hegde P, Wallin J, Mancao C. Predictive Markers of Anti-VEGF and Emerging Role of Angiogenesis Inhibitors as Immunotherapeutics. Semin Cancer Biol (2018) 52:117–24. doi: 10.1016/j.semcancer.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 42. Harter P, Pautier P, Van Nieuwenhuysen E, Reuss A, Redondo A, et al. Atezolizumab in Combination With Bevacizumab and Chemotherapy Versus Bevacizumab and Chemotherapy in Recurrent Ovarian Cancer - A Randomized Phase III Trial (AGO-OVAR 2.29/ENGOT-Ov34). Int J Gynecol Cancer (2020) 30(12):1997–2001. doi: 10.1136/ijgc-2020-001572 [DOI] [PubMed] [Google Scholar]
- 43. Reck M, Wehler T, Orlandi F, Nogami N, Barone C, Moro-Sibilot D, et al. Safety and Patient-Reported Outcomes of Atezolizumab Plus Chemotherapy With or Without Bevacizumab Versus Bevacizumab Plus Chemotherapy in Non-Small-Cell Lung Cancer. J Clin Oncol (2020) 38(22):2530–42. doi: 10.1200/JCO.19.03158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol (2020) 6(7):1003–10. doi: 10.1001/jamaoncol.2020.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mfolfox6+Bevacizumab+PD-1 Monoclonal Antibody in Local Advanced MSS CRC (BASKETII). Nct04895137, Guangdong Province. Available at: https://clinicaltrials.gov/ct2/show/NCT04895137?cond=Nct04895137&draw=1&rank=1. [Google Scholar]
- 46. Chemotherapy and Immunotherapy as Treatment for MSS Metastatic Colorectal Cancer With High Immune Infiltrate (POCHI). Nct04262687. Available at:https://clinicaltrials.gov/ct2/show/NCT04262687?cond=Nct04262687&draw=2&rank=1 [Google Scholar]
- 47. Combination Chemotherapy, Bevacizumab, and/or Atezolizumab in Treating Patients With Deficient DNA Mismatch Repair Metastatic Colorectal Cancer, the COMMIT Study. Nct02997228. Available at: https://clinicaltrials.gov/ct2/show/NCT02997228?cond=Nct02997228.&draw=2&rank=1. [Google Scholar]
- 48. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, Epoc1603). J Clin Oncol (2020) 38(18):2053–61. doi: 10.1200/JCO.19.03296 [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Wei B, Gao J, Cai X, Xu L, Zhong H, et al. Combination of Fruquintinib and Anti-PD-1 for the Treatment of Colorectal Cancer. J Immunol (2020) 205(10):2905–15. doi: 10.4049/jimmunol.2000463 [DOI] [PubMed] [Google Scholar]
- 50. Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol (2020) 6(6):831–8. doi: 10.1001/jamaoncol.2020.0910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D, et al. Mismatch Repair Status and BRAF Mutation Status in Metastatic Colorectal Cancer Patients: A Pooled Analysis of the CAIRO, CAIRO2, COIN, and FOCUS Studies. Clin Cancer Res (2014) 20(20):5322–30. doi: 10.1158/1078-0432.CCR-14-0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fariña-Sarasqueta A, van Lijnschoten G, Moerland E, Creemers GJ, Lemmens VEPP, Rutten HJT, et al. The BRAF V600E Mutation is an Independent Prognostic Factor for Survival in Stage II and Stage III Colon Cancer Patients. Ann Oncol (2010) 21(12):2396–402. doi: 10.1093/annonc/mdq258 [DOI] [PubMed] [Google Scholar]
- 53. Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of BRAF Mutation and Microsatellite Instability on the Pattern of Metastatic Spread and Prognosis in Metastatic Colorectal Cancer. Cancer (2011) 117(20):4623–32. doi: 10.1002/cncr.26086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taieb J, Lapeyre-Prost A, Laurent Puig P, Zaanan A. Exploring the Best Treatment Options for BRAF-Mutant Metastatic Colon Cancer. Br J Cancer (2019) 121(6):434–42. doi: 10.1038/s41416-019-0526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fang C, Lin J, Zhang T, Luo J, Nie D, Li M, et al. Metastatic Colorectal Cancer Patient With Microsatellite Stability and BRAF Mutation Showed a Complete Metabolic Response to PD-1 Blockade and Bevacizumab: A Case Report. Front Oncol (2021) 11:652394. doi: 10.3389/fonc.2021.652394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Asaoka Y, Ijichi H, Koike K. PD-1 Blockade in Tumors With Mismatch-Repair Deficiency. N Engl J Med (2015) 373(20):1979: doi: 10.1056/NEJMc1510353. [DOI] [PubMed] [Google Scholar]
- 57. Molina-Cerrillo J, San Román M, Pozas J, Alonso-Gordoa T, Pozas M, Conde E, et al. BRAF Mutated Colorectal Cancer: New Treatment Approaches. Cancers (Basel) (2020) 12(6). doi: 10.3390/cancers12061571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, et al. Kirsten Ras Mutations in Patients With Colorectal Cancer: The 'RASCAL II' Study. Br J Cancer (2001) 85(5):692–6. doi: 10.1054/bjoc.2001.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Uhlig J, Cecchini M, Sheth A, Stein S, Lacy J, Kim HS. Microsatellite Instability and KRAS Mutation in Stage IV Colorectal Cancer: Prevalence, Geographic Discrepancies, and Outcomes From the National Cancer Database. J Natl Compr Canc Netw (2021) 19(3):307–18. doi: 10.6004/jnccn.2020.7619 [DOI] [PubMed] [Google Scholar]
- 60. Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P, et al. KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell (2019) 35(4):559–572.e7. doi: 10.1016/j.ccell.2019.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu J, Huang X, Liu H, Wei C, Ru H, Qin H, et al. Immune Landscape and Prognostic Immune-Related Genes in KRAS-Mutant Colorectal Cancer Patients. J Transl Med (2021) 19(1):27. doi: 10.1186/s12967-020-02638-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fumet JD, Isambert N, Hervieu A, Zanetta S, Guion JF, Hennequin A, et al. Phase Ib/II Trial Evaluating the Safety, Tolerability and Immunological Activity of Durvalumab (MEDI4736) (Anti-PD-L1) Plus Tremelimumab (Anti-CTLA-4) Combined With FOLFOX in Patients With Metastatic Colorectal Cancer. ESMO Open (2018) 3(4):e000375. doi: 10.1136/esmoopen-2018-000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Damato A, Iachetta F, Antonuzzo L, Nasti G, Bergamo F, Bordonaro R, et al. Phase II Study on First-Line Treatment of NIVolumab in Combination With Folfoxiri/Bevacizumab in Patients With Advanced COloRectal Cancer RAS or BRAF Mutated - NIVACOR Trial (GOIRC-03-2018). BMC Cancer (2020) 20(1):822. doi: 10.1186/s12885-020-07268-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Diederich M. Natural Products Target the Hallmarks of Chronic Diseases. Biochem Pharmacol (2020) 173:113828. doi: 10.1016/j.bcp.2020.113828 [DOI] [PubMed] [Google Scholar]
- 65. Samec M, Liskova A, Koklesova L, Samuel SM, Murin R, Zubor P, et al. The Role of Plant-Derived Natural Substances as Immunomodulatory Agents in Carcinogenesis. J Cancer Res Clin Oncol (2020) 146(12):3137–54. doi: 10.1007/s00432-020-03424-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li TY, Chiang BH. 4-Acetylantroquinonol B From Antrodia Cinnamomea Enhances Immune Function of Dendritic Cells Against Liver Cancer Stem Cells. BioMed Pharmacother (2019) 109:2262–9. doi: 10.1016/j.biopha.2018.11.101 [DOI] [PubMed] [Google Scholar]
- 67. Mahmoud YK, Abdelrazek HMA. Cancer: Thymoquinone Antioxidant/Pro-Oxidant Effect as Potential Anticancer Remedy. BioMed Pharmacother (2019) 115:108783. doi: 10.1016/j.biopha.2019.108783 [DOI] [PubMed] [Google Scholar]
- 68. Ye H, He X, Feng X. Developing Neobavaisoflavone Nanoemulsion Suppresses Lung Cancer Progression by Regulating Tumor Microenvironment. BioMed Pharmacother (2020) 129:110369. doi: 10.1016/j.biopha.2020.110369 [DOI] [PubMed] [Google Scholar]
- 69. Zhao H, Zhang X, Chen X, Li Y, Ke Z, Tang T, et al. Isoliquiritigenin, a Flavonoid From Licorice, Blocks M2 Macrophage Polarization in Colitis-Associated Tumorigenesis Through Downregulating PGE2 and IL-6. Toxicol Appl Pharmacol (2014) 279(3):311–21. doi: 10.1016/j.taap.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 70. Sun Y, Diao F, Niu Y, Li X, Zhou H, Mei Q, et al. Apple Polysaccharide Prevents From Colitis-Associated Carcinogenesis Through Regulating Macrophage Polarization. Int J Biol Macromol (2020) 161:704–11. doi: 10.1016/j.ijbiomac.2020.06.121 [DOI] [PubMed] [Google Scholar]
- 71. Chen Y, Wang B, Yuan X, Lu Y, Hu J, Gao J, et al. Vitexin Prevents Colitis-Associated Carcinogenesis in Mice Through Regulating Macrophage Polarization. Phytomedicine (2021) 83:153489. doi: 10.1016/j.phymed.2021.153489 [DOI] [PubMed] [Google Scholar]
- 72. Lee NY, Kim Y, Kim YS, Shin JH, Rubin LP, Kim Y. β-Carotene Exerts Anti-Colon Cancer Effects by Regulating M2 Macrophages and Activated Fibroblasts. J Nutr Biochem (2020) 82:108402. doi: 10.1016/j.jnutbio.2020.108402 [DOI] [PubMed] [Google Scholar]
- 73. Li D, Zhang Y, Liu K, Zhao Y, Xu B, Xu L, et al. Berberine Inhibits Colitis-Associated Tumorigenesis via Suppressing Inflammatory Responses and the Consequent EGFR Signaling-Involved Tumor Cell Growth. Lab Invest (2017) 97(11):1343–53. doi: 10.1038/labinvest.2017.71 [DOI] [PubMed] [Google Scholar]
- 74. Chung KS, Cheon SY, Roh SS, Lee M, An HJ. Chemopreventive Effect of Aster Glehni on Inflammation-Induced Colorectal Carcinogenesis in Mice. Nutrients (2018) 10(2):202. doi: 10.3390/nu10020202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. James S, James S, Aparna JS, Babu A, Paul AM, Lankadasari MB, Athira SR, et al. Cardamonin Attenuates Experimental Colitis and Associated Colorectal Cancer. Biomolecules (2021) 11(5):661. doi: 10.3390/biom11050661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pan P, Kang S, Wang Y, Liu K, Oshima K, Huang YW, et al. Black Raspberries Enhance Natural Killer Cell Infiltration Into the Colon and Suppress the Progression of Colorectal Cancer. Front Immunol (2017) 8:997. doi: 10.3389/fimmu.2017.00997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Huang YW, Lin CW, Pan P, Shan T, Echeveste CE, Mo YY, et al. Black Raspberries Suppress Colorectal Cancer by Enhancing Smad4 Expression in Colonic Epithelium and Natural Killer Cells. Front Immunol (2020) 11:570683. doi: 10.3389/fimmu.2020.570683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang W, An EK, Park HB, Hwang J, Dhananjay Y, Kim SJ, et al. Ecklonia Cava Fucoidan has Potential to Stimulate Natural Killer Cells In Vivo. Int J Biol Macromol (2021) 185:111–21. doi: 10.1016/j.ijbiomac.2021.06.045 [DOI] [PubMed] [Google Scholar]
- 79. Yang LC, Lai CY, Hsieh CC, Lin WC. Natural Killer Cell-Mediated Anticancer Effects of an Arabinogalactan Derived From Rice Hull in CT26 Colon Cancer-Bearing Mice. Int J Biol Macromol (2019) 124:368–76. doi: 10.1016/j.ijbiomac.2018.11.200 [DOI] [PubMed] [Google Scholar]
- 80. Bhattacharyya S, Md SakibHossain D, Mohanty S, Sankar Sen G, Chattopadhyay S, Banerjee S, et al. Curcumin Reverses T Cell-Mediated Adaptive Immune Dysfunctions in Tumor-Bearing Hosts. Cell Mol Immunol (2010) 7(4):306–15. doi: 10.1038/cmi.2010.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shafabakhsh R, Pourhanifeh MH, Mirzaei HR, Sahebkar A, Asemi Z, Mirzaei H. Targeting Regulatory T Cells by Curcumin: A Potential for Cancer Immunotherapy. Pharmacol Res (2019) 147:104353. doi: 10.1016/j.phrs.2019.104353 [DOI] [PubMed] [Google Scholar]
- 82. Rahimi K, Ahmadi A, Hassanzadeh K, Soleimani Z, Sathyapalan T, Mohammadi A, et al. Targeting the Balance of T Helper Cell Responses by Curcumin in Inflammatory and Autoimmune States. Autoimmun Rev (2019) 18(7):738–48. doi: 10.1016/j.autrev.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 83. Zhao GJ, Lu ZQ, Tang LM, Wu ZS, Wang DW, Zheng JY, et al. Curcumin Inhibits Suppressive Capacity of Naturally Occurring CD4+CD25+ Regulatory T Cells in Mice In Vitro. Int Immunopharmacol (2012) 14(1):99–106. doi: 10.1016/j.intimp.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 84. Xu B, Yu L, Zhao LZ. Curcumin Up Regulates T Helper 1 Cells in Patients With Colon Cancer. Am J Transl Res (2017) 9(4):1866–75 PMID: 28469791. [PMC free article] [PubMed] [Google Scholar]
- 85. Dent P, Dent P, Booth L, Roberts JL, Poklepovic A, Hancock JF. (Curcumin+sildenafil) Enhances the Efficacy of 5FU and Anti-PD1 Therapies In Vivo. J Cell Physiol (2020) 235(10):6862–74. doi: 10.1002/jcp.29580 [DOI] [PubMed] [Google Scholar]
- 86. Li M, Yue GG, Song LH, Huang MB, Lee JK, Tsui SK, et al. Natural Small Molecule Bigelovin Suppresses Orthotopic Colorectal Tumor Growth and Inhibits Colorectal Cancer Metastasis via IL6/STAT3 Pathway. Biochem Pharmacol (2018) 150:191–201. doi: 10.1016/j.bcp.2018.02.017 [DOI] [PubMed] [Google Scholar]
- 87. Ayeka PA, Bian Y, Mwitari PG, Chu X, Zhang Y, Uzayisenga R, et al. Immunomodulatory and Anticancer Potential of Gan Cao (Glycyrrhiza Uralensis Fisch.) Polysaccharides by CT-26 Colon Carcinoma Cell Growth Inhibition and Cytokine IL-7 Upregulation In Vitro. BMC Complement Altern Med (2016) 16:206. doi: 10.1186/s12906-016-1171-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chalons P, Courtaut F, Limagne E, Chalmin F, Cantos-Villar E, Richard T, et al. Red Wine Extract Disrupts Th17 Lymphocyte Differentiation in a Colorectal Cancer Context. Mol Nutr Food Res (2020) 64(11):e1901286. doi: 10.1002/mnfr.201901286 [DOI] [PubMed] [Google Scholar]
- 89. Liang J, Li H, Chen J, He L, Du X, Zhou L, et al. Dendrobium Officinale Polysaccharides Alleviate Colon Tumorigenesis via Restoring Intestinal Barrier Function and Enhancing Anti-Tumor Immune Response. Pharmacol Res (2019) 148:104417. doi: 10.1016/j.phrs.2019.104417 [DOI] [PubMed] [Google Scholar]
- 90. Masuda Y, Ito K, Konishi M, Nanba H. A Polysaccharide Extracted From Grifola Frondosa Enhances the Anti-Tumor Activity of Bone Marrow-Derived Dendritic Cell-Based Immunotherapy Against Murine Colon Cancer. Cancer Immunol Immunother (2010) 59(10):1531–41. doi: 10.1007/s00262-010-0880-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang H, Zou C, Zhao W, Yu Y, Cui Y, Zhang H, et al. Juglone Eliminates MDSCs Accumulation and Enhances Antitumor Immunity. Int Immunopharmacol (2019) 73:118–27. doi: 10.1016/j.intimp.2019.04.058 [DOI] [PubMed] [Google Scholar]
- 92. Zhang J, Shen L, Li X, Song W, Liu Y, Huang L. Nanoformulated Codelivery of Quercetin and Alantolactone Promotes an Antitumor Response Through Synergistic Immunogenic Cell Death for Microsatellite-Stable Colorectal Cancer. ACS Nano (2019) 13(11):12511–24. doi: 10.1021/acsnano.9b02875 [DOI] [PubMed] [Google Scholar]
- 93. Xu H, Van derJeught K, Zhou Z, Zhang L, Yu T, Sun Y, et al. Atractylenolide I Enhances Responsiveness to Immune Checkpoint Blockade Therapy by Activating Tumor Antigen Presentation. J Clin Invest (2021) 131(10):e146832. doi: 10.1172/JCI146832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu F, Ran F, He H, Chen L. Astragaloside IV Exerts Anti-Tumor Effect on Murine Colorectal Cancer by Re-Educating Tumor-Associated Macrophage. Arch Immunol Ther Exp (Warsz) (2020) 68(6):33. doi: 10.1007/s00005-020-00598-y [DOI] [PubMed] [Google Scholar]
- 95. Han K, Nam J, Xu J, Sun X, Huang X, Animasahun O, et al. Generation of Systemic Antitumour Immunity via the in Situ Modulation of the Gut Microbiome by an Orally Administered Inulin Gel. Nat BioMed Eng (2021) 5(11):1377–88. doi: 10.1038/s41551-021-00749-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lee EJ, Kim JH, Kim TI, Kim YJ, Pak ME, et al. Sanguisorbae Radix Suppresses Colorectal Tumor Growth Through PD-1/PD-L1 Blockade and Synergistic Effect With Pembrolizumab in a Humanized PD-L1-Expressing Colorectal Cancer Mouse Model. Front Immunol (2021) 12:737076. doi: 10.3389/fimmu.2021.737076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang SL, Mao YQ, Zhang ZY, Li ZM, Kong CY, Chen HL, et al. Pectin Supplement Significantly Enhanced the Anti-PD-1 Efficacy in Tumor-Bearing Mice Humanized With Gut Microbiota From Patients With Colorectal Cancer. Theranostics (2021) 11(9):4155–70. doi: 10.7150/thno.54476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Liu W, Fan T, Li M, Zhang G, Guo W, Yang X, et al. Andrographolide Potentiates PD-1 Blockade Immunotherapy by Inhibiting COX2-Mediated PGE2 Release. Int Immunopharmacol (2020) 81:106206. doi: 10.1016/j.intimp.2020.106206 [DOI] [PubMed] [Google Scholar]
- 99. Magrì A, Germano G, Lorenzato A, Lamba S, Chilà R, Montone M, et al. High-Dose Vitamin C Enhances Cancer Immunotherapy. Sci Transl Med (2020) 12(532):eaay8707. doi: 10.1126/scitranslmed.aay8707 [DOI] [PubMed] [Google Scholar]
- 100. Huang J, Liu D, Wang Y, Liu L, Li J, Yuan J, et al. Ginseng Polysaccharides Alter the Gut Microbiota and Kynurenine/Tryptophan Ratio, Potentiating the Antitumour Effect of Antiprogrammed Cell Death 1/Programmed Cell Death Ligand 1 (Anti-PD-1/PD-L1) Immunotherapy. Gut (2022) 71(4):734–45. doi: 10.1136/gutjnl-2020-321031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Gao W, Zhang X, Yang W, Dou D, Zhang H, Tang Y, et al. Prim-O-Glucosylcimifugin Enhances the Antitumour Effect of PD-1 Inhibition by Targeting Myeloid-Derived Suppressor Cells. J Immunother Cancer (2019) 7(1):231. doi: 10.1186/s40425-019-0676-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Murphy N, Moreno V, Hughes DJ, Vodicka L, Vodicka P, Aglago EK, et al. Lifestyle and Dietary Environmental Factors in Colorectal Cancer Susceptibility. Mol aspects Med (2019) 69:2–9. doi: 10.1016/j.mam.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 103. Keum N, Giovannucci E. Global Burden of Colorectal Cancer: Emerging Trends, Risk Factors and Prevention Strategies. Nat Rev Gastroenterol Hepatol (2019) 16(12):713–32. doi: 10.1038/s41575-019-0189-8 [DOI] [PubMed] [Google Scholar]
- 104. Gurjao C, Zhong R, Haruki K, Li YY, Spurr LF, Lee-Six H, et al. Discovery and Features of an Alkylating Signature in Colorectal Cancer. Cancer Discovery (2021) 11(10):2446–55. doi: 10.1158/2159-8290.CD-20-1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Song M, Garrett W, Chan A. Nutrients, Foods, and Colorectal Cancer Prevention. Gastroenterology (2015) 148(6):1244–60.e16. doi: 10.1053/j.gastro.2014.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kopp TI, Vogel U, Tjonneland A, Andersen V. Meat and Fiber Intake and Interaction With Pattern Recognition Receptors (TLR1, TLR2, TLR4, and TLR10) in Relation to Colorectal Cancer in a Danish Prospective, Case-Cohort Study. Am J Clin Nutr (2018) 107(3):465–79. doi: 10.1093/ajcn/nqx011 [DOI] [PubMed] [Google Scholar]
- 107. Ringel AE, Drijvers JM, Baker GJ, Catozzi A, García-Cañaveras JC, Gassaway BM, et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell (2020) 183(7):1848–1866.e26. doi: 10.1016/j.cell.2020.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Amitay EL, Carr PR, Jansen L, Roth W, Alwers E, Herpel E, et al. Smoking, Alcohol Consumption and Colorectal Cancer Risk by Molecular Pathological Subtypes and Pathways. Br J Cancer (2020) 122(11):1604–10. doi: 10.1038/s41416-020-0803-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ogino S, Nowak JA, Hamada T, Phipps AI, Peters U, Milner DA, Jr, et al. Integrative Analysis of Exogenous, Endogenous, Tumour and Immune Factors for Precision Medicine. Gut (2018) 67(6):1168–80. doi: 10.1136/gutjnl-2017-315537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Song M, Nishihara R, Cao Y, Chun E, Qian ZR, Mima K, et al. Marine ω-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer Characterized by Tumor-Infiltrating T Cells. JAMA Oncol (2016) 2(9):1197–206. doi: 10.1001/jamaoncol.2016.0605 [DOI] [PMC free article] [PubMed] [Google Scholar]