Abstract
Most screening programs to identify individuals at risk for type 1 diabetes have targeted relatives of people living with the disease to improve yield and feasibility. However, ∼90% of those who develop type 1 diabetes do not have a family history. Recent successes in disease-modifying therapies to impact the course of early-stage disease have ignited the consideration of the need for and feasibility of population screening to identify those at increased risk. Existing population screening programs rely on genetic or autoantibody screening, and these have yielded significant information about disease progression and approaches for timing for screening in clinical practice. At the March 2021 Type 1 Diabetes TrialNet Steering Committee meeting, a session was held in which ongoing efforts for screening in the general population were discussed. This report reviews the background of these efforts and the details of those programs. Additionally, we present hurdles that need to be addressed for successful implementation of population screening and provide initial recommendations for individuals with positive screens so that standardized guidelines for monitoring and follow-up can be established.
Introduction
Combined with work by multiple groups over the past decades to identify those at high risk, the recent positive results of the phase 2 randomized controlled TrialNet TN10 Anti-CD3 (Teplizumab) Prevention Trial have opened opportunities for prevention of type 1 diabetes (1). The TN10 trial reported that a single 14-day course of teplizumab drug therapy delayed the clinical diagnosis of type 1 diabetes in 76 multiple islet autoantibody (AA)–positive relatives without diabetes by a median of 24 months and, in a subsequent analysis, up to 32.5 months (1,2). The relatively rapid time to clinical diabetes in the placebo group fulfilled the predictions from trial planning: a 75% risk of clinical diagnosis in 5 years in the AA-positive relatives with dysglycemia without diabetes and validated methods used in that trial to identify individuals at risk for disease. In addition to teplizumab, prevention trials with other therapies are underway (clinical trial reg. nos. NCT01773707 and NCT03428945, ClinicalTrials.gov).
Type 1 diabetes frequently presents with preventable life-threatening complications (diabetic ketoacidosis [DKA]), and the diagnosis of type 1 diabetes affects longevity, morbidity, and the quality of life for patients and their families (3–6). These and other data highlight an urgent unmet need to develop programs to identify those at risk, with or without a relative with type 1 diabetes, who may benefit from these treatments (7).
Relatives of patients with type 1 diabetes have an ∼15-fold increased risk of disease compared with those without a relative with type 1 diabetes (8–10). Siblings of patients have, on average, a 6–7% lifetime risk of type 1 diabetes, and offspring of mothers and fathers with type 1 diabetes have a 1.3–4% and 6–9% lifetime risk, respectively, compared with 0.4% in the general population (8–10). Because of the enriched risk in relatives, screening programs and clinical trials have often targeted this group.
However, ∼90% of those who will present with new type 1 diabetes do not have a positive family history (11,12). The treatment effects of teplizumab and other immune therapies after the diagnosis of type 1 diabetes in patients without affected family members illustrates the efficacy of these therapies in the general population. Therefore, to identify the most individuals who would benefit from therapies to prevent type 1 diabetes, those without a positive family history must be identified. Several groups have initiated screening of the general population, and there has been interest on the part of academics, advocacy organizations, policy groups, the pharmaceutical industry, and others in evaluating the optimal manner in which to proceed with this large endeavor. At the March 2021 TrialNet Steering Committee meeting, ongoing efforts for screening of the general population were reviewed. This report presents the background on these and other screening efforts, clinical recommendations, the details of selected programs, and challenges for implementation of population screening.
Progression of Type 1 Diabetes in Humans
Type 1 diabetes is caused by the destruction of insulin-producing β-cells by immune mechanisms, involving B, CD4+, and CD8+ T cells, with CD8+ T cells serving as the postulated effectors (11). Some immune cell targets have been identified, such as proinsulin and insulin, glutamic acid decarboxylase 65 (GAD65), islet antigen 2 (IA-2), islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP), zinc transporter 8 (ZnT8), and chromogranin A (13). In model systems and in vitro, T cells that are reactive with peptides from these antigens can elicit β-cell killing, yet a direct causal role for these cells remains to be defined.
Despite the primary role of T cells in β-cell killing, clues to the immune targets in type 1 diabetes originated by finding AAs that are reactive with these proteins in individuals with and prior to the diagnosis of clinical type 1 diabetes. The earliest observations of anti-islet cell antibodies (ICA), in 1974, entailed immunofluorescent detection of immunoglobulins that reacted with islets from the pancreas from a blood type group O donor. The specific molecular targets of AAs have been progressively discovered, with the first being insulin (14,15). Subsequently, other antigens, including GAD65, were recognized, and methods such as radioimmunoprecipitation were used to identify islet cell proteins recognized by antibodies (16–18). The methods to measure biochemically defined AAs to insulin (IAA), GAD65 (GAD antibody [GADA]), ZnT8 (ZnT8A), and a protein tyrosine phosphatase (ICA512A or IA2A) have previously been reviewed (19).
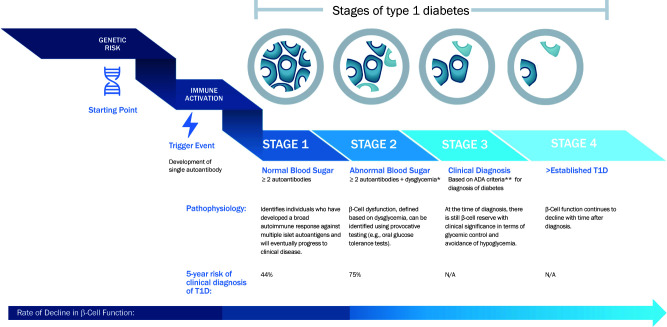
AAs can be found prior to clinical disease (20–24), indicating that there is an asymptomatic period before the typical presentation with clinical type 1 diabetes, which is associated with β-cell functional loss, hyperglycemia, and, often, ketoacidosis (25). The risk for progression to type 1 diabetes is built on the detection of AAs. Beginning with the appearance of two AAs, stages of type 1 diabetes are now defined and identify steps during the progression of disease (Fig. 1) (26). The notions of stages have been useful for identifying cohorts for clinical studies, but there are limitations to their application in the clinical practice setting. First, AAs identify risk but not the speed of progression to clinical type 1 diabetes. The rates of progression for each individual may vary considerably (20–22,27). Risk is modified by age at seroconversion (to AA positivity) and the number of the AAs present in an individual’s serum, although which AAs are found may differ by age. Younger individuals frequently have IAA initially, whereas in teenage years, GADA are frequently found. Second, the stages do not include direct measures of the immune process or β-cell decline (28,29). Finally, discrete stages may not be identified in all individuals. For example, some individuals, particularly children <5 years in stage 1, may progress to overt clinical disease without a period of dysglycemia (i.e., stage 2). This may reflect infrequent glucose monitoring or, alternatively, a more rapid progression than that for older individuals (30).
Figure 1.
Definitions of stages of type 1 diabetes (26,63). *Dysglycemia defined as fasting glucose level of 110–125 mg/dL, or 2-h postprandial plasma glucose of >140 and <200 mg/dL, or an intervening glucose value at 30, 60, or 90 min >200 mg/dL during an OGTT. An HbA1c of 5.7–6.4% or a 10% increase in HbA1c levels in those with multiple AAs has also been suggested as criteria for stage 2 (26). However, in general, increased HbA1c levels have variable performance as a predictive marker for type 1 diabetes (T1D). **Because some patients are actually asymptomatic at the time that they cross the threshold for glucose-based criteria for type 1 diabetes, some investigators have proposed 3a and 3b subtypes of stage 3 based on the presence of clinical symptoms, which may be useful in guiding degree of clinical intervention (i.e., insulin dosing). ADA, American Diabetes Association; N/A, not applicable.
At-risk individuals typically harbor a genetic predisposition to autoimmunity. The strongest genetic determinants of risk are the HLA genotypes, but other non-HLA susceptibility loci have also been identified. Genetic risk scores (GRS), incorporating multiple loci, have been developed and shown to predict islet autoimmunity (31). After development of islet autoimmunity, metabolic features, including BMI and more subtle analyses of β-cell function and insulin secretion, can inform risk and evolution of progression from early-stage disease (32). Other risk indices (e.g., Index 60 and Diabetes Prevention Trial—Type 1 Risk Score [DPTRS]) that incorporate these metabolic data can greatly enhance the prediction of progression from early-stage disease. Reviews that detail the pathophysiology of type 1 diabetes, including AA, genetics, and metabolic measures in type 1 diabetes prediction, are available (19,31–33).
Technological Performance and Improvements in AA Measurements
Most contemporary studies of type 1 diabetes progression have used radiobinding assays (RBA), but newer methods and assays may improve prediction. These are reviewed in So et al. (19) and are summarized in Table 1. In addition to new AA targets, new technologies have improved specificity and sensitivity and may be multiplexed, minimizing the blood volumes needed and enhancing the throughput and accessibility of tests. Some newer assays selectively measure AAs with high binding affinities or truncated peptides (e.g., GAD96-585) and have shown improved assay specificity and type 1 diabetes prediction (19,34,35). The validation of these methods has been supported by the Islet Autoantibody Standardization Program (IASP) workshop, which compares assay performance across different methods (19). The results from this program indicate that the assays are sensitive and sufficiently specific to distinguish patients with type 1 diabetes from control subjects without diabetes, but the program was not designed to evaluate specificity at the level required for population-based screening. In an ongoing comparator study, TrialNet will evaluate the prediction of type 1 diabetes within 5 years with these new assays. Minimization of false-positive rates in individuals without diabetes is a particularly important consideration to minimize risks of unnecessary testing and anxiety in the context of broader screening.
Table 1.
AA methods
| Method | Description |
|---|---|
| Radiobinding assay (RBA) | Radiolabeled antigens detected in antibody-antigen complexes |
| Electrochemiluminescence (ECL) | Biotin and sulfo-TAG–labeled ligands that emit light when activated |
| ELISA | Detection of antigen–antibody complexes by enzyme-linked reagents |
| Luciferase immunoprecipitation (LIPS) | Quantitates serum antibodies by measuring luminescence emitted by the reporter enzyme luciferase fused to an antigen of interest |
| Agglutination PCR (ADAP) | PCR amplification of DNA in DNA–antigen conjugates bound to antibodies to form aggregates |
These methods are reviewed in So et al. (19).
Ongoing Screening Programs
Screening in Relatives of Individuals With Type 1 Diabetes
Both TrialNet (a U.S.-based consortium) and INNODIA (a European private/public partnership) began by screening relatives to maximize efficiency for enrollment in clinical studies (Table 2A). However, both have begun to include monitoring or screening of at-risk individuals from the general population. The Type 1 Diabetes TrialNet Pathway to Prevention Study, initiated in 2004, has screened over 220,000 relatives. Initially, assays for ICA and IAA, IA2A, and GADA (by RBA) were performed. In 2019, after an internal review of data from this study to improve cost and efficiency, screening was changed to GADA and IAA by online consent and optional at-home test kits. Those individuals who tested positive for either AA then underwent testing for ZnT8A, IA2A, and ICA. Overall, TrialNet identified ∼5% of relatives without diabetes to have at least one AA, and about half of these had multiple AAs (i.e., stage 1 or stage 2). INNODIA, a European private/public partnership, screens for four AAs by RBA and has screened more than 4,400 first-degree relatives. Consistent with the TrialNet data, the most frequently found AAs are GADA and IAA, with 2.6% of the individuals tested having multiple AAs.
Table 2.
Ongoing screening programs
| A: Selected type 1 diabetes screening programs using screening of relatives for eligibility to participate in clinical studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Program | Population screened | Location | Screening site(s) | Number screened | Screening material | Screening assay(s) | Rate(s) of positive scree(s) | Comment(s) |
| TrialNet Pathway to Prevention (TN01) | Relatives aged 3–45 years | U.S., Canada, Europe, Australia | TrialNet centers and affiliates | >250,000 | Serum or capillary sample | RBA: IAA and GADA, followed by IA-2A, ZnT8A, and ICA if positive | AA+: 5% • ≥2 AA+: 2.5% | Objective is to identify participants eligible for clinical trials |
| Monitors nonrelatives identified through other programs | ||||||||
| INNODIA | Relatives and general population | Europe | Academic sites | >4,400 | Serum | RBA | AA+: 379 | Of AA+ |
| • 1 AA+: 6.0% | • IAA: 184 (49.9%) | |||||||
| • >2 AA+: 1.0% | • GADA: 242 (65.2%) | |||||||
| • 3 AA+: 0.9% | • IA-2A: 81 (21.8%) | |||||||
| • 4 AA+: 0.8% | • ZnT8A: 94 (25.1%) | |||||||
| • ≥2 AA+: 2.6% | ||||||||
| Bart’s Oxford (BOX) Family Study | Relatives | U.K. | Diabetes clinics/ at home | 6,000 | Capillary blood since 2015 | RBA: IAA, GADA, IA2A, ZnT8A | 470 AA+: • 1 AA+: 6% • ≥2 AA+: 2% | Family members are recruited at diagnosis of a proband (<21 years old) in the study area |
| Type1Screen | Relatives aged 2–30 years | Australia and New Zealand | Community collection centers and in-home collection | >700 | Capillary or venous blood | IAA: RBA or ADAP; GADA, IA-2A, ZNT8A, ELISA, or ADAP | AA+: 34 (5%) • 1 AA+: 13 (1.9%) • ≥2 AA+: 21 (3.9%) | Family members recruited by health professionals, emails, and social media |
| Of AA+: | ||||||||
| • IAA 3 (9%) | ||||||||
| • GADA 25 (74%) | ||||||||
| • IA-2A 18 (53%) | ||||||||
| • ZNT8A 22 (65%) | ||||||||
| B: General population screening programs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Program | Population screened | Location | Screening site(s) | Number screened | Screening material | Screening assay(s) | Rate(s) of positive screens | Comment(s) |
| Genetic prescreening with follow-up for AA | ||||||||
| DIPP | Age 0.25–15 years with high- risk HLA genotypes | Finland | Three university hospitals | >250,000 | Serum | HLA genotyping followed by RBA: IAA, GADA, IA-2A, ZnT8A | ∼10% of screens with high-risk HLA ≥2 AA+: • by 2 years: 2.2% • by 5 years: 3.5% • by 15 years: 5.0% | All newborns with parental consent (∼25% of birth cohort) receive cord blood HLA screening; guardians of ∼19,000 at-risk infants have agreed to follow-up AA screening at 3- to 12- month intervals up to age 15 years |
| BABY- SCREEN | Newborns to 3 years with high- risk HLA for type 1 diabetes and/or celiac disease | Helsinki, Finland | University hospital | Target for HLA screening: 30,000; >9,000 tested | Serum | HLA genotyping followed by RBA: IAA, GADA, IA-2A, ZnT8A, tTGA | By 1 year: • 1 AA+: 5.3% • ≥2 AA+: 1.8% By 2 years: • 1 AA+: 6.5% • ≥2 AA+: 3.7% | HLA screening from cord blood followed by AA screening at age 1, 2, and 3 years Type 1 diabetes in first- degree relative in 3.1% |
| GPPAD | Infants <1 month of age | Germany, U.K., Poland, Belgium, and Sweden | Around delivery or PCP visits | >275,000 (1.72% first-degree relatives) | Capillary blood spots | 47-SNP GRS to identify those with >10% risk of ≥2 AA+ by age 6 years | 1.1% with increased genetic risk | Guardians of at-risk infants are offered participation in a primary prevention trial |
| PLEDGE | Age <6 years | North and South Dakota and Minnesota, U.S. | Integrated health system clinics and laboratories | Target = 33,000 | Capillary blood spot for GRS, serum for AA | GRS, RBA | N/A | GRS with newborn screen or study entry; AA testing at ∼2 and 5 years Uses EHR for tracking/communication |
| CASCADE | Age ≥1 year | Northwest U.S. | Newborn screens and elementary schools | Target = 60,000 | Serum | GRS, RBA: GADA, IAA, ZnT8A, tTGA; LIPS for IA2A | N/A | Initial GRS screen, at-risk infants followed for type 1 diabetes and celiac disease |
| PRiMeD | Age 2–16 years | Virginia, U.S. | Pediatric clinics | 3,477 | Saliva for GRS, serum for AA | 82-SNP GRS, RBA: IAA, GADA, IA-2A, ZnT8A | 461 (1.3%) with high GRS (10× over expected) AA testing in progress | AA screening offered to those with high GRS, ≥2 AA+ invited to contact TrialNet or obtain CGM locally |
| Screening for AA | ||||||||
| Fr1da | Age 1.75–10.99 years | Bavaria, then Lower Saxony, Hamburg, Saxony, Germany | PCP clinics | >150,000 | Capillary blood | ELISA: GADA, IA2A, ZnT8A/ LIPS: IAA; confirm with RBA: IAA, GADA, IA-2A, ZnT8A | ≥2 AA+: 0.3% | Positive screens invited for metabolic staging by OGTT; >80% of these with stage 1 |
| Fr1dolin | Age 2–6 years | Lower Saxony and Hamburg, Germany | PCP clinics | >15,000 | Capillary blood | ELISA: GADA, IA-2A, ZnT8A; confirm with RBA: IAA, GADA, IA2A, ZnT8A | ≥2 AA+: 0.35% | Combined screening for type 1 diabetes risk and familial hypercholesterolemia |
| Positive screens invited for staging with OGTT | ||||||||
| T1Detect (JDRF) | Age ≥1 year | Most U.S. states | At home | Up to 2,000/ month | Capillary blood spot | ADAP: GADA, IA-2A, IAA | Nonrelatives • 1 AA+: 12% • ≥2 AA+: 5.4% Relatives • 1 AA+: 12% • ≥2 AA+: 5.7% | Direct access to participants through the JDRF website Of the first 800 tests, 203 (25.4%) were from the general population |
| ASK | Age 1–17 years | Colorado, U.S. | PCP and hospital specialty clinics, emergency departments | 25,738 | Serum | RBA with ECL confirmation: IA-2A, GADA, IAA, ZnT8A, tTGA | AA+: 3.4% • ≥2 AA+: 0.52% • Single high-affinity AA+: 0.58% | Screening for type 1 diabetes, celiac disease, and SARS-CoV-2 Ab 4.84% with first-degree relative with type 1 diabetes |
| Screening programs in development | ||||||||
| T1Early | Preschool age: 3.5–4 years | U.K. | Preschool vaccination PCP visit | N/A | Capillary blood | LIPS: GADA, IA-2A, ZnT8A | N/A | Positive screens using the LIPS assay will undergo metabolic staging |
| ADIR | Age 9–18 months and 5 years | Israel | PCP visit with hemoglobin screening | Target of up to 50,000 | Capillary or venous blood | ADAP: GADA, IA-2A, IAA | N/A | Due to start October 2021 |
| JDRF Australia General Population Screening Pilot | Newborns, infants, and 2–6 years | Australia | Maternity hospitals, general population | Target of 3,000 in each cohort | Capillary blood and saliva | GRS, ADAP for IAA,GADA, IA-2A, ZNT8A | N/A | Starting in 2022; will compare GRS approach to cross-sectional AA screening in older children |
ECL, enhanced chemiluminescence; EHR, electronic health record; LIPS, luciferase immunoprecipitation; N/A, not applicable.
Screening in the General Population
In total, the number of individuals without a relative with type 1 diabetes who have been screened is greater than the number of relatives. Table 2B summarizes data from selected programs ongoing and under development for the general population. Supplementary Table 1 describes completed programs. These generally fall into the categories of birth cohorts or AA-based screening programs. Some differences in positive screen rates between programs exist; these are likely multifactorial and related to background prevalence, overall screening strategy, inclusion of individuals with relatives with type 1 diabetes, and the assays used.
Birth Cohorts
Birth cohorts use a combined approach to initially identify individuals at increased genetic risk for type 1 diabetes. Genetic screening can enrich for individuals who are appropriate for targeted AA screening. Using screening for HLA, the TEDDY (The Environmental Determinants of Diabetes in the Young) study is gathering data from >8,000 HLA genetically at risk newborns, most (∼90%) without a known relative with type 1 diabetes (22) (Supplementary Table 1). These newborns are followed for 15 years for the appearance of AAs and diabetes, with documentation of environmental factors that could contribute to disease. The Type 1 Diabetes Prediction and Prevention Study (DIPP) has been active in three Finnish university hospitals since 1994, screening >250,000 infants (36). All newborn infants from these hospitals (∼25% of the national birth cohort) are screened for HLA-conferred susceptibility to type 1 diabetes, with parental consent, using cord blood. Almost 10% of those screened carry such HLA genotypes and are invited for follow-up until 15 years of age or type 1 diabetes diagnosis. The Newborn Screening for Genetic Susceptibility to Type 1 Diabetes and Celiac Disease and Prospective Follow-up Study (BABYSCREEN), initiated in 2018 in Helsinki, Finland, screens cord blood cells for HLA alleles conferring high risk for type 1 diabetes and celiac disease. Participants carrying increased risk for either disease are invited to AA testing at 1, 2, and 3 years of age. Of the 9,000 children screened, 6.0% were considered at high genetic risk for type 1 diabetes, 15.0% at high genetic risk for celiac disease, and 4.1% at high genetic risk for both diseases. The Global Platform for the Prevention of Autoimmune Diabetes (GPPAD) tests newborn blood spots collected from cord blood or at primary care provider (PCP) visits and calculates GRS to identify those at ≥10% risk for multiple AAs by 6 years of age. Those at increased genetic risk are offered the opportunity to enroll in a primary prevention study (37). Over 279,000 infants have been screened as of July 2021, with a positive screen rate of 1.1% with increased genetic risk.
Three recently initiated programs in the U.S., the Combined Antibody Screening for Celiac and Diabetes Evaluation (CASCADE) program, the Sanford Population Level Estimation of T1D Risk GEnes in Children (PLEDGE) project, and the Precision Individualized Medicine for Diabetes (PRiMeD) project, also use GRS (38) from dried blood spots or saliva. Those with “positive” GRS screens are offered AA screening (39). In follow-up of the newborn and study entry samples for GRS testing, the PLEDGE study performs AA testing at 2 years and prekindergarten visits, with an emphasis on integrating study processes into routine pediatric care and integration with the electronic health record system. Children with positive AAs are offered ongoing monitoring according to principles described in Table 3 or offered the opportunity to participate in a TrialNet clinical trial for at-risk individuals (www.trialnet.org).
Table 3.
Recommendations for practice
| 1. When asked about screening for type 1 diabetes risk: |
| a. Available screening tools: genetic, AAs, glucose levels, symptoms. |
| b. The overall risk for development of type 1 diabetes is greater for those with a relative with type 1 diabetes than for those without such relatives because of shared genetic and environmental factors. However, the risk for type 1 diabetes in children who have ≥2 AAs is the same whether or not they have an affected relative. |
| c. Screening initiatives are available in North America, Europe, Australia, and New Zealand (Table 2). |
| d. There are risks and benefits of screening. The former may involve anxiety about the findings, but the latter may include reassurance of a negative test, avoidance of DKA at diagnosis, and access to clinical studies and therapies to delay or prevent type 1 diabetes. |
| 2. Information, with the assurance of privacy, testing for antibodies, and ongoing monitoring or enrollment in trials is available (e.g., through the National Institutes of Health–funded research network TrialNet or through the Innovative Medicines Initiative–funded research network INNODIA and other programs in Europe) |
| a. For relatives, TrialNet, INNODIA, and Type1Screen provide free, confidential AA testing and ongoing monitoring for relatives who are AA positive. |
| b. For nonrelatives, see regional initiatives (Table 2). If testing shows that they have one or more AA, the test should be confirmed. TrialNet/INNODIA/Type1Screen will provide confirmation of positive AA tests conducted outside a research study. AA-positive individuals can be referred to TrialNet/INNODIA/Type1Screen for a confirmation test whether or not they have a relative with diabetes. |
| 3. The optimal time for cross-sectional screening is ages 2 and 5–7 years, but screening school-age children, particularly at the time of other laboratory tests, may be the most practical. |
| 4. Follow-up of positive tests is needed to reduce rates of DKA and avoid the unexpected diagnosis of type 1 diabetes. Follow-up may include the following: |
| a. Discussing the results and the implications. |
| b. Explaining signs and symptoms of diabetes. |
| c. Standards for metabolic follow-up have not been established but may involve HbA1c levels, random glucose levels, OGTTs, or continuous glucose monitoring. |
| d. Discussing clinical studies available through TrialNet and INNODIA. |
Screening After the Neonatal Period
Several programs use AAs for primary screening in children after the neonatal period, including ASK (Autoimmunity Screening for Kids, Colorado), T1Detect (U.S.), and Early Detection of Type 1 Diabetes (Fr1da) and Early Detection of Type 1 diabetes and Hypercholesterolemia in Lower Saxony (Fr1dolin) (Germany) (Table 2B) (40–44). Relatives are not excluded from participating in these programs. AA screening alone is more costly when conducted without genetic prescreening, but it is specific for stage 1 or stage 2 disease. Multiple methods for AA detection have been used (40–44). Unique approaches to optimize enrollment and follow-up have been used (40–44).
The goals of the U.S.-based ASK program, available to residents of Colorado aged 1–17 years, are early diagnosis, DKA prevention, prevention study enrollment, and referral. Diabetes AA testing is combined with screening for celiac disease by measuring tissue transglutaminase antibodies (tTGA) and, more recently, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies. Children who have a positive test are invited for confirmatory testing. Of 25,738 participants, 3.4% were positive for any AA on the initial screening, 0.52% were positive for multiple islet AA, and 0.58% were positive for a single high-affinity AA (M.R., personal communication, 2021).
The T1Detect program was initiated by the JDRF in 2020 (42). T1Detect provides online links for individuals ≥1 years of age to a commercial laboratory (Enable Biosciences). That laboratory uses an online portal to provide screening at home with a blood spot testing approach. Participants receive test kits for collection of dried blood spots that are mailed for measurement of GADA, IA2A, and IAA using the antibody detection by agglutination-PCR (ADAP) assay (19). Participants screening AA positive are contacted by the laboratory and offered one-on-one and/or online support. Of the 800 initial screens (of which 74% are first-degree relatives of individuals with type 1 diabetes), 12.0% are positive for 1 AA, 4.0% for 2 AAs, and 1.63% for 3 AAs.
Some programs have successfully established partnerships with community PCPs. The Fr1da program, initiated in 2015, screens for AAs in children 1.75–10.99 years of age in Bavaria at well-child visits and, more recently, was extended to Saxony and northern Germany and to include screening for SARS-CoV-2 antibodies (45,46). Consistent with the predicted frequency, 0.31% of the 90,632 children screened were positive for ≥2 AAs (43). Of the 196 participants found to have stage 1 disease, 28.7% developed stage 2 or 3 type 1 diabetes in 3 years of follow-up. Through this program, factors were identified in this screening program that predicted progression from stage 1 to stage 2 or type 1 diabetes, including obesity, IA2A positivity, and HbA1c >5.7%, and from 60-min oral glucose tolerance test (OGTT) glucose levels in the highest tertile. The type 1 diabetes GRS was predictive of AAs but not predictive of progression of stages (43).
Other programs are in development (Table 2B). The Australian General Population Screening Pilot, set to launch in 2022, will compare uptake, feasibility, and cost of screening children using three different strategies: genetic testing at birth, genetic testing in infancy, and AA testing of participants between 2 and 6 years of age. Recruitment will be through dedicated maternity hospitals and by direct mail-out to defined regions. In the T1Early program under development in the U.K., AAs will be measured in capillary blood at a preschool vaccination visit (between 3.5 and 4 years of age) by PCPs. The Screening for Islet Autoantibodies in the Israeli Pediatric General Population for Detection of Presymptomatic Type 1 Diabetes (ADIR) program starting in 2021 in Israel will coordinate capillary blood AA screening with scheduled PCP hemoglobin screening.
Considerations for Clinical Practice
Benefits and Risks of Screening for Early-Stage Type 1 Diabetes
The early identification, monitoring, and regular follow-up of high-risk individuals can reduce DKA rates at the time of diagnosis of stage 3 type 1 diabetes (Table 3). DKA rates fall from 25–62% to 4–6% with monitoring, with potential longer-term impacts to reduce HbA1c levels and risk of complications (40,41,47).
Some studies have described a risk of negative psychological impact on those who screen AA positive, but this stress appears to wane over time. Post-diagnosis adjustment for subjects diagnosed through screening and monitoring compares favorably to those diagnosed with clinical symptoms (43,48,49). In addition, screening enables access to medical expertise to discuss results and provide ongoing education and monitoring. Importantly, the majority (∼95%) of relatives of individuals with type 1 diabetes are AA negative at screening, which can be reassuring, particularly for families with an affected family member.
Perception of benefit is an important consideration for program success. One study from the U.S. suggested that both parents and pediatricians valued screening programs associated with monitoring that minimize the risk of DKA and enable treatment options or access to clinical studies to delay the onset of clinical type 1 diabetes (50). Thus, studies should be highlighted as part of outreach.
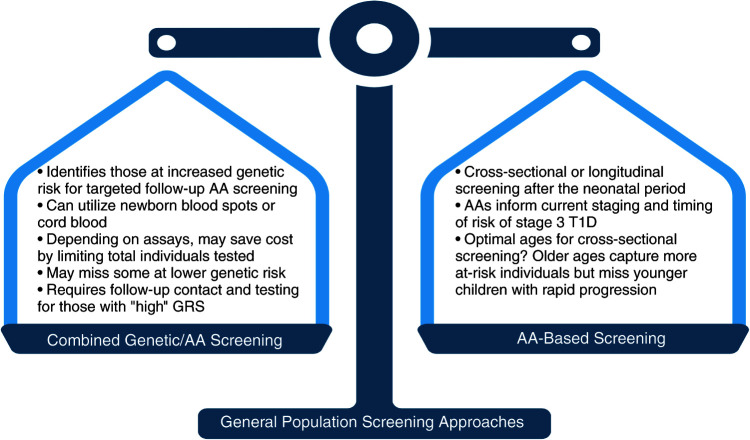
What Is the Optimal Timing and Approach to Screening for Type 1 Diabetes?
Genetics-based testing versus AA-based screening has the benefit of enriching for individuals who are most likely to have AAs (Fig. 2). By selecting these individuals for AA screening, costs may be reduced, yet the potential losses to follow-up and serologic testing and costs of recontact need to be considered. Analysis of birth cohorts have shown that peak rates of AA seroconversion occur around 1.5 years in those who progress to clinical type 1 diabetes, and most individuals seroconvert by 2–3 years of age (51,52). Thus, if a single AA test is performed, testing at ages 3–4 should maximize case capture. However, early-onset type 1 diabetes, where severe DKA rates are highest, as well as older adolescent and adult seroconverters would be missed. If two tests can be done, straddling the 3- to 4-year age-group (i.e., at 2 and 5–7 years of age) has been suggested (52,53). Most genetically high-risk young children who convert from single to multiple AA positivity do so within 2 years after initial seroconversion, suggesting that a single AA-positive individual should be rescreened after this interval (52). For practical considerations, timing AA screening with primary care visits may expand participation. The optimal strategies for identification of at-risk adults need to be studied. The ASK and T1Detect programs have taken a broader approach to screening older individuals and will capture the smaller proportion of individuals that become AA positive after early childhood, but they may miss children who progress to stage 3 disease at an early age. Involvement of pediatric and adult PCPs might not only improve initial community engagement but also facilitate follow-up, monitoring, and ultimate care coordination for those that screen positive. Pediatric testing may coincide with other laboratory screenings performed routinely, such as those for anemia, lead, or lipid levels, but because children, in general, infrequently undergo routine laboratory testing in general care, capillary blood testing with multiplexed or dried blood spot testing can facilitate screening with referral to a diabetes center if the test results are positive (43,54).
Figure 2.
Considerations for approaches to general population screening: combined genetic/AA-based screening versus an AA-based approach. T1D, type 1 diabetes.
Which Tests Should Be Used?
AA screening tests need to be standardized, since sensitivities, thresholds for positive tests, and other characteristics may differ between assays. The RBA was used in the successful prevention trial (TN10). Many programs use assays that have been validated in the IASP, but only one assay system (Kronus) is approved by the U.S. Food and Drug Administration as a diagnostic, and this assay has not been tested for identifying risk for type 1 diabetes. Home testing with dried blood spots or capillary microsamples rather than serum-based assays may enable a much broader outreach and acceptance for patients, but like other assays, validation with the RBA and their ability to predict stage 3 disease need to be confirmed.
What Is the Optimal Follow-Up for Positive Screens?
As noted, the biomarkers of risk do not give information about the rate of progression to type 1 diabetes. Importantly, prevention of DKA and enrollment in clinical trials are not achieved with screening alone; follow-up is needed and requires input from health care professionals familiar with the significance of laboratory findings and the clinical disease (55,56). Some programs use monitoring of HbA1c, random glucose levels, or OGTTs for those at high risk. Home glucose meters and continuous glucose monitoring (CGM) have also been suggested as options (57), and CGM has been tested in TrialNet (M. Pietropaolo, personal communication). INNODIA is testing whether repeated home measurements of C-peptide, using dried blood spots, is useful for assessing β-cell loss.
Optimal methods or frequencies for monitoring have not been established. Furthermore, communication of risk associated with positive screens and treatment options is complicated even among those with a family history and baseline knowledge of type 1 diabetes (48). Understanding optimal communication about risk and treatment of early-stage type 1 diabetes is essential. Finally, referral to clinical trials through networks such as INNODIA or TrialNet should be considered. In these consortia, patients will have access to the most advanced and potentially beneficial options for delay or prevention.
How Can the General Public Be Made Aware of These Opportunities?
Currently, general population screening requires the participation of PCPs. Screening in the Fr1da, PLEDGE, PRiMeD, and T1Early programs are performed in primary care clinics. In the U.K., the T1Early program is using a creative design agency to inform communication with the general public and engage leading pediatric diabetologists and the National Children’s and Young People’s Network to raise awareness about preclinical diabetes, aid recruitment, and embed screening within the U.K. health system.
Outreach to Minority Communities Is an Unmet Need
The rates of type 1 diabetes among minority ethnic/racial group members are significant and, in total, are comparable to the frequency among non-Hispanic White (NHW) individuals: NHW 2.55/1,000, non-Hispanic Black (NHB) 1.63/1,000, Hispanic 1.29/1,000, and non-Hispanic Asian 0.6/1,000 (58). Recent analyses in the U.S. have suggested that type 1 diabetes incidence is increasing most rapidly among minority groups (increases of incidence of 4.0% per year in Hispanics, 2.7% per year in NHB, 4.4% per year in Asian/Pacific Islanders, and 0.7% per year in NHW) (59). There is a higher frequency of DKA at diagnosis among these populations (41) who would, therefore, benefit from early detection and monitoring. However, groups of non-European ancestry are underrepresented in type 1 diabetes research (60). Of the 226,553 initial screens in the TrialNet Pathway to Prevention Study, only 3.75% and 13.58% are African American and Hispanic, respectively. In the T1Detect program, 5.5% (of 800) are Hispanic and 1.4% are African American. More success has been seen in the ASK program, in which more than half are from minority groups (35% are NHW, 51% Hispanic, and 8% African American). Obstacles such as engagement with PCPs and specialists in underserved neighborhoods and out-of-pocket costs remain hurdles that need to be addressed so that all who can benefit have access.
Does Screening Have Economic Benefits?
Screening costs vary by the types of assays and the expenditures needed to identify participants. Clinical charges for AA tests can range from $131 (ICA) to $528 (ZnT8A) (61), but multiplexing and selective AA measurements (e.g., GADA and IAA) can reduce these costs. The current cost for AA screening in the ASK study is $47 and in the JDRF T1Detect program is $55. Based on the frequencies of positive screenings in the ASK program, the cost of AA screening per case of type 1 diabetes detected before diagnosis is $4,700 (62).
A major goal of general population screening, through attentive follow-up of individuals who test positive, is to reduce the rate of life-threatening DKA at the time of diagnosis, a complication that is associated with long-term sequelae and outcomes (40,41,47). It is estimated that screening and follow-up would be cost-effective even if it would reduce the rate of DKA by 20%, which would also lower HbA1c by 0.1% over a lifetime (62). An approved treatment to delay type 1 diabetes would eliminate the cost of insulin, supplies for administration, and glucose monitoring. In addition to impacts on patient outcomes, a clear understanding of cost savings of successful screening programs will be important to achieve buy-in and coverage from medical payers. Further analyses testing cost-effectiveness at multiple levels will be key for payer engagement and long-term integration into health systems.
Summary and Conclusions
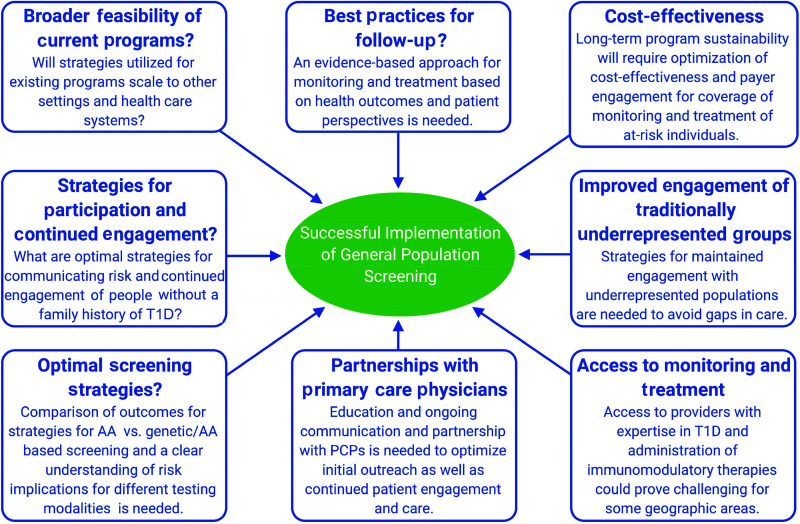
Criteria have been proposed to be applied for the justification of population screening (Table 4) (64), and the programs listed in Table 2 are working toward fulfilling these criteria. It is now possible to identify the majority of children and adults who will develop type 1 diabetes and to take action to delay or prevent the disease prior to needing insulin. Recently, a report from the Milken Institute identified hurdles and suggested changes needed in U.S. health care policy, recommendations for screening, and a unified framework for policy implementation (https://milkeninstitute.org/reports/diabetes-pediatric-autoantibody-screening). Clearly, a number of logistical uncertainties remain before screening and monitoring can be applied as part of clinical care (Fig. 3) (7). Understanding the implications of positive screens from different testing methods on ultimate risk of clinical progression will be important to guide these protocols. Education and partnership with community PCPs will be essential for continued engagement and monitoring of at-risk individuals.
Table 4.
Wilson and Jungner’s guidelines for screening as applied to type 1 diabetes
| Principle | Application to screening for type 1 diabetes |
|---|---|
| 1. Identify an important health problem | Type 1 diabetes is one of the most common and consequential chronic illnesses of children but also affects individuals of all ages. |
| 2. There should be an accepted treatment for the condition | Teplizumab was shown to delay the diagnosis of individuals at risk. Other agents are under evaluation. |
| 3. Facilities for diagnosis and treatment are available | Diagnosis and treatment can be done in medical offices. |
| 4. There should be a recognizable latent or early symptomatic period | Stages of progression of type 1 diabetes in those at genetic risk have been defined. High-risk individuals (stage 2) have a 75% risk of diagnosis within 5 years. |
| 5. There should be a suitable test or examination | AAs can define risk. Newer technologies to improve prediction are under study. AAs can be measured in many laboratories. |
| 6. The test should be acceptable to the population | |
| 7. The natural history of the condition should be understood | Although many specifics remain uncertain, results from immune therapy trials indicate that type 1 diabetes is due to immune-mediated killing of β-cells. |
| 8. There should be an agreed policy on whom to treat as patients | Children and adolescents, during developmental years, have the highest unmet need. |
| 9. The cost of case finding should be economically balanced in relation to expenditure on medical care as a whole | The lifetime costs for type 1 diabetes after onset in childhood are great, even without the additional costs associated with disease-related complications. |
| 10. Case finding should be a continuing process | Projects across the globe are piloting strategies for case identification. |
Guidelines are as described by Wilson and Jungner (64).
Figure 3.
Logistical needs and uncertainties that remain to be answered for optimal implementation and sustainability of large-scale general population screening for type 1 diabetes (T1D).
The value of the prevention or even delay of the diagnosis of type 1 diabetes to the lives of families and those who would have otherwise been diagnosed with type 1 diabetes, as well as to their development, emotional, physical, and mental health, should not be underestimated. The ability to intervene in the disease course during a presymptomatic phase is a key tenant of population screening, but likewise, identifying effective therapies and applying them in clinical settings depends on identifying those at risk who are most likely to benefit from them. Collaborations between groups involved in screening and therapeutics will be needed to fulfill this objective.
In conclusion, screening for type 1 diabetes for purposes of delay or prevention of clinical disease has entered a new phase. With the availability of new therapies that can delay or prevent type 1 diabetes, the opportunity for dramatically changing the future of this disease is enormous. Attention to hurdles discussed in Figs. 3 and 4 and the Milken Institute Report should be considered a high priority for stakeholders in our field, taking advantage of knowledge gained from current successful efforts so that thoughtful coordinated larger-scale approaches can be implemented and interventions provided to all who stand to benefit.
Article Information
Acknowledgments. S.S.R. acknowledges the assistance of Kristin A. Guertin, Department of Public Health Sciences, and David R. Repaske and Julia F. Taylor, Department of Pediatrics, University of Virginia.
Funding. This work was funded in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (U01DK106993). The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded through a cooperative agreement by the National Institutes of Health through the NIDDK, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and JDRF.
Duality of Interest. E.K.S. and M.R. received compensation from Medscape for a continuing medical education event focused on general population screening. M.R. has consulted for Provention Bio and Janssen Research & Development. R.E.J.B. received speaking honoraria from Springer Healthcare and Eli Lilly and reports sitting on the Novo Nordisk UK Foundation Research Selection Committee on a voluntary basis. C.D. has served on advisory boards for Provention Bio, Quell Therapeutics, and Viela Bio. K.C.H. has consulted for Provention Bio, Viela Bio, and Merck; is on the scientific advisory board for Nextimmune; is named as a co-inventor on a patent application to use teplizumab for delay of type 1 diabetes; and was the principle investigator on a trial of AG019 (Precigen) in the U.S. K.C.H. is also a co-inventor on a patent for delay or prevention of T1D with teplizumab but has assigned all rights. No other potential conflicts of interest relevant to this article were reported.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.18018407.
A complete list of members of NIDDK Type 1 Diabetes TrialNet Study Group can be found in the supplementary material.
References
- 1. Herold KC, Bundy BN, Long SA, et al.; Type 1 Diabetes TrialNet Study Group . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sims EK, Bundy BN, Stier K, et al.; Type 1 Diabetes TrialNet Study Group . Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci Transl Med 2021;13:eabc8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rawshani A, Sattar N, Franzén S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet 2018;392:477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Livingstone SJ, Levin D, Looker HC, et al.; Scottish Diabetes Research Network epidemiology group; Scottish Renal Registry . Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA 2015;313:37–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLoS One 2010;5:e11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenbaum CJ. A key to T1D prevention: screening and monitoring relatives as part of clinical care. Diabetes 2021;70:1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Battaglia M, Anderson MS, Buckner JH, et al. Understanding and preventing type 1 diabetes through the unique working model of TrialNet. Diabetologia 2017;60:2139–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mathieu C, Lahesmaa R, Bonifacio E, Achenbach P, Tree T. Immunological biomarkers for the development and progression of type 1 diabetes. Diabetologia 2018;61:2252–2258 [DOI] [PubMed] [Google Scholar]
- 10. Redondo MJ, Steck AK, Pugliese A. Genetics of type 1 diabetes. Pediatr Diabetes 2018;19:346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet 2018;391:2449–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karges B, Prinz N, Placzek K, et al. A comparison of familial and sporadic type 1 diabetes among young patients. Diabetes Care 2021;44:1116–1124 [DOI] [PubMed] [Google Scholar]
- 13. Purcell AW, Sechi S, DiLorenzo TP. The evolving landscape of autoantigen discovery and characterization in type 1 diabetes. Diabetes 2019;68:879–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 1974;2:1279–1283 [DOI] [PubMed] [Google Scholar]
- 15. Palmer JP, Asplin CM, Clemons P, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 1983;222:1337–1339 [DOI] [PubMed] [Google Scholar]
- 16. Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 1990;347:151–156 [DOI] [PubMed] [Google Scholar]
- 17. Arvan P, Pietropaolo M, Ostrov D, Rhodes CJ. Islet autoantigens: structure, function, localization, and regulation. Cold Spring Harb Perspect Med 2012;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hagopian WA, Karlsen AE, Gottsäter A, et al. Quantitative assay using recombinant human islet glutamic acid decarboxylase (GAD65) shows that 64K autoantibody positivity at onset predicts diabetes type. J Clin Invest 1993;91:368–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. So M, Speake C, Steck AK, et al. Advances in type 1 diabetes prediction using islet autoantibodies: beyond a simple count. Endocr Rev 2021;42:584–604 [DOI] [PubMed] [Google Scholar]
- 20. Kulmala P, Savola K, Petersen JS, et al.; The Childhood Diabetes in Finland Study Group . Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes. A population-based study. J Clin Invest 1998;101:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steck AK, Vehik K, Bonifacio E, et al.; TEDDY Study Group . Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015;38:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Colman PG, McNair P, Margetts H, et al. The Melbourne Pre-Diabetes Study: prediction of type 1 diabetes mellitus using antibody and metabolic testing. Med J Aust 1998;169:81–84 [DOI] [PubMed] [Google Scholar]
- 24. Verge CF, Gianani R, Kawasaki E, et al. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 1996;45:926–933 [DOI] [PubMed] [Google Scholar]
- 25. Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 1986;314:1360–1368 [DOI] [PubMed] [Google Scholar]
- 26. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Orban T, Sosenko JM, Cuthbertson D, et al.; Diabetes Prevention Trial-Type 1 Study Group . Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial–Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sosenko JM, Skyler JS, Herold KC; Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups . The metabolic progression to type 1 diabetes as indicated by serial oral glucose tolerance testing in the Diabetes Prevention Trial–Type 1. Diabetes 2012;61:1331–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS; DPT-1 Study Group . Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes 2010;59:679–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pöllänen PM, Lempainen J, Laine AP, et al. Characterisation of rapid progressors to type 1 diabetes among children with HLA-conferred disease susceptibility. Diabetologia 2017;60:1284–1293 [DOI] [PubMed] [Google Scholar]
- 31. Oram RA, Redondo MJ. New insights on the genetics of type 1 diabetes. Curr Opin Endocrinol Diabetes Obes 2019;26:181–187 [DOI] [PubMed] [Google Scholar]
- 32. Sims EK, DiMeglio LA. Cause or effect? A review of clinical data demonstrating beta cell dysfunction prior to the clinical onset of type 1 diabetes. Mol Metab 2019;27S:S129–S138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bluestone JA, Buckner JH, Herold KC. Immunotherapy: building a bridge to a cure for type 1 diabetes. Science 2021;373:510–516 [DOI] [PubMed] [Google Scholar]
- 34. Gu Y, Zhao Z, Waugh K, et al. High-throughput multiplexed autoantibody detection to screen type 1 diabetes and multiple autoimmune diseases simultaneously. EBioMedicine 2019;47:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams AJ, Lampasona V, Wyatt R, et al. Reactivity to N-terminally truncated GAD65(96-585) identifies GAD autoantibodies that are more closely associated with diabetes progression in relatives of patients with type 1 diabetes. Diabetes 2015;64:3247–3252 [DOI] [PubMed] [Google Scholar]
- 36. Pöllänen PM, Ryhänen SJ, Toppari J, et al. Dynamics of islet autoantibodies during prospective follow-up from birth to age 15 years. J Clin Endocrinol Metab 2020;105:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Winkler C, Haupt F, Heigermoser M, et al.; GPPAD Study Group . Identification of infants with increased type 1 diabetes genetic risk for enrollment into Primary Prevention Trials-GPPAD-02 study design and first results. Pediatr Diabetes 2019;20:720–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharp SA, Rich SS, Wood AR, et al. Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care 2019;42:200–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ferrat LA, Vehik K, Sharp SA, et al.; TEDDY Study Group Committees . A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat Med 2020;26:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alonso GT, Coakley A, Pyle L, Manseau K, Thomas S, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado children, 2010-2017. Diabetes Care 2020;43:117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care 2017;40:1249–1255 [DOI] [PubMed] [Google Scholar]
- 42. JDRF . T1Detect: learn why you should be screened, 2021. Accessed 15 November 2021. Available from https://www.jdrf.org/t1d-resources/t1detect/
- 43. Ziegler AG, Kick K, Bonifacio E, et al.; Fr1da Study Group . Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA 2020;323:339–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kordonouri O, Lange K, Boettcher I, et al. New approach for detection of LDL-hypercholesterolemia in the pediatric population: the Fr1dolin trial in Lower Saxony, Germany. Atherosclerosis 2019;280:85–91 [DOI] [PubMed] [Google Scholar]
- 45. Raab J, Haupt F, Scholz M, et al.; Fr1da Study Group . Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open 2016;6:e011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hippich M, Holthaus L, Assfalg R, et al. A public health antibody screening indicates a 6-fold higher SARS-CoV-2 exposure rate than reported cases in children. Med (N Y) 2021;2:149–163.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes 2012;13:308–313 [DOI] [PubMed] [Google Scholar]
- 48. Johnson SB. Psychological impact of screening and prediction in type 1 diabetes. Curr Diab Rep 2011;11:454–459 [DOI] [PubMed] [Google Scholar]
- 49. Smith LB, Liu X, Johnson SB, et al.; TEDDY study group . Family adjustment to diabetes diagnosis in children: can participation in a study on type 1 diabetes genetic risk be helpful? Pediatr Diabetes 2018;19:1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dunne JL, Koralova A, Sutphin J, et al. Parent and pediatrician preferences for type 1 diabetes screening in the U.S. Diabetes Care 2021;44:332–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parikka V, Näntö-Salonen K, Saarinen M, et al. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia 2012;55:1926–1936 [DOI] [PubMed] [Google Scholar]
- 52. Chmiel R, Giannopoulou EZ, Winkler C, Achenbach P, Ziegler AG, Bonifacio E. Progression from single to multiple islet autoantibodies often occurs soon after seroconversion: implications for early screening. Diabetologia 2015;58:411–413 [DOI] [PubMed] [Google Scholar]
- 53. Bonifacio E, Weiß A, Winkler C, et al.; TEDDY Study Group . An age-related exponential decline in the risk of multiple islet autoantibody seroconversion during childhood. Diabetes Care 2021;44:2260–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu Y, Rafkin LE, Matheson D, et al.; Type 1 Diabetes TrialNet Study Group . Use of self-collected capillary blood samples for islet autoantibody screening in relatives: a feasibility and acceptability study. Diabet Med 2017;34:934–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barker JM, Goehrig SH, Barriga K, et al.; DAISY Study . Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care 2004;27:1399–1404 [DOI] [PubMed] [Google Scholar]
- 56. Hekkala AM, Ilonen J, Toppari J, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes: effect of prospective studies with newborn genetic screening and follow up of risk children. Pediatr Diabetes 2018;19:314–319 [DOI] [PubMed] [Google Scholar]
- 57. Steck AK, Dong F, Taki I, et al. Continuous glucose monitoring predicts progression to diabetes in autoantibody positive children. J Clin Endocrinol Metab 2019;104:3337–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Imperatore G, Mayer-Davis EJ, Orchard TJ, Zhong VW. Prevalence and incidence of type 1 diabetes among children and adults in the United States and comparison with non-U.S. countries. In Diabetes in America. Cowie CC, Casagrande SS, Menke A, et al., Eds. Bethesda, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [PubMed] [Google Scholar]
- 59. Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in incidence of type 1 and type 2 diabetes among youths—selected counties and indian reservations, United States, 2002-2015. MMWR Morb Mortal Wkly Rep 2020;69:161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sims EK, Geyer S, Johnson SB, et al.; Type 1 Diabetes TrialNet Study Group . Who is enrolling? The path to monitoring in Type 1 Diabetes TrialNet’s Pathway to Prevention. Diabetes Care 2019;42:2228–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kanner L, Bekx MT. Targeting IA-2 and GAD65 as a cost-saving approach for antibody testing in children with new-onset diabetes. Clin Diabetes 2019;37:90–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McQueen RB, Geno Rasmussen C, Waugh K, et al. Cost and cost-effectiveness of large-scale screening for type 1 diabetes in Colorado. Diabetes Care 2020;43:1496–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Couper JJ, Haller MJ, Greenbaum CJ, et al. ISPAD clinical practice consensus guidelines 2018: stages of type 1 diabetes in children and adolescents. Pediatr Diabetes 2018;19(Suppl. 27):20–27 [DOI] [PubMed] [Google Scholar]
- 64. Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. Geneva, World Health Organization, 1968 [Google Scholar]