Abstract
OBJECTIVE
To assess the association of sodium–glucose cotransporter 2 (SGLT2) inhibitors with diabetic ketoacidosis compared with dipeptidyl peptidase 4 (DPP-4) inhibitors and sulfonylureas in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
We conducted a new-user active comparator cohort study to examine two pairwise comparisons: 1) SGLT2 inhibitors versus DPP-4 inhibitors and 2) SGLT2 inhibitors versus sulfonylureas. The main outcome was diabetic ketoacidosis present on hospital admission. We adjusted for confounders through propensity score matching. We used Cox proportional hazards regression with a robust variance estimator to estimate hazard ratios (HRs) and corresponding 95% CIs while adjusting for calendar time.
RESULTS
In cohort 1 (n = 85,125 for SGLT2 inhibitors and n = 85,125 for DPP-4 inhibitors), the incidence rates of diabetic ketoacidosis per 1,000 person-years were 6.0 and 4.3 for SGLT2 inhibitors and DPP4 inhibitors, respectively. In cohort 2 (n = 72,436 for SGLT2 inhibitors and n = 72,436 for sulfonylureas), the incidence rates of diabetic ketoacidosis per 1,000 person-years were 6.3 and 4.5 for SGLT2 inhibitors and sulfonylureas, respectively. In Cox proportional hazards regression models, the use of SGLT2 inhibitors was associated with a higher rate of diabetic ketoacidosis compared with DPP-4 inhibitors (adjusted HR [aHR] 1.63; 95% CI 1.36, 1.96) and sulfonylureas (aHR 1.56; 95% CI 1.30, 1.87).
CONCLUSIONS
In this comparative safety study using real-world data, patients with type 2 diabetes who were newly prescribed SGLT2 inhibitors had a higher rate of diabetic ketoacidosis compared with DPP-4 inhibitors and sulfonylureas. Clinicians should be vigilant about this association.
Introduction
Type 2 diabetes affects ∼34 million individuals in the U.S. (1). The use of antidiabetes drugs can prevent or delay the development of macrovascular and microvascular complications (2). Evidence from randomized clinical trials (RCTs) showed that sodium–glucose cotransporter 2 (SGLT2) inhibitors (vs. placebo) exhibit cardioprotective (e.g., reduction in hospitalization due to heart failure) and metabolic benefits (e.g., reduction in body weight) (2,3). Observational analyses evaluating SGLT2 inhibitors compared with other commonly used antidiabetes drugs (e.g., dipeptidyl peptidase 4 [DPP-4] inhibitors and sulfonylureas) reported findings consistent with these (4,5). However, concerns about the association between these agents and risk of diabetic ketoacidosis remain unresolved (6).
In 2015, the U.S. Food and Drug Administration (FDA) issued a safety warning that use of SGLT2 inhibitors, including canagliflozin, dapagliflozin, and empagliflozin, may lead to diabetic ketoacidosis, a serious condition where the body produces high levels of ketones, which often leads to hospitalization (6). The FDA later added new warnings to the labels of all SGLT2 inhibitors and required manufacturers of SGLT2 inhibitors to conduct postmarketing assessment of this safety concern (7). A post hoc analysis of an RCT suggested an increased rate of diabetic ketoacidosis, although not statistically significant, with canagliflozin compared with placebo (0.6 vs. 0.3 events per 1,000 person-years; hazard ratio [HR] 2.33; 95% CI 0.76, 7.17) (8). In the Canagliflozin and Renal Events in Diabetes and Nephropathy Clinical Evaluation (CREDENCE) trial, the rate of diabetic ketoacidosis was significantly higher in the canagliflozin versus placebo group (2.2 vs. 0.2 per 1,000 patient-years; HR 10.80; 95% CI 1.39, 83.65) (9). Similarly, in the Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial, diabetic ketoacidosis was more common with dapagliflozin versus placebo (0.3% vs. 0.1%, P = 0.02) (10). Observational studies that reported conflicting findings adjusted for a small number of variables (11) or included insulin in the comparator group (12). It is worth noting that the absolute increase in the incidence rate of diabetic ketoacidosis was relatively small (crude rate difference 1.2 per 1,000 person-years) (13).
Given the limited evidence, comparative safety studies are needed to guide treatment selection in clinical practice. In this study, we aimed to investigate the risk of diabetic ketoacidosis with SGLT2 inhibitors compared with DPP-4 inhibitors and sulfonylureas using a large U.S.-based health insurance database that included sociodemographic data, mortality records, and laboratory measures for a subset of the cohort.
Research Design and Methods
Database
We used Optum’s deidentified Clinformatics Data Mart Database. The data include privately insured individuals in the U.S. and provide information on enrollment, patient demographics, outpatient claims, inpatient claims, and prescription drug claims. Laboratory data were available for a subset of beneficiaries. At the University of Pennsylvania, studies using the Optum Clinformatics Data Mart Database are categorized as exempt from requiring institutional review board approval.
Study Design
We performed a retrospective new-user active comparator cohort study of patients with type 2 diabetes who had one or more prescriptions dispensed for SGLT2 inhibitors, DPP-4 inhibitors, or sulfonylureas between January 2013 and December 2019. We included patients who were 1) aged >18 years at cohort entry, 2) new users of SGLT2 inhibitors or DPP-4 inhibitors in cohort 1 and SGLT2 inhibitors or sulfonylureas in cohort 2, 3) had 12 months of continued enrollment before their first prescription (i.e., lookback period), and 4) had a diagnosis of type 2 diabetes during the lookback period (ICD-9 codes 250.x0 or 250.x2 and ICD-10 code E11.x) (14) (Supplementary Fig. 1). We assigned the date of the first eligible prescription as the cohort entry date. We excluded patients who as of the index date had end-stage renal disease, type 1 diabetes, diabetic ketoacidosis (to minimize the capture of prevalent events), initiated SGLT2 inhibitors or comparators on the same date, or used combined products that constituted SGLT2 inhibitors and DPP-4 inhibitors (in cohort 1 only).
Exposure Ascertainment
We identified new users of the study drugs, including SGLT2 inhibitors (i.e., dapagliflozin, canagliflozin, empagliflozin), DPP-4 inhibitors (i.e., saxagliptin, linagliptin, sitagliptin, alogliptin), and sulfonylureas (i.e., chlorpropamide, tolbutamide, tolazamide, glimepiride, glipizide, glyburide), who did not have prior use during the lookback period. Patients were considered exposed if they filled at least one prescription of one of the study drugs. We selected DPP-4 inhibitors and sulfonylureas as the active comparators since they are both commonly used second-line alternatives to SGLT2 inhibitors. We examined the within-class effect of the individual SGLT2 inhibitors, including dapagliflozin, canagliflozin, empagliflozin, compared with DPP-4 inhibitors and sulfonylureas. For the latter analysis, the initiation of an SGLT2 inhibitor different from the one at cohort entry led to censoring (i.e., termination of follow-up).
Outcome Ascertainment
The primary outcome measure was diabetic ketoacidosis, defined on the basis of the presence of diagnosis codes on hospital admission (principal position) (ICD-9 code 250.1 and ICD-10 code E1x.1) (15). This algorithm was validated in the inpatient setting and had a positive predictive value of 88.9% (95% CI 71.9, 96.1) (15).
Follow-up
Eligible patients were followed from the cohort entry date (i.e., treatment initiation) until the occurrence of the first of the following: development of diabetic ketoacidosis, treatment discontinuation defined by the presence of a gap >30 days between consecutive refills, end of enrollment, initiation of study comparator, death, or end of study period (30 June 2019).
Adjustment for Confounding
We ascertained the following confounders during the lookback period: 1) demographics (i.e., age, sex, education level [census block level], race, income [census block level], geographic location); 2) comorbidities (e.g., heart failure, peripheral vascular disease [PVD], hypertension, cancer, stroke); 3) medications (e.g., ACE inhibitors, β-blockers, diuretics); 4) use of other antidiabetes drugs, excluding the study drugs (e.g., insulin, metformin, glucagon-like peptide 1 receptor agonist); 5) Diabetes Complications Severity Index (DCSI) (16); and 6) measures of intensity of health care utilization (e.g., total hospitalizations during the lookback period).
Statistical Analysis
We used propensity score (PS) matching (PSM) to adjust for confounding. We calculated PSs through a logistic regression model (PROC LOGISTIC in SAS [SAS Institute, Cary, NC) that included all covariates listed in Table 1. We did not include laboratory values for measures such as cholesterol, hemoglobin A1c (HbA1c), AST, ALT, or triglyceride levels in the PS since the information was available for a small subset of the cohort. No other data were missing in our study. The estimated PSs were used to match patients in each comparison (SGLT2 inhibitors vs. DPP-4 inhibitors and SGLT2 inhibitors vs. sulfonylureas) using 1:1 fixed ratio matching to the nearest neighbor on the basis of a maximum caliper width of 0.1 of the SD of the logit of PS. We examined the distribution of PS values (PROC SGPLOT in SAS) and assessed the balance before and after PS using standardized mean differences. We considered variables balanced between treatment groups if they met a threshold <0.1.
Table 1.
Demographics and clinical characteristics of new users of SGLT2 inhibitors, DPP-4 inhibitors, and sulfonylureas after PSM
| Characteristic | SGLT2 inhibitors (n = 85,125) |
DPP-4 inhibitors (n = 85,125) |
SMD | SGLT2 inhibitors (n = 72,436) |
Sulfonylureas (n = 72,436) |
SMD |
|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 60.7 (11.0) | 60.4 (12.1) | 0.02 | 59.8 (11.1) | 59.5 (12.9) | 0.04 |
| Male sex | 48,629 (57.2) | 48,816 (57.4) | 0.00 | 40,701 (56.2) | 40,776 (56.2) | 0.00 |
| Education level | 0.00 | 0.00 | ||||
| <12th grade | 806 (1.0) | 794 (1.0) | 650 (0.8) | 699 (1.0) | ||
| High school diploma | 30,207 (35.4) | 30,037 (35.2) | 24,693 (34.0) | 24,762 (34.2) | ||
| Less than bachelor’s degree | 44,566 (52.4) | 44,709 (52.6) | 38,176 (52.8) | 38,040 (52.6) | ||
| Bachelor’s degree plus | 9,239 (10.8) | 9,264 (10.8) | 8,665 (12.0) | 8,674 (12.0) | ||
| Race | 0.00 | 0.00 | ||||
| Asian | 3,258 (3.8) | 3,344 (4.0) | 2,980 (4.2) | 3,035 (4.2) | ||
| Black | 11,520 (13.6) | 11,520 (13.6) | 9,600 (13.2) | 9,533 (13.2) | ||
| Hispanic | 14,453 (17.0) | 14,356 (16.8) | 12,146 (16.8) | 12,203 (16.8) | ||
| White | 55,894 (65.6) | 55,905 (65.6) | 47,710 (65.8) | 47,665 (65.8) | ||
| Unknown | 12,891 (15.2) | 12,760 (15.0) | 11,102 (15.4) | 11,122 (15.4) | ||
| Income | 0.02 | 0.00 | ||||
| <$40,000 | 18,955 (22.2) | 18,629 (21.8) | 15,395 (21.2) | 15,260 (21.0) | ||
| $40,000–$49,000 | 5,923 (7.0) | 5,858 (6.8) | 4,797 (6.6) | 4,776 (6.6) | ||
| $50,000–$59,000 | 6,219 (7.4) | 6,214 (7.2) | 5,081 (7.0) | 5,038 (7.0) | ||
| $60,000–$74,000 | 8,578 (10.0) | 8,572 (10.0) | 7,191 (10.0) | 7,138 (9.8) | ||
| $75,000–$99,000 | 12,324 (14.4) | 12,525 (14.8) | 10,451 (14.4) | 10,618 (14.6) | ||
| ≥$100,000 | 20,235 (23.8) | 20,567 (24.2) | 18,419 (25.4) | 18,484 (25.6) | ||
| U.S. region | 0.00 | 0.00 | ||||
| East north central | 11,464 (13.4) | 11,613 (13.6) | 9,428 (13.0) | 9,401 (13.0) | ||
| East south central | 4,638 (5.4) | 4,685 (5.6) | 3,977 (5.4) | 3,965 (5.4) | ||
| Middle Atlantic | 4,678 (5.4) | 4,478 (5.2) | 4,446 (6.2) | 4,384 (6.0) | ||
| Mountain | 6,146 (7.2) | 6,103 (7.2) | 5,356 (7.4) | 5,398 (7.4) | ||
| New England | 1,823 (2.2) | 1,758 (2.0) | 1,527 (2.2) | 1,495 (2.0) | ||
| South Atlantic | 23,740 (27.8) | 23,913 (28.0) | 19,982 (27.6) | 20,090 (27.8) | ||
| West north central | 7,078 (8.4) | 7,001 (8.2) | 5,788 (8.0) | 5,698 (7.8) | ||
| West south central | 18,537 (21.8) | 18,539 (21.8) | 16,077 (22.2) | 16,160 (22.4) | ||
| Baseline comorbidities | ||||||
| Heart failure | 5,063 (6.0) | 4,807 (5.6) | 0.02 | 3,949 (5.4) | 3,932 (5.4) | 0.00 |
| PVD | 5,964 (7.0) | 5,897 (7.0) | 0.00 | 4,615 (6.4) | 4,610 (6.4) | 0.00 |
| Hypertension | 58,568 (68.8) | 58,287 (68.4) | 0.00 | 48,126 (66.4) | 48,133 (66.4) | 0.00 |
| Cancer | 3,867 (4.6) | 3,786 (4.4) | 0.00 | 3,326 (4.6) | 3,267 (4.6) | 0.00 |
| Chronic lung disease | 11,565 (13.6) | 11,471 (13.4) | 0.00 | 9,666 (13.4) | 9,778 (13.4) | 0.00 |
| Hyperlipidemia | 39,183 (46.0) | 39,034 (45.8) | 0.00 | 32,220 (44.4) | 32,215 (44.4) | 0.00 |
| Chronic kidney disease | 10,021 (11.8) | 9,378 (11.0) | 0.02 | 7,917 (11.0) | 7,748 (10.6) | 0.00 |
| Stroke | 56 (0.1) | 67 (0.1) | 0.00 | 45 (0.1) | 38 (0.1) | 0.00 |
| Tobacco use | 5,688 (6.6) | 5,870 (6.8) | 0.00 | 4,704 (6.4) | 4,721 (6.6) | 0.00 |
| Arthritis | 3,638 (4.2) | 3,617 (4.2) | 0.00 | 2,984 (4.2) | 2,921 (4.0) | 0.00 |
| Liver disease | 3,991 (4.6) | 4,048 (4.8) | 0.00 | 3,454 (4.8) | 3,455 (4.8) | 0.00 |
| Alcohol abuse | 880 (1.0) | 878 (1.0) | 0.00 | 753 (1.0) | 739 (1.0) | 0.00 |
| Drug abuse | 1,132 (1.4) | 1,117 (1.4) | 0.00 | 951 (1.4) | 919 (1.2) | 0.00 |
| VTE | 1,449 (1.8) | 1,381 (1.6) | 0.00 | 1,179 (1.6) | 1,148 (1.6) | 0.00 |
| Depression | 3,153 (3.8) | 2,994 (3.6) | 0.00 | 2,721 (3.8) | 2,636 (3.6) | 0.00 |
| Baseline medications | ||||||
| ACE inhibitors | 33,589 (39.4) | 33,463 (39.4) | 0.00 | 26,345 (36.4) | 26,276 (36.2) | 0.00 |
| α-Adrenergic blockers | 5,087 (6.0) | 4,948 (5.8) | 0.00 | 3,978 (5.4) | 3,995 (5.6) | 0.00 |
| ARBs | 20,518 (24.2) | 20,447 (24.0) | 0.00 | 17,462 (24.2) | 17,402 (24.0) | 0.00 |
| β-Blockers | 19,170 (22.6) | 18,834 (22.2) | 0.00 | 15,176 (21.0) | 15,156 (21.0) | 0.00 |
| CCBs | 17,199 (20.2) | 16,839 (19.8) | 0.02 | 13,862 (19.2) | 13,931 (19.2) | 0.00 |
| Direct vasodilators | 1,093 (1.2) | 1,064 (1.2) | 0.00 | 826 (1.2) | 792 (1.0) | 0.00 |
| Thiazide diuretics | 22,639 (26.6) | 22,716 (26.6) | 0.00 | 18,417 (25.4) | 18,337 (25.4) | 0.00 |
| Loop diuretics | 7,479 (8.8) | 7,092 (8.4) | 0.02 | 5,756 (8.0) | 5,842 (8.0) | 0.00 |
| Potassium diuretics | 2,409 (2.8) | 2,390 (2.8) | 0.00 | 2,007 (2.8) | 1,989 (2.8) | 0.00 |
| Aldosterone antagonists | 2,301 (2.8) | 2,276 (2.6) | 0.00 | 1,905 (2.6) | 1,884 (2.6) | 0.00 |
| PPIs | 16,610 (19.6) | 16,358 (19.2) | 0.00 | 13,883 (19.2) | 13,731 (19.0) | 0.00 |
| SSRIs | 11,681 (13.8) | 11,592 (13.6) | 0.00 | 9,838 (13.6) | 9,812 (13.6) | 0.00 |
| Statins | 50,136 (58.8) | 49,988 (58.8) | 0.00 | 41,079 (56.8) | 41,175 (56.8) | 0.00 |
| Metformin | 55,263 (65.0) | 53,129 (62.4) | 0.06 | 45,701 (63.0) | 46,061 (63.6) | 0.02 |
| Amylin | 90 (0.2) | 34 (0.1) | 0.02 | 73 (0.2) | 11 (0.1) | 0.02 |
| TZDs | 6,112 (7.2) | 5,119 (6.0) | 0.04 | 4,051 (5.6) | 4,075 (5.6) | 0.00 |
| Insulin | 18,383 (21.6) | 17,586 (20.6) | 0.02 | 14,762 (20.4) | 14,214 (19.6) | 0.02 |
| GLP-1 | 7,272 (8.6) | 6,422 (7.6) | 0.04 | 7,082 (9.8) | 6,381 (8.8) | 0.04 |
| DCSI | 0.02 | 0.00 | ||||
| 0 | 61,936 (72.8) | 62,486 (73.4) | 54,478 (75.2) | 54,709 (75.6) | ||
| 1 | 10,172 (12.0) | 10,077 (11.8) | 7,928 (11.0) | 7,787 (10.8) | ||
| 2 | 7,324 (8.6) | 7,145 (8.4) | 5,712 (7.8) | 5,637 (7.8) | ||
| >2 | 5,693 (6.6) | 5,417 (6.4) | 4,318 (6.0) | 4,303 (6.0) | ||
| Measures of health care use | ||||||
| Inpatient visits, mean (SD) | 0.1 (0.5) | 0.1 (0.4) | 0.02 | 0.1 (0.5) | 0.1 (0.4) | 0.00 |
| Prescriptions, mean (SD) | 34.1 (33.7) | 33.5 (33.3) | 0.02 | 31.9 (32.5) | 31.7 (34.8) | 0.00 |
Data are n (%) unless otherwise indicated. We considered variables balanced between treatment groups if they met a threshold <0.1. ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; GLP-1, glucagon-like peptide 1; PPI, proton pump inhibitor; SMD, standardized mean difference; SSRI, selective serotonin reuptake inhibitor; TZD, thiazolidinedione; VTE, venous thromboembolism.
We estimated the incidence rate of diabetic ketoacidosis in each pairwise comparison and reported the total number of events per 1,000 person-years. We estimated marginal HRs and corresponding 95% CIs by Cox proportional hazards regression (PROC PHREG in SAS) using a robust variance estimator to account for the dependence in matched cohorts while adjusting for calendar time (17). We assessed proportional hazards assumptions using Schoenfeld residuals. We estimated the absolute increase in the risk of diabetic ketoacidosis with SGLT2 inhibitors versus DPP-4 inhibitors and versus sulfonylureas at 1 month, 6 months, and 1 year of treatment initiation (PROC LIFETEST in SAS).
Sensitivity and Subgroup Analyses
We conducted several sensitivity analyses, including 1) increasing the permissible gap between consecutive refills from 30 days to 60 and 90 days (to account for stockpiling of diabetes medications), 2) trimming the PS tails (95th and 5th, 85th and 15th, 75th and 25th), 3) adjusting for PS in the outcome model, and 4) limiting the end of the study period to December 2015 to minimize the impact of FDA safety communication. We examined the balance before and after matching for the following laboratory values: cholesterol, HbA1c, AST, ALT, and triglyceride levels. In subgroup analyses using Cox proportional hazards regression models, we examined whether differential associations existed for SGLT2 inhibitors versus comparators by age, sex, baseline insulin (including basal and prandial insulin), stroke, and PVD. To assess the potential for effect modification, we included an interaction term between exposure status and indicators for each subgroup. To account for multiple testing in subgroup analyses, we used Bonferroni adjustment, which considered results statistically significant if the corresponding P value was ≤ α (i.e., 0.05)/n where n = total number of subgroup analyses (18). We assessed for the potential of unmeasured confounders using the E-value, which provides, conditional on measured confounders, the minimum needed strength of association among an unmeasured confounder, exposure, and outcome to move the observed effect estimates toward the null (https://www.evalue- calculator.com) (19). All analyses were conducted using SAS 9.4 statistical software.
Results
Description of Study Cohorts
SGLT2 Inhibitors Versus DPP-4 Inhibitors
We identified 100,046 new users of SGLT2 inhibitors and 212,311 new users of DPP-4 inhibitors (Supplementary Fig. 2). Patient demographic and clinical characteristics before PSM are summarized in Supplementary Table 1. Supplementary Fig. 3A depicts the distribution of PSs before and after PSM. After PSM (n = 85,125 for SGLT2 inhibitors and n = 85,125 for DPP-4 inhibitors), patient characteristics were balanced between the two groups, including mean age (61 vs. 60 years), education (less than bachelor’s degree 52% vs. 53%), race (White 66% vs. 66%), income (≥$100,000 24% vs. 24%), heart failure (6% vs. 6%), hypertension (69% vs. 68%), chronic kidney disease (12% vs. 11%), ACE inhibitor use (39% vs. 39%), β-blocker use (23% vs. 22%), insulin use (22% vs. 21%), and mean inpatient visits (0.1 vs. 0.1) (Table 1). The median follow-up time was 131 days (25th–75th percentile 54–321 days) among SGLT2 inhibitor users and 131 days (55–325 days) among DPP-4 inhibitor users.
SGLT2 Inhibitors Versus Sulfonylureas
We identified 84,583 new users of SGLT2 inhibitors and 296,947 new users of sulfonylureas who met inclusion criteria (Supplementary Fig. 2). Patient demographic and clinical characteristics before PSM are summarized in Supplementary Table 1. Supplementary Fig. 3B depicts the distribution of PSs before and after PSM. After matching (n = 72,436 for SGLT2 inhibitors and n = 72,436 for sulfonylureas), patient characteristics were well-balanced between the two groups, including mean age (60 vs. 60 years), education (less than bachelor’s degree 53% vs. 53%), race (White 66% vs. 66%), income (≥$100,000 25% vs. 26%), heart failure (5% vs. 5%), hypertension (66% vs. 66%), chronic kidney disease (11% vs. 11%), ACE inhibitor use (36% vs. 36%), β-blocker use (21% vs. 21%), insulin use (20% vs. 20%), and mean inpatient visits (0.1 vs. 0.1) (Table 1). The median follow-up time was 138 days (25th–75th percentile 57–333 days) among SGLT2 inhibitor users and 128 days (56–322 days) among sulfonylurea users.
Rates of Diabetic Ketoacidosis With SGLT2 Inhibitors Versus DPP-4 Inhibitors
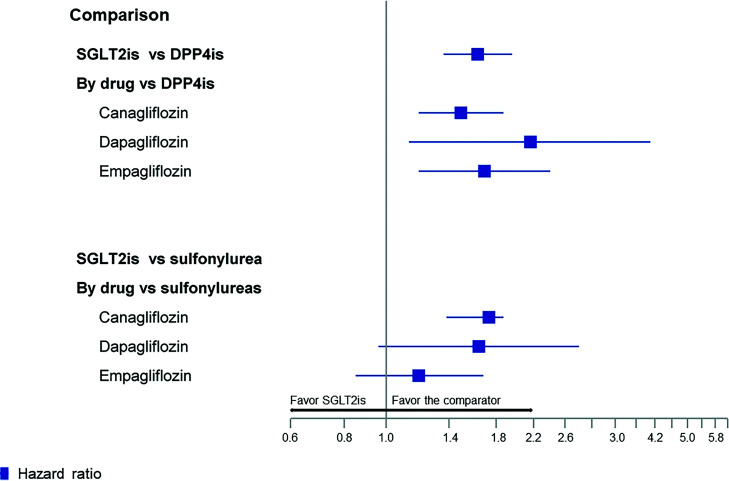
In the matched sample, 343 patients had diabetic ketoacidosis among 85,125 SGLT2 inhibitor users (6.0 events per 1,000 person-years) compared with 256 among 85,125 DPP-4 inhibitor users (4.3 events per 1,000 person-years; adjusted HR [aHR] 1.63; 95% CI 1.36, 1.96) (Fig. 1 and Supplementary Table 2). The absolute increase in the risk of diabetic ketoacidosis with SGLT2 inhibitors compared with DPP-4 inhibitors per 1,000 person-years within 1 month, 6 months, and 1 year of SGLT2 inhibitor initiation was 0.20, 0.95, and 1.73, respectively. We found a similar increased rate of diabetic ketoacidosis when we stratified the primary analysis by individual SGLT2 inhibitors (vs. DPP-4 inhibitors), including canagliflozin (aHR 1.49; 95% CI 1.19, 1.87), dapagliflozin (aHR 2.16; 95% CI 1.13, 4.10), and empagliflozin (aHR 1.69; 95% CI 1.19, 2.40).
Figure 1.
Rate of diabetic ketoacidosis with SGLT2 inhibitors (SGLT2is) compared with DPP-4 inhibitors (DPP-4is) and sulfonylureas among patients with type 2 diabetes. The x-axis displays HRs and 95% CIs on the logarithmic scale. The absolute increase in the incidence of diabetic ketoacidosis with SGLT2is per 1,000 person-years was 0.20 within 1 month, 0.95 within 6 months, and 1.73 within 1 year of treatment initiation for SGLT2is vs. DPP-4is, and 0.20 within 1 month, 0.96 within 6 months, and 1.72 within 1 year of treatment initiation for SGLT2is vs. sulfonylureas.
Rates of Diabetic Ketoacidosis With SGLT2 Inhibitors Versus Sulfonylureas
In the matched sample, 313 patients had diabetic ketoacidosis among 72,436 SGLT2 inhibitor users (6.3 events per 1,000 person-years) compared with 227 among 72,436 sulfonylurea users (4.5 events per 1,000 person-years; aHR 1.56; 95% CI 1.30, 1.87) (Fig. 1 and Supplementary Table 2). The absolute increase in the risk of diabetic ketoacidosis with SGLT2 inhibitors compared with sulfonylureas per 1,000 person-years within 1 month, 6 months, and 1 year of SGLT2 inhibitor initiation was 0.20, 0.96, and 1.72, respectively. We found a similar increased rate of diabetic ketoacidosis when we stratified the primary analysis by individual SGLT2 inhibitors (vs. sulfonylureas), including canagliflozin (aHR 1.73; 95% CI 1.38, 2.18), dapagliflozin (aHR 1.64; 95% CI 0.96, 2.80), and empagliflozin (aHR 1.19; 95% CI 0.85, 1.68).
Sensitivity Analyses
The results from the sensitivity analyses were similar to the primary findings when changing the length of the allowable gap between refills, trimming the tails of the PS, and adjusting for PS as a variable in the model instead of matching (Table 2). Although laboratory values were not included in the PS model, our examination of a subset of the cohort with complete laboratory values showed well-balanced measures after matching (Supplementary Table 3). The E-value for diabetic ketoacidosis in cohort 1 was 2.64 for the estimate and 2.06 for the CI (Supplementary Fig. 4, top). In cohort 1, an unmeasured confounder associated with diabetic ketoacidosis and SGLT2 inhibitors by a risk ratio of 2.64-fold each would be needed to explain away the lower confidence limit. Similarly, the E-value for diabetic ketoacidosis in cohort 2 was 2.49 for the estimates and 1.92 for the CI (Supplementary Fig. 4, bottom). In cohort 2, an unmeasured confounder associated with diabetic ketoacidosis and SGLT2 inhibitors by a risk ratio of 2.49-fold each would be needed to explain away the lower confidence limit.
Table 2.
Summary of results from subgroup and sensitivity analyses
| SGLT2 inhibitor vs. DPP-4 inhibitor | SGLT2 inhibitor vs. sulfonylurea | |||||
|---|---|---|---|---|---|---|
| Sensitivity analysis | SGLT2 inhibitor, n | DPP-4 inhibitor, n | aHR (95% CI) | SGLT2 inhibitor, n | Sulfonylurea, n | aHR (95% CI) |
| Increasing permissible grace period between consecutive refills from 30 to 60 days | 85,125 | 85,125 | 1.77 (1.48, 2.12) | 72,436 | 72,436 | 1.60 (1.34, 1.92) |
| Increasing permissible grace period between consecutive refills from 30 to 60 days | 85,125 | 85,125 | 1.78 (1.49, 2.13) | 72,436 | 72,436 | 1.62 (1.35, 1.94) |
| Trimming PS distribution tail to 95th and 5th percentile | 84,992 | 84,992 | 1.62 (1.34, 1.94) | 71,592 | 71,592 | 1.56 (1.30, 1.87) |
| Trimming PS distribution tail to 85th and 15th percentile | 79,510 | 79,510 | 1.67 (1.38, 2.02) | 58,495 | 58,495 | 1.45 (1.20, 1.76) |
| Trimming PS distribution tail to 75th and 25th percentile | 63,126 | 63,126 | 1.67 (1.35, 2.05) | 39,063 | 39,063 | 1.49 (1.20, 1.85) |
| Adjusting for PS in the primary outcome model | 100,046 | 212,311 | 1.53 (1.29, 1.84) | 84,583 | 296,947 | 1.47 (1.18, 1.82) |
| Limiting the study period to 2015 | 26,333 | 26,333 | 1.26 (1.02, 1.57) | 22,968 | 22,968 | 1.53 (1.21, 1.93) |
Subgroup Analyses
We did not find evidence of heterogeneity of treatment effect by age, sex, baseline insulin use, stroke, and PVD after accounting for multiple testing (Table 3).
Table 3.
Examination of effect modification within clinically relevant subgroups
| P for interaction | ||
|---|---|---|
| SGLT2 inhibitors vs. DPP-4 inhibitors | SGLT2 inhibitors vs. sulfonylureas | |
| Age-group | 0.06 | 0.51 |
| Sex | 0.10 | 0.02 |
| Insulin | 0.98 | 0.14 |
| Stroke | 0.99 | 0.99 |
| PVD | 0.17 | 0.14 |
Bonferroni adjustment was used to account for multiple testing. Results were considered statistically significant if the corresponding P value was ≤ α (i.e., 0.05) / n, where n = total number of subgroup analyses.
Conclusions
In this large population-based study of patients with type 2 diabetes, we found that the use of SGLT2 inhibitors was associated with an increased rate of diabetic ketoacidosis compared with the use of DPP-4 inhibitors or sulfonylureas. The results were consistent for the individual SGLT2 inhibitors, including canagliflozin (vs. DPP-4 inhibitors or sulfonylureas), dapagliflozin, and empagliflozin (vs. DPP-4 inhibitors). However, the CIs for the comparisons between dapagliflozin and empagliflozin (vs. sulfonylureas) crossed the null, presumably because of the lack of power for individual agents. These findings have important clinical implications for patients with type 2 diabetes. Diabetic ketoacidosis is a major complication of diabetes that can be life threatening (1). Despite the advancement in the treatment of diabetes and its complications, diabetic ketoacidosis remains a significant burden and requires prompt diagnosis and treatment (20,21).
Several potential mechanisms may explain the association between SGLT2 inhibitors and diabetic ketoacidosis. SGLT2 inhibitors may indirectly enhance ketone bodies level in the plasma by reducing sodium reabsorption and enhancing glucosuria production and renal ketone reabsorption (22). An RCT of patients with type 2 diabetes treated with dapagliflozin or placebo found that dapagliflozin (vs. placebo) caused a 16% decline in glucose oxidation and a 14% increase in lipid oxidation (23). The increase in glucagon-to-insulin ratio can stimulate ketone production (23). Others proposed that SGLT2 inhibitors might reduce renal tubular clearance of ketone bodies through their effect on the glomerular filtration rate (24). Several case reports have suggested a link between risk of diabetic ketoacidosis with SGLT2 inhibitors and low-calorie diet and volume depletion, especially in the perioperative period (25). This prompted the FDA in 2020 to recommend that patients temporarily stop taking SGLT2 inhibitors before any scheduled surgery. Similar to the results reported in prior studies, the increase in the absolute incidence of diabetic ketoacidosis with SGLT2 inhibitors in our study was relatively low (1.7 per 1,000 person-years within 1 year of treatment initiation). The Dapagliflozin in Patients With Cardiometabolic Risk Factors Hospitalized With COVID-19 (DARE-19) trial reported only two cases (0.3%) of diabetic ketoacidosis in patients receiving dapagliflozin (26). However, the trial focused on patients with COVID-19 and included a small number of patients relative to our study (n = 1,250 vs. ∼300,000 in each pairwise comparison).
Because of their efficacy in maintaining glycemic control and weight reduction, SGLT2 inhibitors as an add-on to metformin therapy are widely used for the treatment of type 2 diabetes. The class protects against major cardiovascular diseases (2) and heart failure (27). However, concerns emerged regarding their association with an increased risk of adverse events, including diabetic ketoacidosis. Several case reports in the medical literature and FDA adverse drug reporting system suggested a potential link between SGLT2 inhibitors and diabetic ketoacidosis. These cases prompted the FDA and European Medicines Agency to issue statements warning about the potential link between SGLT2 inhibitors and diabetic ketoacidosis. A post hoc analysis of an RCT suggested an increased rate of diabetic ketoacidosis, although not statistically significant, with canagliflozin compared with placebo (0.6 vs. 0.3 events per 1,000 person-years; HR 2.33; 95% CI 0.76, 7.17) (8). Similarly, in the DECLARE-TIMI 58 trial, the rate of diabetic ketoacidosis was more common with dapagliflozin compared with placebo (0.3% vs. 0.1%, P = 0.02) (10). The increased rate of diabetic ketoacidosis with SGLT2 inhibitors was observed in cardiovascular outcomes trials (8,9) but not in those examining SGLT2 inhibitors in patients with heart failure (28). This difference may be related to the fact that patients in the cardiovascular outcomes trials all had diabetes, whereas many of the patients in the heart failure trials did not, which might suggest less risk of euglycemic diabetic ketoacidosis when SGLT2 inhibitors are used in patients without diabetes.
Several observational studies evaluated the risk of diabetic ketoacidosis with SGLT2 inhibitors compared with other glucose-lowering drugs (11–13,29–31). Fralick et al. (11) found that the use of SGLT2 inhibitors was associated with a twofold increased risk of diabetic ketoacidosis compared with DPP-4 inhibitors (HR 2.2; 95% CI 1.14, 3.6). However, the study adjusted for a relatively small number of variables. For example, confounders such as baseline use of medications (other than diabetes drugs) and diabetes comorbidity index were not included. Other observational studies reported conflicting findings. Wang et al. (12) compared the rate of diabetic ketoacidosis with SGLT2 inhibitors versus other glucose-lowering agents. The study found no association between SGLT2 inhibitors (vs. other glucose-lowering drugs) and diabetic ketoacidosis. However, the study had major limitations, including inclusion of insulin in the comparator group and omitting the adjustment of important variables such as prior medication use, baseline comorbidities, and measures of health care utilization. Of note, none of the prior studies adjusted for sociodemographic factors, including race, education, and income. Adjusting for those variables in our study attenuated the observed treatment effect, suggesting that residual confounding by sociodemographic status is likely to have been present in prior analyses. Finally, unlike the findings reported in an earlier analysis (13), our subgroup analyses showed an absence of effect modification by prior insulin use.
Strengths of this study include the large sample size of individuals with commercial health care coverage from a large representative sample in the U.S. and the availability of sociodemographic data, mortality records, and laboratory measures, including data on HbA1c for a subset of the cohort. Limitations of the study include the absence of data on lifestyle variables, such as exercise and diet. Furthermore, laboratory measures were missing for a large subset of the cohort. However, our examination of HbA1c, cholesterol, AST, ALT, and triglyceride levels in a subset of the cohort showed that those variables were balanced after matching in both comparisons. Additionally, an E-value of 2.46 for the point estimate for SGLT2 inhibitors vs. DPP-4 inhibitors and 2.49 for SGLT2 inhibitors vs. sulfonylureas indicates that a fairly large degree of unmeasured confounding would be needed to explain the observed results in the absence of an effect of the exposure (21). Although we lacked a direct measure of diabetes severity, we used DCSI to adjust for differences in diabetes complications and severity in administrative data. DCSI is a useful tool in predicting the risk of hospitalization and mortality in patients with diabetes (16). Outcome misclassification is possible since our outcome definition was based on ICD diagnoses codes only. Diabetic ketoacidosis is characterized by a serum glucose level ≥250 mg/dL, arterial pH <7.3, and a serum bicarbonate level ≤18 mEq/L. Although those measures are not available in the current data, we relied on an outcome definition that has been validated previously (15). Any outcome misclassification is likely nondifferential since we used the same outcome definition across treatment groups. Furthermore, it is possible that our study underestimated the risk of diabetic ketoacidosis since only cases resulting in hospitalization were included. Given the lack of information on serum glucose level, we were not able to ascertain whether diabetic ketoacidosis cases in the current study were euglycemic. Exposure misclassification is possible because our definition was based on prescription refills. However, the study results were consistent when we changed the gap between refills from 30 days to 60 and 90 days. Although we censored patients if they died, the available death records were limited to the month and year of death only. There is the potential for surveillance bias since physicians may be more likely to detect diabetic ketoacidosis cases in the SGLT2 inhibitor group following the FDA safety communication. Although such bias would have overestimated the relative risk of diabetic ketoacidosis with SGLT2 inhibitors compared with other agents, study results in the sensitivity analysis limiting the study period to December 2015 were consistent with those of the primary analysis. Finally, the study results may not be generalizable to patients with type 2 diabetes with governmental health insurance or those without health care coverage.
In conclusion, in this comparative safety study using real-world data, patients with type 2 diabetes who were newly prescribed SGLT2 inhibitors had a higher rate of diabetic ketoacidosis compared with DPP-4 inhibitors and sulfonylureas. Clinicians need to be vigilant about this safety signal.
Article Information
Funding and Duality of Interest. This work was supported by a career development award from the National Institutes of Health (grant K99HL159230). G.K.D. receives funding from the American Society of Hematology and National Institutes of Health. J.H.F. has consulted for Genentech, Eli Lilly, and Boehringer Ingelheim on matters unrelated to the topic of this article. S.H. has consulted for the Medullary hyroid Cancer Consortium (Novo Nordisk, AstraZeneca, and Eli Lilly), Provention Bio Inc., Novo Nordisk, Arbor Pharmaceuticals, and Intercept Pharmaceuticals on matters unrelated to the topic of this article. C.E.L. serves on the Executive Committee of the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training, which receives funding from Pfizer and Sanofi to support trainee education; receives research funding from the FDA, Department of Veterans Affairs, and National Institutes of Health; consults for the Reagan-Udall Foundation; and has received honoraria from the University of Massachusetts, University of Florida, and the American College of Clinical Pharmacy Foundation. C.E.L.’s spouse is employed by Merck, but neither C.E.L. nor his spouse hold Merck stock. J.D.L. has served as a consultant for Janssen Pharmaceuticals, Samsung Bioepis, Bristol-Myers Squibb, Merck, Celgene, and Bridge Biotherapeutics; has served as a paid member of a data monitoring committee for Pfizer, UCB, Gilead, Arena Pharmaceuticals, Protagonist Therapeutics, and Amgen; has received research funding from Janssen Pharmaceuticals and Takeda Pharmaceuticals; and has received educational grant funding from Takeda Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. G.K.D. created the study concept and design, analyzed data, and drafted the manuscript. J.H.F., S.H., C.E.L., and J.D.L. contributed to the interpretation of the results and writing and revision of the final manuscript. G.K.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the virtual 37th International Conference on Pharmacoepidemiology and Therapeutic Risk Management, 23–25 August 2021.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.18834131.
References
- 1. Centers for Disease Control and Prevention . National diabetes statistics report. Updated 2020. Accessed 9 November 2020. Available from https://www.cdc.gov/diabetes/data/statistics- report/index.html
- 2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 3. Rosenstock J, Aggarwal N, Polidori D, et al.; Canagliflozin DIA 2001 Study Group . Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 2012;35:1232–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xie Y, Bowe B, Gibson AK, McGill JB, Maddukuri G, Al-Aly Z. Comparative effectiveness of sodium-glucose cotransporter 2 inhibitors vs sulfonylureas in patients with type 2 diabetes. JAMA Intern Med 2021;181:1043–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dawwas GK, Smith SM, Park H. Cardiovascular outcomes of sodium glucose cotransporter-2 inhibitors in patients with type 2 diabetes. Diabetes Obes Metab 2019;21:28–36 [DOI] [PubMed] [Google Scholar]
- 6. U.S. Food and Drug Administration . FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Published 15 May 2015. Accessed 17 September 2021. Available from https://www.fda.gov/files/drugs/published/Drug-Safety-Communication-- FDA-warns-that-SGLT2-inhibitors-for-diabetes- may-result-in-a-serious-condition-of-too-much- acid-in-the-blood.pdf
- 7. U.S. Food and Drug Administration . FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. Updated 19 March 2020. Accessed 17 September 2021. Available from https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels- sglt2-inhibitors-diabetes-include-warnings- about-too-much-acid-blood-and-serious
- 8. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 9. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 10. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 11. Fralick M, Schneeweiss S, Patorno E. Risk of diabetic ketoacidosis after initiation of an SGLT2 inhibitor. N Engl J Med 2017;376:2300–2302 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Desai M, Ryan PB, et al. Incidence of diabetic ketoacidosis among patients with type 2 diabetes mellitus treated with SGLT2 inhibitors and other antihyperglycemic agents. Diabetes Res Clin Pract 2017;128:83–90 [DOI] [PubMed] [Google Scholar]
- 13. Douros A, Lix LM, Fralick M, et al.; Canadian Network for Observational Drug Effect Studies (CNODES) Investigators . Sodium–glucose cotransporter-2 inhibitors and the risk for diabetic ketoacidosis. Ann Intern Med 2020;173:417–425 [DOI] [PubMed] [Google Scholar]
- 14. Chi GC, Li X, Tartof SY, Slezak JM, Koebnick C, Lawrence JM. Validity of ICD-10-CM codes for determination of diabetes type for persons with youth-onset type 1 and type 2 diabetes. BMJ Open Diabetes Res Care 2019;7:e000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bobo WV, Cooper WO, Epstein RA Jr, Arbogast PG, Mounsey J, Ray WA. Positive predictive value of automated database records for diabetic ketoacidosis (DKA) in children and youth exposed to antipsychotic drugs or control medications: a Tennessee Medicaid Study. BMC Med Res Methodol 2011;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008;14:15–23 [PMC free article] [PubMed] [Google Scholar]
- 17. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noble WS. How does multiple testing correction work? Nat Biotechnol 2009;27:1135–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med 2017;167:268–274 [DOI] [PubMed] [Google Scholar]
- 20. Umpierrez G, Korytkowski M. Diabetic emergencies - ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol 2016;12:222–232 [DOI] [PubMed] [Google Scholar]
- 21. Desai D, Mehta D, Mathias P, Menon G, Schubart UK. Health care utilization and burden of diabetic ketoacidosis in the U.S. over the past decade: a nationwide analysis. Diabetes Care 2018;41:1631–1638 [DOI] [PubMed] [Google Scholar]
- 22. Goldenberg RM, Berard LD, Cheng AYY, et al. SGLT2 inhibitor–associated diabetic ketoacidosis: Clinical review and recommendations for prevention and diagnosis. Clin Ther 2016;38:2654–2664.e1 [DOI] [PubMed] [Google Scholar]
- 23. Daniele G, Xiong J, Solis-Herrera C, et al. Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Diabetes Care 2016;39:2036–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev 2017;33:e2886. [DOI] [PubMed] [Google Scholar]
- 25. Meyer EJ, Mignone E, Hade A, Thiruvenkatarajan V, Bryant RV, Jesudason D. Periprocedural euglycemic diabetic ketoacidosis associated with sodium-glucose cotransporter 2 inhibitor therapy during colonoscopy. Diabetes Care 2020;43:e181–e184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2021;9:586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet 2020;396:819–829 [DOI] [PubMed] [Google Scholar]
- 28. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. New Engl J Med 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 29. Kim YG, Jeon JY, Han SJ, Kim DJ, Lee KW, Kim HJ. Sodium-glucose co-transporter-2 inhibitors and the risk of ketoacidosis in patients with type 2 diabetes mellitus: A nationwide population-based cohort study. Diabetes Obes Metab 2018;20:1852–1858 [DOI] [PubMed] [Google Scholar]
- 30. Wang L, Voss EA, Weaver J, et al. Diabetic ketoacidosis in patients with type 2 diabetes treated with sodium glucose co-transporter 2 inhibitors versus other antihyperglycemic agents: an observational study of four US administrative claims databases. Pharmacoepidemiol Drug Saf 2019;28:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ 2018;363:k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]