Abstract
In the past decade, the incidence of recurrent pregnancy loss (RPL) has increased significantly, and immunological disorders have been considered as one of the possible causes contributing to RPL. The presence of antinuclear antibodies (ANAs) is regarded as a typical antibody of autoimmunity. However, the relationship between the presence of ANAs and RPL, the underlying mechanism, and the possible role of immunotherapy is still controversial. The aim of this mini review is to assess the association between ANAs and RPL and the effects of immunotherapy on pregnancy outcomes in women with positive ANAs and a history of RPL from the available data and to provide a relevant reference basis for clinical application in this group of women.
Keywords: antinuclear antibodies (ANAs), recurrent pregnancy loss (RPL), prognostic value, pregnancy outcome, immunotherapy
Introduction
Pregnancy loss, or the spontaneous death of a pregnancy before the fetus reaches viability, affects up to 20% of women who conceive, making it one of the commonest complications of pregnancy (1). Currently, the definitions of recurrent pregnancy loss (RPL) vary in different countries and regions internationally. Based on the European Society of Human Reproduction and Embryology (ESHRE) guideline, RPL was defined as the loss of two or more pregnancies before 24 weeks of pregnancy (1), while the Royal College of Obstetricians and Gynaecologists (RCOG) guideline used a stricter criterion, which was defined as three or more fetal losses before 24 weeks of pregnancy, including biochemical pregnancy (2). Compared with pregnancy loss, RPL is less prevalent and affects approximately 1 to 3% of women who are trying to conceive (3). RPL has a significant negative impact on the physiological and psychological health of women and brings great emotional frustration to couples.
There are several recognized causes related to RPL, namely, genetic factors, uterine abnormalities (congenital malformations, endometrial polyps, uterine fibroids, etc.), hormonal and metabolic disorders (thyroid dysfunction, diabetes, polycystic ovary syndrome, hyper-prolactinemia, etc.), thrombophilia, immunological disorders, and male factors. However, approximately half of RPL remains unexplained in etiology, which is referred to as unexplained RPL (uRPL) (4).
Antinuclear antibodies (ANAs) are a group of autoantibodies that target components of the cell nucleus and bind to proteins, nucleic acids, and protein–nucleic acid complexes (5). ANA detection may be performed by immunofluorescence (IF) on human epithelial laryngeal carcinoma type 2 cells or by solid-phase ANA screening immunoassay with at least equivalent performance (6). Indirect immunofluorescence is an extensively used laboratory test for detecting ANAs. The result is usually expressed in titers, which are used to describe the antibody concentration in peripheral blood. Positive ANAs expressed in low titers are commonly found in healthy women, whereas the presence of high titers (>1:160) is closely related to autoimmune diseases, namely, systemic lupus erythematosus, systemic sclerosis, and Sjögren’s syndrome, which are related to adverse pregnancy outcomes (7, 8). A previous cross-sectional analysis including 4,754 individuals from the US showed the prevalence of positive ANAs could reach up to 13.8% and vary widely in healthy populations, ranging from 5.92% in Chinese to 30.8% in African Americans, which was higher in women (17.8%), compared with that in men (9.6%) (9).
There is evidence that autoimmunity is an important risk factor for pregnancy loss. A series of studies have tried to elucidate the association between ANAs and RPL, but the relationship between ANAs and RPL pregnancy outcomes and whether the treatments for ANA-positive affect pregnancy outcomes are still highly controversial. However, the prognostic value of ANAs for subsequent pregnancy outcomes is unclear as well. Furthermore, the underlying patho-physiological link and mechanism that the presence of ANAs plays in women with RPL has not yet been fully understood.
Therefore, given the importance of the potential association between the presence of antinuclear antibodies and pregnancy loss, the aim of this mini review was to provide evidence on the relationship between positive ANAs and recurrent pregnancy loss and the possible underlying mechanism. Given the possible role of immunotherapy in improving pregnancy outcomes in women with a history of RPL, we also reviewed the available clinical studies on the effects of different types of immunotherapy, focusing on positive ANAs.
Presence of ANAs and its Prognostic Value in RPL
The presence of ANAs has been regarded as a typical feature of autoimmunity. There is growing evidence suggesting that ANAs can play a role in both early pregnancy and pregnancy loss. Although how the ANAs are present in women with RPL remains unclear, it is possible that the presence of ANAs in RPL indicates that there may be an underlying autoimmune disorder in RPL women, at least in a subgroup of patients, which affects the development of the trophoblast and can lead to early pregnancy loss. Therefore, RPL women with previous autoimmune diseases are likely to have a higher prevalence of positive ANAs. One previous study showed that in women with autoimmune disorders, a history of RPL is independently associated with reactivity against three distinct Ro antigen-related reactivities (a subtype of autoantibody of ANAs), suggesting that cumulative autoimmune responses correlate with the risk of spontaneous miscarriage (10). However, even in RPL women without autoimmune disorders, ANAs still need to be screened. A recent meta-analysis, including 2,683 women with RPL without defined autoimmune diseases and 2,355 controls, found that the total positive rate of ANAs was significantly higher in the RPL group, compared with the control group (22.0% vs 8.3%, OR = 2.97, 95% CI 1.91–4.64, P <0.001) (11). Additionally, subgroup analysis demonstrated a significant association between high ANA titers (≥1:160) and RPL (OR = 45.89, 95% CI 8.44–249.45, P <0.001), while there was no significant relationship between low titers of ANAs (1:40 ≤ANA ≤1:80) and RPL (OR = 2.44, 95% CI 0.42–14.06, P = 0.32).
In the other previous studies, most of them did not provide definite information on the past history of autoimmune disorders, and the results showed that the prevalence of positive ANAs in women with a history of RPL varied ( Table 1 ). Several previous studies have found a significantly increased prevalence of positive ANAs in women with a history of RPL. There was a significantly higher proportion of women with RPL who had ANAs at ≥1:80 compared with controls (21, 23, 30). A case–control study including 294 women showed that women with RPL had a three-fold higher prevalence of positive ANAs (50%) and higher serum titers of ANAs (≥1:80) when compared with women without reproductive disorders (21). Similarly, another study including 560 Iranian women showed that the ANA-positive rate in women with a history of two or more unexplained pregnancy losses (13.21%) was significantly higher than that in control women (0.9%) (24). This observation was also supported by another systematic review and meta-analysis (31). Their results showed that the prevalence of positive ANAs in the RPL women (20.6%, 288/1,400) was significantly higher than it was non-pregnant women with no history of pregnancy loss (6.7%, 72/1,080) (31).
Table 1.
The prevalence of positive ANAs in women with a history of RPL in different studies.
| Author | Year | Ethnic/Country | Study subjects | Definition of RPL (the number of pregnancy loss) | ANA detection methods (cut-off dilution) | Prevalence of ANA+ (case group) | Prevalence of ANA+ (control group) |
|---|---|---|---|---|---|---|---|
| Hefler-Frischmuth et al. (12) | 2017 | Caucasian | Case: 114 RPL | ≥3 | ELISA (unclear) | NA | NA |
| Control: 107 age-matched healthy controls | |||||||
| Sakthiswary et al. (13) | 2015 | Malaysia | Case: 68 uRPL | ≥2 | IF (1:80) | 35.3% | 13.3% |
| Control: 60 non-pregnant women without pregnancy | |||||||
| Molazadeh et al. (14) | 2014 | Iran | Case: 560 uRPL | ≥2 | IF (1:40) | 13.2% | 0.9% |
| Control: 560 healthy controls | |||||||
| Roye-Green et al. (15) | 2011 | Jamaica | Case: 50 RPL | ≥2 | IF (unclear) | 2% | 2.2% |
| Control: 135 multiparous women without pregnancy loss | |||||||
| Ticconi et al. (16) | 2010 | Caucasian | Case: 194 RPL | ≥2 | IF (1:80) | 50% | 16% |
| Control: 100 non-pregnant controls | |||||||
| Giasuddin (17) | 2010 | Bangladesh | Case: 35 RPL | ≥3 | ELISA (unclear) | 20% | 0.54% |
| Control: 37 normal pregnant women | |||||||
| Bustos et al. (18) | 2006 | Argentina | Case: 118 RPL | ≥3 | IF (1:40) | 16% | 14% |
| Control: 125 fertile control women without abortions and two children | |||||||
| Habara et al. (19) | 2002 | Japan | Case: 49 uRPL | ≥3 | IF (unclear) | NA | NA |
| Control: 72 normal women with sterility caused by male factor | |||||||
| Matsubayashi et al. (20) | 2001 | Japan | Case: 273 RPL | ≥2 | IF (1:80) | 23.4% | 13% |
| Control: 200 healthy non-pregnant women | |||||||
| Kaider et al. (21) | 1999 | USA | Case: 302 RPL | ≥3 | ELISA (unclear) | 45.7% | 10% |
| Control: 20 healthy fertile women | |||||||
| Kovács et al. (22) | 1999 | Hungary | Case: 59 uRPL | ≥2 | IF (unclear) | 3.39% | 8% |
| Control: 25 non-pregnant women without pregnancy | |||||||
| Stern et al. (23) | 1998 | New Zealand | Case: 97 RPL | ≥3 | IF (1:80) | 22.7% | 9.4% |
| Control: 106 fertile controls | |||||||
| Konidaris et al. (24) | 1994 | Greece | Case: 44 uRPL | ≥3 | IF (1:40) | 9.1% | 2.9% |
| Control: 4 non-pregnant healthy women without pregnancy loss | |||||||
| Bahar et al. (25) | 1993 | Kuwait | Case: 103 uRPL | ≥3 | IF (1:40) | 13.6% | 1.2% |
| Control: 85 multiparous non-pregnant women without pregnancy loss | |||||||
| Kwak et al. (26) | 1992 | USA | Case: 153 uRPL | ≥3 | IF (1:40) | 19.0% | 14.0% |
| Control: 90 normal controls | |||||||
| Harger et al. (27) | 1989 | USA | Case: 277 RPL | ≥2 | IF (1:40) | 16.3% | 16.8%/16.6% |
| Control: 199 non-pregnant/299 pregnant women | |||||||
| Petri et al. (28) | 1987 | USA | Case: 44 uRPL | ≥3 | IF (1:40) | 16% | 20% |
| Control: 40 Volunteers | |||||||
| Garcia-De La Torre et al. (29) | 1984 | Mexico | Case: 20 uRPL | ≥3 | IF (1:20) | 30% | 6.6% |
| Control: 30 women with normal pregnancy |
NA, Not Applicable.
Nevertheless, some other studies failed to find such a significant difference between women with RPL and controls. A case–control study including 72 Bangladeshi women showed that the mean serum levels of ANAs in women with RPL (1.07 ± 0.34) were similar in cases compared with controls (0.92 ± 0.15) (17). Another study including 243 Caucasian Argentine healthy women showed that the ANA-positive rate in women with a history of three or more unexplained pregnancy losses (16%) was similar to that in control women (14%), and the median titers (1:40) (16). A recent study including 114 women with RPL and 107 healthy controls found no significant differences were ascertained regarding serum levels of ANAs (0.32 vs 0.39, P = 0.2) (27).
It is also interesting to evaluate the association of ANAs with gene polymorphisms of the hemostasis system and RPL. Hereditary thrombophilia, namely, factor V Leiden mutation, prothrombin mutation, protein C, protein S, and antithrombin deficiency, could be associated with adverse obstetric outcomes. There was one previous study investigating the presence of autoimmune antibodies (antithyroid antibodies and ANAs) and polymorphism genotypes for factor V Leiden, prothrombin gene mutation, and MTHFR in women with RPL (14). The results showed that only one out of 39 subjects had a combination of hereditary thrombophilia and positive autoimmune antibodies, suggesting a weak association between ANAs and gene polymorphisms of the hemostasis system and RPL (14). Another study evaluating thrombophilia and immunological disorders in pregnancies found that the presence of ANAs was significantly elevated in pregnancies complicated by small for gestational age, while the prevalence of inherited thrombophilia did not differ significantly. However, the authors did not provide information on the history of miscarriage of the participants (12).
Regarding the prognostic value of ANAs, there are several studies reporting different results. An earlier study found a higher subsequent miscarriage rate in ANA-positive women with RPL as compared with ANA-negative subjects (36). In another previous prospective study, Ticconi et al. investigated the ANA status in a cohort of women with unexplained RPL before pregnancy, repeated the test during the first trimester of the subsequent pregnancy, and correlated the result with the pregnancy outcome (23). Interestingly, the authors found that subsequent miscarriages occurred in women who had ANAs positive before pregnancy and remained positive in the first trimester, whereas no miscarriages were observed in women who had ANAs positive before pregnancy but turned negative in the first trimester, which suggested that the disappearance of ANA in early pregnancy could have a favorable prognostic value in the subsequent pregnancies (23).
However, some studies did not find that the presence of ANAs could predict new pregnancy losses. One study found that the live birth rate of the next pregnancy (untreated) in the RPL patients with positive ANAs at ≥1:80 (52%) was not significantly different from that in RPL patients with negative ANAs (65.6%) (30). Likewise, Ogasawara et al. observed that the ANA-positive rate in women with RPL was 17%, and the miscarriage rate in the next pregnancy was similar to that of women who had RPL and tested negative for ANAs (32). Additionally, it was reported that the occurrence of subsequent live births was not affected by ANA levels or associated thrombophilia (25).
There are several possible causes contributing to the observed difference derived from the above studies. Firstly, different definitions of RPL were used in different studies; some studies used two or more pregnancy losses (18, 21, 24, 30, 33–35) while others used three or more pregnancy losses (13, 15–17, 19, 20, 22, 26–29) to define RPL. Secondly, the subjects recruited in the above studies were from different ethnic populations, which may have led to variability. Thirdly, these studies used different methods or assays to detect ANAs; some used IF (12, 14, 16, 19–22, 24, 26, 28–30, 36) while others used ELISA (15, 17, 27). Furthermore, different criteria were applied to define positive for ANAs; some used 1:40 (16, 19, 22, 24, 26, 28, 30, 34, 35) while others used 1:80 (14, 19, 20, 36).
Possible Mechanisms for the Presence of ANAs in RPL
Generally, autoimmune disorders may impair all stages of pregnancy, leading to implantation failure or pregnancy loss via different putative mechanisms (37). It has been suggested that antiphospholipid antibody (aPL) and anti-beta(2)-glycoprotein I antibody (A-β2-GPI) can lead to placental vascular thrombosis, trophoblast dysfunction, and maternal hormone imbalance (38–40) and the presence of thyroid autoantibodies may result in dysregulation of the immune system activity at the fetal-maternal interface (41–43). Although the effects of ANAs on reproductive health are widely recognized, unlike aPL, A-β2-GPI, and thyroid autoantibodies, the exact mechanism of action of ANA in RPL is not yet clear.
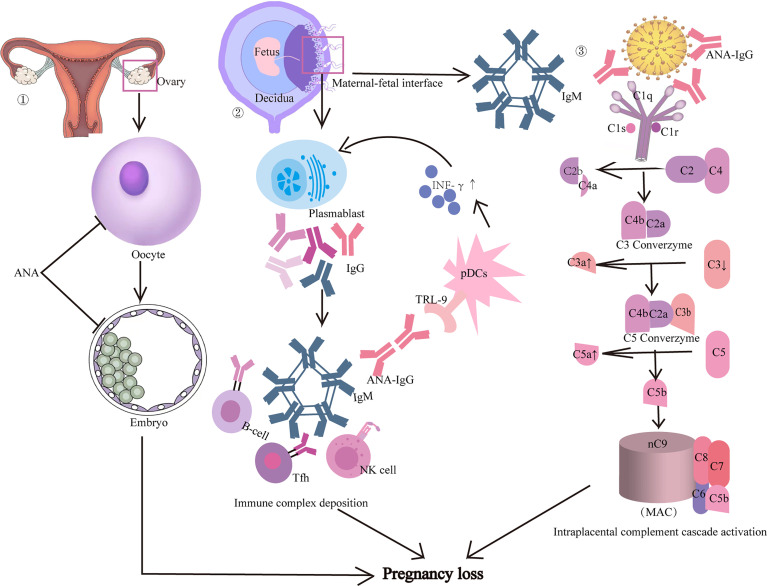
Previous studies have suggested several possible mechanisms that ANAs play in pregnancy failure ( Figure 1 ) (44–48). Firstly, ANAs might have a direct adverse effect on the quality and development of oocytes and embryos, resulting in reduced pregnancy and implantation rates (49). Although there was no nuclear antigen on the zona pellucida, in vitro studies indicated that ANAs could bind to the embryos directly and it was proposed that ANAs might recognize the glycerol moiety or protein cofactor (50). An earlier study showed that the development of embryos that were co-cultured with immunoglobulins from ANA-positive women was severely impaired (51). Another study recruiting women undergoing in vitro fertilization-embryo transfer (IVF-ET) treatment found that the proportion of mature oocytes and number of higher embryos and pregnancy rates in the ANA-positive group was significantly lower than in the ANA-negative group, which suggested that the presence of ANAs significantly interferes with oocyte and embryo development and therefore impairs the pregnancy outcome (49).
Figure 1.
Possible mechanisms that ANA may play in pregnancy loss. Firstly, ANA might have a direct adverse effect on the quality and development of oocytes and embryos, resulting in reduced pregnancy and implantation rates. Secondly, the precipitation of immune complex tissues at the maternal–fetal interface may be one of the possible mechanisms. Thirdly, the immune complex tissues may also induce local complement activation with inflammatory infiltration, leading to miscarriage.
Secondly, the precipitation of immune complex tissues in the maternal–fetal interface may be one of the possible mechanisms leading to miscarriage in ANA-positive women (51). An animal study showed that mice treated with ANA-positive IgG obtained from RPL women had a remarkably higher embryonic absorption rate, reduced complement 3 (C3) and increased C3a serum levels, compared with those treated with IgG obtained from normal healthy women (52). Interestingly, increased C3 deposition and immune complex staining in placental tissues were also found in mice treated with ANA-positive IgG fraction from women with RPL (52). Additionally, it has been shown that ANAs can also induce the activation of plasmacytoid dendritic cells via Toll‐like receptor‐9,which can result in increased production of inflammatory cytokines (such as interferon‐α) that stimulate the humoral immune response and lead to further production of ANAs (53, 54).
Furthermore, the immune complex tissue may induce local complement activation with inflammatory infiltration (52). Although there was no direct evidence of the association between ANAs and complement activation, a previous study using a mouse model of the antiphospholipid syndrome induced by passive transfer of human aPL antibodies showed that mice deficient in C3 were resistant to fetal injury induced by aPL antibodies (55). Studies defining the downstream effectors of complement activation have shown a rapid increase in decidual and systemic tumor necrosis factor-a levels, which appears to be the mediator that links complement activation to fetal damage (21, 56). The recruitment of inflammatory cells accelerates the activated pathway and creates a pro-inflammatory amplification loop that enhances C3 activation and deposition, generates additional C3a and C5a, and results in a further flow of inflammatory cells into the placenta, ultimately leading to pregnancy loss (56).
Potential Treatments for ANA-Positive Women With RPL
Interventions for ANA-positive women with a history of RPL were recommended on the basis of the possible adverse effect of ANAs on the subsequent pregnancy outcome in this group of women. However, there is no consensus on treatment regimens yet.
Aspirin has both anti-platelet and anti-inflammatory effects, and glucocorticoids exhibit a beneficial clinical effect in most autoimmune diseases. Therefore, they are considered potential therapies in ANA-positive women with RPL, which has a suspected immune etiology. In a placebo-controlled trial, prednisolone and low-dose aspirin were used to treat women with RPL and positivity for antiphospholipid, antinuclear, anti-DNA, or anti-lymphocyte antibodies (57). Although the live birth rate was higher in the treatment group, it was not significantly different from controls (OR 1.5; 95% CI 0.8–2.6). However, the treated patients had a significantly higher risk of preterm birth (62% versus 12%, p <0.001) and higher risks for diabetes and hypertension, which are well known to be associated with high and prolonged administration of prednisolone. However, another case–control study, including more than 200 women who were diagnosed with unexplained RPL and tested positive for ANAs at a titer of 1:80 or more, showed that live birth rates were comparable in women receiving prednisone plus aspirin and women prescribed aspirin only. Additionally, no previous preterm delivery, fetal growth restriction, or placental abruption occurred in any subject (58).
In addition to glucocorticoids, there are other types of immunotherapy used to treat RPL with immunological causes, namely, intravenous immunoglobulin (IVIG), lymphocyte immunization treatment (LIT), etc. IVIG is a fractionated blood product that is used to treat certain autoimmune diseases and RPL. Two randomized controlled trials indicated that IVIG increases live birth rates in secondary RPL patients but not significantly in patients with primary RPL (59, 60). In contrast, recent meta-analyses found that IVIG did not improve the live birth rates of RPL women (61, 62). As for RPL women with positive ANAs, there is a limited study evaluating the therapeutic effect of IVIG in this group of women. One earlier study showed that low-dose IVIG therapy is beneficial for older women with immunologic abnormalities and RPL by increasing the successful pregnancy rate, in which 28% of the participants were found to have positive ANAs (63). The possible mechanisms of action of IVIG for treating RPL are multifactorial, namely, the modulation of various immune cells, the down-regulation of primary antibody production, and the modulation of complement activation (64–66).
LIT is another immunotherapy used in RPL. A review demonstrated that RPL women treated with paternal LIT had more successful outcomes (68%) than untreated women (54%, p <0.02) (67). As for those with positive ANAs, a retrospective observational study showed that the presence of ANAs is one of the risk factors for further pregnancy loss in patients with RPL treated with LIT (68). However, some previous studies have shown that patients with positive ANAs and antithyroid antibodies after receiving LIT have a higher risk of miscarriage and do not benefit from LIT (69, 70). Although the exact mechanisms of LIT have yet to be elucidated, the possible mechanisms consist of inducing the production of humoral antibodies to mask the fetal human leukocyte antigens (71), regulating Th2 cell transition (72), and decreasing NK cell activity (73).
Plasmapheresis has been used for decades for treating autoimmune disease as it is thought to have a profound modulation of the immune system, namely, the removal of circulating immune complexes, immunoglobulins, and complement components. Plasmapheresis has also been used in pregnant women with autoimmune diseases, such as Sjoegren’s Syndrome or systemic lupus erythematosus, to treat congenital fetal heart block (74, 75). Several reports have suggested that plasmapheresis may also treat pregnant women with anti-phospholipid syndrome (76, 77). For women with RPL, plasmapheresis was used to prevent future miscarriage in pregnant women immunized with anti-P or anti-PP1Pk and a history of RPL (78, 79). However, as far as we are aware, there is no study reporting the use of plasmapheresis in RPL women with positive ANAs.
Heparin is effective for its anticoagulant and anti-inflammatory properties (80). Due to the evidence from randomized controlled trials that heparin appears beneficial in treating women with RPL and other autoimmune antibodies (81, 82), heparin has been increasingly administered in clinic to RPL women with positive ANAs. However, there is a limited clinical trial investigating the therapeutic effect of heparin alone in this group of women. Overall, different results showed that the effect of different therapies on maternal and fetal pregnancy outcomes in patients with RPL is still controversial, and therefore large sample size randomized controlled trials are needed.
Future Direction
As discussed above, several issues should be taken into account in future studies. Firstly, due to the different definitions used for recurrent pregnancy loss among these studies, standardization of the definition is in urgent need. Secondly, since the methods for ANA detection varied as well, a standardized methodology should be proposed in the future. Thirdly, clinical pregnancy outcomes are assumed to be followed up, namely, clinical pregnancy, miscarriage, pregnancy complications (gestational hypertension, intrauterine fetal restriction, etc.), and live birth in future studies focusing on the correlation between the presence of positive ANAs and pregnancy outcomes in women with RPL. Moreover, although a series of studies have suggested the possible roles that ANAs play in pregnancy loss, the exact mechanisms are still unclear and further mechanistic investigations are needed. In vitro co-culture models of endometrial and trophoblast cells may provide more information in this regard.
Conclusion
Recurrent pregnancy loss is a challenging disease in the field of reproductive medicine that can cause great emotional frustration to the suffering couples. Although the exact mechanism that ANAs plays in women with RPL is still unclear, most studies suggest that the presence of ANAs not only correlates significantly with RPL but also has a prognostic value for the subsequent pregnancy outcome in this group of women. Interventions for ANA-positive women with a history of RPL include aspirin, glucocorticoids, and heparin. However the therapeutic effect of these regimens is still controversial and large-scale randomized controlled trials are needed.
Author Contributions
TL, YZ, and XC: Substantial contribution to the conception and design of the work. TL, XG, YLia, and XC: participation in acquisition of the literature. TL, YLiu, YZ, and XC: manuscript drafting. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by Shenzhen Key Medical Discipline Construction Fund (SZXK028), Shenzhen Science and Technology Program (JCYJ20210324141403009) and Basic and Applied Basic Research Foundation of Guangdong Province of China (2020A1515110082).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. NHS Direct Wales Encyclopaedia . Miscarriage (2010). Available at: http://www.nhsdirect.wales.nhs.uk/encyclopaedia/m/article/miscarriage/.
- 2. Royal College of Obstetricians and Gynaecologists . The Investigation and Treatment of Couples with Recurrent First-Trimester and Second-Trimester Miscarriage. In: Green-Top Guideline No.17 (2011). [Google Scholar]
- 3. Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal Age and Fetal Loss: Population Based Register Linkage Study. BMJ (2000) 320:1708–12. doi: 10.1136/bmj.320.7251.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liang PY, Diao LH, Huang CY, Lian RC, Chen X, Li GG, et al. The Pro-Inflammatory and Anti-Inflammatory Cytokine Profile in Peripheral Blood of Women With Recurrent Implantation Failure. Reprod BioMed Online (2015) 31:823–6. doi: 10.1016/j.rbmo.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 5. Pisetsky DS. Antinuclear Antibodies in Rheumatic Disease: A Proposal for a Function-Based Classification. Scand J Immunol (2012) 76:223–8. doi: 10.1111/j.1365-3083.2012.02728.x [DOI] [PubMed] [Google Scholar]
- 6. Aringer M, Costenbader K, Daikh D, Brinks R, Mosca M, Ramsey-Goldman R, et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol (2019) 71:1400–12. doi: 10.1002/art.40930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tebo AE. Recent Approaches To Optimize Laboratory Assessment of Antinuclear Antibodies. Clin Vaccine Immunol (2017) 24:e00270-17. doi: 10.1128/cvi.00270-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Damoiseaux J, Andrade LEC, Carballo OG, Conrad K, Francescantonio PLC, Fritzler MJ, et al. Clinical Relevance of HEp-2 Indirect Immunofluorescent Patterns: The International Consensus on ANA Patterns (ICAP) Perspective. Ann Rheum Dis (2019) 78:879–89. doi: 10.1136/annrheumdis-2018-214436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, et al. Prevalence and Sociodemographic Correlates of Antinuclear Antibodies in the United States. Arthritis Rheum (2012) 64:2319–27. doi: 10.1002/art.34380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mavragani CP, Ioannidis JP, Tzioufas AG, Hantoumi IE, Moutsopoulos HM. Recurrent Pregnancy Loss and Autoantibody Profile in Autoimmune Diseases. Rheumatol (Ox) (1999) 38:1228–33. doi: 10.1093/rheumatology/38.12.1228 [DOI] [PubMed] [Google Scholar]
- 11. Chen S, Yang G, Wu P, Sun Y, Dai F, He Y, et al. Antinuclear Antibodies Positivity Is a Risk Factor of Recurrent Pregnancy Loss: A Meta-Analysis. Semin Arthritis Rheum (2020) 50:534–43. doi: 10.1016/j.semarthrit.2020.03.016 [DOI] [PubMed] [Google Scholar]
- 12. Hefler-Frischmuth K, Walch K, Hefler L, Tempfer C, Grimm C. Serologic Markers of Autoimmunity in Women With Recurrent Pregnancy Loss. Am J Reprod Immunol (2017) 77:e12635. doi: 10.1111/aji.12635 [DOI] [PubMed] [Google Scholar]
- 13. Sakthiswary R, Rajalingam S, Norazman MR, Hussein H. Antinuclear Antibodies Predict a Higher Number of Pregnancy Loss in Unexplained Recurrent Pregnancy Loss. Clin Ter (2015) 166:e98-101. doi: 10.7417/ct.2015.1827 [DOI] [PubMed] [Google Scholar]
- 14. Molazadeh M, Karimzadeh H, Azizi MR. Prevalence and Clinical Significance of Antinuclear Antibodies in Iranian Women With Unexplained Recurrent Miscarriage. Iran J Reprod Med (2014) 12:221–6. [PMC free article] [PubMed] [Google Scholar]
- 15. Roye-Green K, Frederick J, Wharfe G, Choo-Kang E, DaCosta V, Fletcher H, et al. Antiphospholipid and Other Autoantibodies in a Cohort of Habitual Aborters and Healthy Multiparous Women in Jamaica. Hum Antibodies (2011) 20:1–5. doi: 10.3233/hab20110236 [DOI] [PubMed] [Google Scholar]
- 16. Ticconi C, Rotondi F, Veglia M, Pietropolli A, Bernardini S, Ria F, et al. Antinuclear Autoantibodies in Women With Recurrent Pregnancy Loss. Am J Reprod Immunol (2010) 64:384–92. doi: 10.1111/j.1600-0897.2010.00863.x [DOI] [PubMed] [Google Scholar]
- 17. Giasuddin AS, Mazhar I, Haq AM. Prevalence of Anticardiolipin Antibody in Bangladeshi Patients With Recurrent Pregnancy Loss. Bangladesh Med Res Counc Bull (2010) 36:10–3. doi: 10.3329/bmrcb.v36i1.5446 [DOI] [PubMed] [Google Scholar]
- 18. Bustos D, Moret A, Tambutti M, Gogorza S, Testa R, Ascione A, et al. Autoantibodies in Argentine Women With Recurrent Pregnancy Loss. Am J Reprod Immunol (2006) 55:201–7. doi: 10.1111/j.1600-0897.2005.00349.x [DOI] [PubMed] [Google Scholar]
- 19. Habara T, Nakatsuka M, Konishi H, Asagiri K, Noguchi S, Kudo T. Elevated Blood Flow Resistance in Uterine Arteries of Women With Unexplained Recurrent Pregnancy Loss. Hum Reprod (2002) 17:190–4. doi: 10.1093/humrep/17.1.190 [DOI] [PubMed] [Google Scholar]
- 20. Matsubayashi H, Sugi T, Arai T, Kondo A, Suzuki T, Izumi S, et al. Different Antiphospholipid Antibody Specificities Are Found in Association With Early Repeated Pregnancy Loss Versus Recurrent IVF-Failure Patients. Am J Reprod Immunol (2001) 46:323–9. doi: 10.1034/j.1600-0897.2001.d01-19.x [DOI] [PubMed] [Google Scholar]
- 21. Kaider AS, Kaider BD, Janowicz PB, Roussev RG. Immunodiagnostic Evaluation in Women With Reproductive Failure. Am J Reprod Immunol (1999) 42:335–46. doi: 10.1111/j.1600-0897.1999.tb00110.x [DOI] [PubMed] [Google Scholar]
- 22. Kovács L, Szabó J, Molnár K, Kovács A, Pokorny G. Antineutrophil Cytoplasmic Antibodies and Other Immunologic Abnormalities in Patients With Habitual Abortion. Am J Reprod Immunol (1999) 41:264–70. doi: 10.1111/j.1600-0897.1999.tb00437.x [DOI] [PubMed] [Google Scholar]
- 23. Stern C, Chamley L, Hale L, Kloss M, Speirs A, Baker HW. Antibodies to Beta2 Glycoprotein I Are Associated With In Vitro Fertilization Implantation Failure as Well as Recurrent Miscarriage: Results of a Prevalence Study. Fertil Steril (1998) 70:938–44. doi: 10.1016/s0015-0282(98)00312-4 [DOI] [PubMed] [Google Scholar]
- 24. Konidaris S, Papadias K, Gregoriou O, Gargaropoulos A, Dalamaga A, Mantzavinos T. Immune Dysfunction in Patients With Unexplained Repeated Abortions. Int J Gynaecol Obstet (1994) 45:221–6. doi: 10.1016/0020-7292(94)90246-1 [DOI] [PubMed] [Google Scholar]
- 25. Bahar AM, Alkarmi T, Kamel AS, Sljivic V. Anticardiolipin and Antinuclear Antibodies in Patients With Unexplained Recurrent Abortions. Ann Saudi Med (1993) 13:535–40. doi: 10.5144/0256-4947.1993.535 [DOI] [PubMed] [Google Scholar]
- 26. Kwak JY, Gilman-Sachs A, Beaman KD, Beer AE. Autoantibodies in Women With Primary Recurrent Spontaneous Abortion of Unknown Etiology. J Reprod Immunol (1992) 22:15–31. doi: 10.1016/0165-0378(92)90003-m [DOI] [PubMed] [Google Scholar]
- 27. Harger JH, Rabin BS, Marchese SG. The Prognostic Value of Antinuclear Antibodies in Women With Recurrent Pregnancy Losses: A Prospective Controlled Study. Obstet Gynecol (1989) 73:419–24. [PubMed] [Google Scholar]
- 28. Petri M, Golbus M, Anderson R, Whiting-O'Keefe Q, Corash L, Hellmann D. Antinuclear Antibody, Lupus Anticoagulant, and Anticardiolipin Antibody in Women With Idiopathic Habitual Abortion. A Controlled, Prospective Study of Forty-Four Women. Arthritis Rheum (1987) 30:601–6. doi: 10.1002/art.1780300601 [DOI] [PubMed] [Google Scholar]
- 29. Garcia-De La Torre I, Hernandez-Vazquez L, Angulo-Vazquez J, Romero-Ornelas A. Prevalence of Antinuclear Antibodies in Patients With Habitual Abortion and in Normal and Toxemic Pregnancies. Rheumatol Int (1984) 4:87–9. doi: 10.1007/bf00541202 [DOI] [PubMed] [Google Scholar]
- 30. Ticconi C, Pietropolli A, Borelli B, Bruno V, Piccione E, Bernardini S, et al. Antinuclear Autoantibodies and Pregnancy Outcome in Women With Unexplained Recurrent Miscarriage. Am J Reprod Immunol (2016) 76:396–99. doi: 10.1111/aji.12560 [DOI] [PubMed] [Google Scholar]
- 31. Cavalcante MB, Cavalcante C, Sarno M, da Silva ACB and Barini R. Antinuclear Antibodies and Recurrent Miscarriage: Systematic Review and Meta-Analysis. Am J Reprod Immunol (2020) 83:e13215. doi: 10.1111/aji.13215 [DOI] [PubMed] [Google Scholar]
- 32. Ogasawara M, Aoki K, Kajiura S, Yagami Y. Are Antinuclear Antibodies Predictive of Recurrent Miscarriage? Lancet (1996) 347:1183–4. doi: 10.1016/s0140-6736(96)90641-8 [DOI] [PubMed] [Google Scholar]
- 33. Ineke Krabbendam I, Dekker GA. Pregnancy Outcome in Patients With a History of Recurrent Spontaneous Miscarriages and Documented Thrombophilias. Gynecol Obstet Invest (2004) 57:127–31. doi: 10.1159/000075702 [DOI] [PubMed] [Google Scholar]
- 34. Harger JH, Archer DF, Marchese SG, Muracca-Clemens M, Garver KL. Etiology of Recurrent Pregnancy Losses and Outcome of Subsequent Pregnancies. Obstet Gynecol (1983) 62:574–81. [PubMed] [Google Scholar]
- 35. Koubi M, Rossi P, Arcani R, Gomes De Pihno Q, Chau C, Blanc J, et al. Relevance of Systematic Anti-Nuclear Antibodies Testing After Obstetrical Complications. J Reprod Immunol (2021) 148:103437. doi: 10.1016/j.jri.2021.103437 [DOI] [PubMed] [Google Scholar]
- 36. Verspyck E, Le Cam-Duchez V, Goffinet F, Tron F, Marpeau L, Borg JY. Thrombophilia and Immunological Disorders in Pregnancies as Risk Factors for Small for Gestational Age Infants. BJOG (2002) 109:28–33. doi: 10.1111/j.1471-0528.2002.00411.x [DOI] [PubMed] [Google Scholar]
- 37. Carp HJ, Selmi C, Shoenfeld Y. The Autoimmune Bases of Infertility and Pregnancy Loss. J Autoimmun (2012) 38:J266–74. doi: 10.1016/j.jaut.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 38. Gharavi AE, Cucurull E, Tang H, Silver RM, Branch DW. Effect of Antiphospholipid Antibodies on Beta(2) Glycoprotein I-Phospholipid Interaction. Am J Reprod Immunol (1998) 39:310–5. doi: 10.1111/j.1600-0897.1998.tb00523.x [DOI] [PubMed] [Google Scholar]
- 39. Chamley LW, Duncalf AM, Mitchell MD, Johnson PM. Action of Anticardiolipin and Antibodies to Beta2-Glycoprotein-I on Trophoblast Proliferation as a Mechanism for Fetal Death. Lancet (1998) 352:1037–8. doi: 10.1016/s0140-6736(05)60080-3 [DOI] [PubMed] [Google Scholar]
- 40. Katsuragawa H, Kanzaki H, Inoue T, Hirano T, Mori T, Rote NS. Monoclonal Antibody Against Phosphatidylserine Inhibits In Vitro Human Trophoblastic Hormone Production and Invasion. Biol Reprod (1997) 56:50–8. doi: 10.1095/biolreprod56.1.50 [DOI] [PubMed] [Google Scholar]
- 41. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and Pregnancy. Reprod Sci (2009) 16:206–15. doi: 10.1177/1933719108329095 [DOI] [PubMed] [Google Scholar]
- 42. Mascanfroni I, Montesinos Mdel M, Susperreguy S, Cervi L, Ilarregui JM, Ramseyer VD, et al. Control of Dendritic Cell Maturation and Function by Triiodothyronine. FASEB J (2008) 22:1032–42. doi: 10.1096/fj.07-8652com [DOI] [PubMed] [Google Scholar]
- 43. Matsuo H, Maruo T, Murata K, Mochizuki M. Human Early Placental Trophoblasts Produce an Epidermal Growth Factor-Like Substance in Synergy With Thyroid Hormone. Acta Endocrinol (Copenh) (1993) 128:225–9. doi: 10.1530/acta.0.1280225 [DOI] [PubMed] [Google Scholar]
- 44. Roussev RG, Kaider BD, Price DE, Coulam CB. Laboratory Evaluation of Women Experiencing Reproductive Failure. Am J Reprod Immunol (1996) 35:415–20. doi: 10.1111/j.1600-0897.1996.tb00503.x [DOI] [PubMed] [Google Scholar]
- 45. Cervera R, Font J, Carmona F, Balasch J. Pregnancy Outcome in Systemic Lupus Erythematosus: Good News for the New Millennium. Autoimmun Rev (2002) 1:354–9. doi: 10.1016/s1568-9972(02)00082-4 [DOI] [PubMed] [Google Scholar]
- 46. Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, et al. Complement C3 Activation is Required for Antiphospholipid Antibody-Induced Fetal Loss. J Exp Med (2002) 195:211–20. doi: 10.1084/jem.200116116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Girardi G, Prohászka Z, Bulla R, Tedesco F, Scherjon S. Complement Activation in Animal and Human Pregnancies as a Model for Immunological Recognition. Mol Immunol (2011) 48:1621–30. doi: 10.1016/j.molimm.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 48. Østensen M, Andreoli L, Brucato A, Cetin I, Chambers C, Clowse MEB, et al. State of the Art: Reproduction and Pregnancy in Rheumatic Diseases. Autoimmun Rev (2015) 14:376–86. doi: 10.1016/j.autrev.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 49. Ying Y, Zhong YP, Zhou CQ, Xu YW, Wang Q, Li J, et al. Antinuclear Antibodies Predicts a Poor IVF-ET Outcome: Impaired Egg and Embryo Development and Reduced Pregnancy Rate. Immunol Invest (2012) 41:458–68. doi: 10.3109/08820139.2012.660266 [DOI] [PubMed] [Google Scholar]
- 50. Sthoeger ZM, Mozes E, Tartakovsky B. Anti-Cardiolipin Antibodies Induce Pregnancy Failure by Impairing Embryonic Implantation. Proc Natl Acad Sci USA (1993) 90:6464–7. doi: 10.1073/pnas.90.14.6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kaider BD, Coulam CB, Roussev RG. Murine Embryos as a Direct Target for Some Human Autoantibodies In Vitro. Hum Reprod (1999) 14:2556–61. doi: 10.1093/humrep/14.10.2556 [DOI] [PubMed] [Google Scholar]
- 52. Veglia M, D'Ippolito S, Marana R, Di Nicuolo F, Castellani R, Bruno V, et al. Human IgG Antinuclear Antibodies Induce Pregnancy Loss in Mice by Increasing Immune Complex Deposition in Placental Tissue: In Vivo Study. Am J Reprod Immunol (2015) 74:542–52. doi: 10.1111/aji.12429 [DOI] [PubMed] [Google Scholar]
- 53. Zeng M, Wen P, Duan J. Association of Antinuclear Antibody With Clinical Outcome of Patients Undergoing In Vitro Fertilization/Intracytoplasmic Sperm Injection Treatment: A Meta-Analysis. Am J Reprod Immunol (2019) 82:e13158. doi: 10.1111/aji.13158 [DOI] [PubMed] [Google Scholar]
- 54. Papadimitraki ED, Choulaki C, Koutala E, Bertsias G, Tsatsanis C, Gergianaki I, et al. Expansion of Toll-Like Receptor 9-Expressing B Cells in Active Systemic Lupus Erythematosus: Implications for the Induction and Maintenance of the Autoimmune Process. Arthritis Rheum (2006) 54:3601–11. doi: 10.1002/art.22197 [DOI] [PubMed] [Google Scholar]
- 55. Girardi G, Redecha P, Salmon JE. Heparin Prevents Antiphospholipid Antibody-Induced Fetal Loss by Inhibiting Complement Activation. Nat Med (2004) 10:1222–6. doi: 10.1038/nm1121 [DOI] [PubMed] [Google Scholar]
- 56. Girardi G. Complement Inhibition Keeps Mothers Calm and Avoids Fetal Rejection. Immunol Invest (2008) 37:645–59. doi: 10.1080/08820130802191615 [DOI] [PubMed] [Google Scholar]
- 57. Laskin CA, Bombardier C, Hannah ME, Mandel FP, Ritchie JW, Farewell V, et al. Prednisone and Aspirin in Women With Autoantibodies and Unexplained Recurrent Fetal Loss. N Engl J Med (1997). doi: 10.1056/nejm199707173370302 [DOI] [PubMed] [Google Scholar]
- 58. Sun S, Li C, Kou X, Chen C, Guo F, Zhao A. Association of Prednisone and Antinuclear Antibodies With Pregnancy Outcomes in Women With Unexplained Recurrent Pregnancy Loss. Int J Gynaecol Obstet (2021) 154:492–99. doi: 10.1002/ijgo.13556 [DOI] [PubMed] [Google Scholar]
- 59. Hutton B, Sharma R, Fergusson D, Tinmouth A, Hebert P, Jamieson J, et al. Use of Intravenous Immunoglobulin for Treatment of Recurrent Miscarriage: A Systematic Review. BJOG (2007) 114:134–42. doi: 10.1111/j.1471-0528.2006.01201.x [DOI] [PubMed] [Google Scholar]
- 60. Christiansen OB, Pedersen B, Rosgaard A, Husth M. A Randomized, Double-Blind, Placebo-Controlled Trial of Intravenous Immunoglobulin in the Prevention of Recurrent Miscarriage: Evidence for a Therapeutic Effect in Women With Secondary Recurrent Miscarriage. Hum Reprod (2002) 17:809–16. doi: 10.1093/humrep/17.3.809 [DOI] [PubMed] [Google Scholar]
- 61. Egerup P, Lindschou J, Gluud C, Christiansen OB. The Effects of Intravenous Immunoglobulins in Women With Recurrent Miscarriages: A Systematic Review of Randomised Trials With Meta-Analyses and Trial Sequential Analyses Including Individual Patient Data. PloS One (2015) 10:e0141588. doi: 10.1371/journal.pone.0141588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rasmark Roepke E, Hellgren M, Hjertberg R, Blomqvist L, Matthiesen L, Henic E, et al. Treatment Efficacy for Idiopathic Recurrent Pregnancy Loss - a Systematic Review and Meta-Analyses. Acta Obstet Gynecol Scand (2018) 97:921–41. doi: 10.1111/aogs.13352 [DOI] [PubMed] [Google Scholar]
- 63. Stricker RB, Steinleitner A, Bookoff CN, Weckstein LN, Winger EE. Successful Treatment of Immunologic Abortion With Low-Dose Intravenous Immunoglobulin. Fertil Steril (2000) 73:536–40. doi: 10.1016/s0015-0282(99)00572-5 [DOI] [PubMed] [Google Scholar]
- 64. Ruiz JE, Kwak JY, Baum L, Gilman-sachs A, Beaman KD, Kim YB, et al. Effects of Intravenous Immunoglobulin G on Natural Killer Cell Cytotoxicity In Vitro in Women With Recurrent Spontaneous Abortion. J Reprod Immunol (1996) 31:125–41. doi: 10.1016/0165-0378(96)00969-2 [DOI] [PubMed] [Google Scholar]
- 65. Kwak JY, Kwak FM, Ainbinder SW, Ruiz AM, Beer AE. Elevated Peripheral Blood Natural Killer Cells Are Effectively Suppressed by Immunoglobulin G Infusions in Women With Recurrent Spontaneous Abortion. Am J Reprod Immunol (1996) 35:363–69. doi: 10.1111/j.1600-0897.1996.tb00495.x [DOI] [PubMed] [Google Scholar]
- 66. Morikawa M, Yamada H, Kato EH, Shimada S, Kishi T, Yamada T, et al. Massive Intravenous Immunoglobulin Treatment in Women With Four or More Recurrent Spontaneous Abortions of Unexplained Etiology: Down-Regulation of NK Cell Activity and Subsets. Am J Reprod Immunol (2001) 46:399–404. doi: 10.1034/j.1600-0897.2001.d01-31.x [DOI] [PubMed] [Google Scholar]
- 67. Pandey MK, Thakur S, Agrawal S. Lymphocyte Immunotherapy and its Probable Mechanism in the Maintenance of Pregnancy in Women With Recurrent Spontaneous Abortion. Arch Gynecol Obstet (2004) 269:161–72. doi: 10.1007/s00404-003-0560-3 [DOI] [PubMed] [Google Scholar]
- 68. Cavalcante MB, Costa Fda S, Araujo Júnior E, Barini R. Risk Factors Associated With a New Pregnancy Loss and Perinatal Outcomes in Cases of Recurrent Miscarriage Treated With Lymphocyte Immunotherapy. J Matern Fetal Neonatal Med (2015) 28:1082–6. doi: 10.3109/14767058.2014.943175 [DOI] [PubMed] [Google Scholar]
- 69. Cauchi MN, Lim D, Young DE, Kloss M, Pepperell RJ. Treatment of Recurrent Aborters by Immunization With Paternal Cells–Controlled Trial. Am J Reprod Immunol (1991) 25:16–7. doi: 10.1111/j.1600-0897.1991.tb01057.x [DOI] [PubMed] [Google Scholar]
- 70. Malinowski A, Szpakowski M, Wilczyński J, Oszukowski P, Puchała B, Włodarczyk B. Antinuclear Antibodies in Women With Recurrent Pregnancy Wastage and Their Prognostic Value for Immunotherapy. Zentralbl Gynakol (1994) 116:631–5. [PubMed] [Google Scholar]
- 71. Pandey MK, Saxena V, Agrawal S. Characterization of Mixed Lymphocyte Reaction Blocking Antibodies (MLR-Bf) in Human Pregnancy. BMC Pregnancy Childbirth (2003) 3:2. doi: 10.1186/1471-2393-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Check JH, Arwitz M, Gross J, Peymer M, Szekeres-Bartho J. Lymphocyte Immunotherapy (LI) Increases Serum Levels of Progesterone Induced Blocking Factor (PIBF). Am J Reprod Immunol (1997) 37:17–20. doi: 10.1111/j.1600-0897.1997.tb00188.x [DOI] [PubMed] [Google Scholar]
- 73. Kwak JY, Gilman-Sachs A, Moretti M, Beaman KD, Beer AE. Natural Killer Cell Cytotoxicity and Paternal Lymphocyte Immunization in Women With Recurrent Spontaneous Abortions. Am J Reprod Immunol (1998) 40:352–8. doi: 10.1111/j.1600-0897.1998.tb00065.x [DOI] [PubMed] [Google Scholar]
- 74. Ruffatti A, Cerutti A, Favaro M, Del Ross T, Calligaro A, Hoxha A, et al. Plasmapheresis, Intravenous Immunoglobulins and Bethametasone - A Combined Protocol to Treat Autoimmune Congenital Heart Block: A Prospective Cohort Study. Clin Exp Rheumatol (2016) 34:706–13. [PubMed] [Google Scholar]
- 75. Martínez-Sánchez N, Robles-Marhuenda Á, Álvarez-Doforno R, Viejo A, Antolín-Alvarado E, Deirós-Bronte L, et al. The Effect of a Triple Therapy on Maternal Anti-Ro/SS-A Levels Associated to Fetal Cardiac Manifestations. Autoimmun Rev (2015) 14:423–8. doi: 10.1016/j.autrev.2015.01.005 [DOI] [PubMed] [Google Scholar]
- 76. Fulcher D, Stewart G, Exner T, Trudinger B, Jeremy R. Plasma Exchange and the Anticardiolipin Syndrome in Pregnancy. Lancet (1989) 2:171. doi: 10.1016/s0140-6736(89)90242-0 [DOI] [PubMed] [Google Scholar]
- 77. El-Haieg DO, Zanati MF, El-Foual FM. Plasmapheresis and Pregnancy Outcome in Patients With Antiphospholipid Syndrome. Int J Gynecol Obstet (2007) 99:236–41. doi: 10.1016/j.ijgo.2007.05.045 [DOI] [PubMed] [Google Scholar]
- 78. Fernández-Jiménez MC, Jiménez-Marco MT, Hernández D, González A, Omeñaca F, de la Cámara C. Treatment With Plasmapheresis and Intravenous Immunoglobulin in Pregnancies Complicated With Anti-PP1Pk or Anti-K Immunization: A Report of Two Patients. Vox Sang (2001) 80:117–20. doi: 10.1046/j.1423-0410.2001.00021.x [DOI] [PubMed] [Google Scholar]
- 79. Lépine MS, Goua V, Debouverie OS, Giraud C, Rafat C, Thonier V, et al. Multidisciplinary Management of Anti-PP1P K or Anti-P Alloimmunization During Pregnancy: A New Case With Anti-P and a Literature Review. Transfusion (2021) 61:1972–79. doi: 10.1111/trf.16384 [DOI] [PubMed] [Google Scholar]
- 80. Mastrolia SA, Mazor M, Holcberg G, Leron E, Beharier O, Loverro G, et al. The Physiologic Anticoagulant and Anti-Inflammatory Role of Heparins and Their Utility in the Prevention of Pregnancy Complications. Thromb Haemost (2015) 113:1236–46. doi: 10.1160/th14-10-0848 [DOI] [PubMed] [Google Scholar]
- 81. Rai R, Cohen H, Dave M, Regan L. Randomised Controlled Trial of Aspirin and Aspirin Plus Heparin in Pregnant Women With Recurrent Miscarriage Associated With Phospholipid Antibodies (or Antiphospholipid Antibodies). BMJ (1997) 314:253–7. doi: 10.1136/bmj.314.7076.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yu X, He L. Aspirin and Heparin in the Treatment of Recurrent Spontaneous Abortion Associated With Antiphospholipid Antibody Syndrome: A Systematic Review and Meta-Analysis. Exp Ther Med (2021) 21:57. doi: 10.3892/etm.2020.9489 [DOI] [PMC free article] [PubMed] [Google Scholar]