Abstract
Genome-wide association studies have identified single nucleotide polymorphisms (SNPs) associated with waist circumference (WC) and waist-to-hip ratio (WHR) adjusted for BMI (WCadjBMI and WHRadjBMI), but it remains unclear whether these SNPs relate to change in WCadjBMI or WHRadjBMI with lifestyle intervention for weight loss. We hypothesized that polygenic scores (PS) comprised of 59 SNPs previously associated with central adiposity would predict less of a reduction in WCadjBMI or WHRadjBMI at 8–10 weeks in two lifestyle intervention trials, NUGENOB and DiOGenes, and at 1 year in five lifestyle intervention trials, Look AHEAD, Diabetes Prevention Program, Diabetes Prevention Study, DIETFITS, and PREDIMED-Plus. One-SD higher PS related to a smaller 1-year change in WCadjBMI in the lifestyle intervention arms at year 1 and thus predicted poorer response (β = 0.007; SE = 0.003; P = 0.03) among White participants overall and in White men (β = 0.01; SE = 0.004; P = 0.01). At average weight loss, this amounted to 0.20–0.28 cm per SD. No significant findings emerged in White women or African American men for the 8–10-week outcomes or for WHRadjBMI. Findings were heterogeneous in African American women. These results indicate that polygenic risk estimated from these 59 SNPs relates to change in WCadjBMI with lifestyle intervention, but the effects are small and not of sufficient magnitude to be clinically significant.
Introduction
Obesity is a well-established risk factor for numerous diseases (1–3). Independent of elevated BMI, measures of central adiposity, such as waist circumference (WC), also predict many common diseases, including type 2 diabetes, cardiovascular disease, cancers, and early mortality (1,4–8). Indeed, it may be beneficial to measure WC clinically to more accurately reflect adiposity-related risk (9).
Genome-wide association studies (GWAS) have successfully identified genetic regions related to both WC and waist-to-hip ratio (WHR), even after statistical adjustment for BMI (WCadjBMI and WHRadjBMI, respectively) (10,11). Whereas several genes at loci associated with BMI are expressed in the central nervous system, many genes at loci related to WCadjBMI and WHRadjBMI reflect adipose tissue and insulin signaling pathways, highlighting a distinct genetic architecture for BMI and measures of central adiposity (10). In Mendelian randomization analyses, genetic markers related to higher WCadjBMI and WHRadjBMI were associated with type 2 diabetes, cardiovascular disease, and certain cancers (12–14), suggesting a causal role for central adiposity, independent of BMI, in the most common causes of morbidity and mortality in high- and middle-income countries.
Lifestyle interventions (LI) involving calorie restriction and often physical activity to promote weight loss reduce WC and WHR compared with minimal intervention control groups (e.g., placebo pill or brief health education alone) (15–18). Indeed, WC may serve as a better predictor of some of the health benefits of LI than change in weight per se (19,20). Knowledge about predictors of change in WC and WHR in response to LI could help tailor interventions for reducing central adiposity and identify actionable targets for WC or WHR reduction in the context of LI. This article examines whether polygenic scores (PS), comprised of 59 single nucleotide polymorphisms (SNPs) associated with WC, WHR, WCadjBMI, or WHRadjBMI in prior GWAS (10), predict the magnitude of change in these measures at two time points: 8–10 weeks and 1 year of LI. This analysis brings together data from seven LI trials, allowing testing of our central hypothesis that genetic variation associated with elevated WCadjBMI or WHRadjBMI will predict less of a reduction in these variables with lifestyle-induced weight loss intervention.
Research Design and Methods
Research Design
This is a meta-analysis of LI trials for weight loss (16,21–28). The research designs and interventions are presented in Supplementary Table 1. Changes in WCadjBMI and WHRadjBMI were examined at 8–10 weeks in the Diet, Obesity and Genes (DiOGenes) Dietary Study and Nutrient-Gene Interactions in Human Obesity (NUGENOB) Study and at 1 year in Look AHEAD (Action for Health in Diabetes), Diabetes Prevention Program (DPP), Diabetes Prevention Study (DPS), Diet Intervention Examining the Factors Interacting with Treatment Success (DIETFITS), and PREDIMED (Prevención con Dieta Mediterránea)-Plus. The time points were selected to maximize common outcomes across multiple trials. Funding sources and an extended author list for the trials can be found in Supplementary Document 1.
Analyses were conducted in the two most populous racial groups, White and African American, to reduce the risk of confounding due to population stratification (29). The response options for self-reporting these ancestries differed across trials and included African American/Black (Look AHEAD) African American or Black (DPP), African American (DIETFITS), White (Look AHEAD, DPP, and DIETFITS), Caucasian/White (DioGenes), Caucasian European (NUGENOB), White/European (PREDIMED-Plus), and Finnish/European (DPS). In this article, we describe African American or Black self-reported race as African American and White or European self-reported race as White to have consistent language across trials. Individuals self-identifying as Hispanic were excluded from the African American or White subgroups.
All trials included an LI that incorporated calorie restriction to produce weight loss. The approach to macronutrient content and physical activity in the intervention differed across studies. Of the interventions with 8–10-week outcomes, DiOGenes used an 8-week low-calorie diet with meal replacements and extra vegetable provision; NUGENOB tested two low-calorie diets, one with 20–25% fat and the second with 40–45% fat over 10 weeks. Of the trials contributing 1-year outcomes, DPP LI, Look AHEAD intensive LI, and the “Healthy, low-fat” treatment arm of DIETFITS used a restriction of dietary fat intake. The DPS LI also used a restriction of fat intake but with increased fiber intake. The “Healthy, low-carbohydrate” arm of DIETFITS used a low-carbohydrate approach, and PREDIMED-Plus used an energy-restricted Mediterranean diet. The trials contributing 1-year data also included a physical activity promotion component. LI were pooled at 8–10 weeks and 1 year, respectively.
DPS, DPP, PREDIMED-Plus, and Look AHEAD included minimal contact control groups (education group, a placebo pill, Mediterranean diet without caloric restriction, and diabetes support and education, respectively). Although the nature of these control groups differs, we refer to these arms as “control” and test for PS × treatment arm and SNP × treatment arm interactions across these trials. Additional treatment arms that were not an LI or minimal-contact control arm, such as the DPP metformin treatment arm, were not included.
The primary independent variables are weighted PS comprised of the additive effects of associated alleles for 59 SNPs associated with WC, WHR, WCadjBMI, or WHRadjBMI in prior GWAS, weighted by the strength of association in published meta-analysis in ancestry-combined analyses (10). Of the 59 SNPs, 49 were initially associated with WHRadjBMI at genome-wide significance (5 × 10−8), including 39 SNPs in sex-combined White samples, 8 additional SNPs in White women, 1 additional SNP in White men, and 1 additional SNP in ancestry- and sex-combined analyses. Seven additional SNPs were initially associated with WCadjBMI in sex-combined analysis, and three additional SNPs were initially associated with WHR. As these phenotypes show substantial overlap and many of the SNPs were associated with multiple phenotypes, PS were created incorporating the 59 SNPs using weights for WCadjBMI and WHRadjBMI from the sex-combined, female, and male samples of combined ancestry presented in Supplementary Table 4 from Shungin et al. (10)
Participants
A total of 7,646 participants were included in at least one analysis. At 8–10 weeks, 1,542 participants from LI were analyzed. No data from control groups were available (Supplementary Table 2A). At year 1, 6,104 contributed to at least one analysis, including 2,755 White participants in LI, 2,354 White participants in control arms, 506 African American participants in LI, and 489 African American participants in control arms (Supplementary Table 2B). Analyses were further conducted in men and women due to differences in effect sizes of genetic associations with WC and WHR by biological sex (10).
BMI entrance criteria ranged from ≥25 (DPP, DPS, Look AHEAD), ≥26 (NUGENOB), ≥27 (PREDIMED-Plus, DiOGenes), and ≥28 (DIETFITS). Additional inclusion criteria specific to trials included impaired glucose tolerance (DPP and DPS), metabolic syndrome (PREDIMED-Plus), and type 2 diabetes (Look AHEAD). Additional details of the study designs, inclusion and exclusion criteria, and intervention and control groups are summarized in Supplementary Table 1.
Measures
Anthropometrics
At a minimum, all cohorts included a measure of WC and height and weight to calculate BMI. In addition, DPP, DPS, PREDIMED-Plus, DiOGenes, and NUGENOB included hip circumference for calculation of WHR. WC was measured in centimeters in duplicate using a tape measure midway between the bottom of the ribs and the top of the hips; hip circumference was measured in duplicate at the level of maximal hip protrusion. Weight was measured in duplicate with a digital scale or a balance beam scale, and height was measured in duplicate using a stadiometer. Details of anthropometric measurement are available for each cohort (16,21–26).
Genotyping
Genotyping platforms are listed in Supplementary Table 1. Briefly, Look AHEAD and DPS contributed SNPs directly genotyped on the Cardiometabochip (30); the remaining cohorts used genome-wide arrays. SNPs selected for inclusion in the PS and individual analyses included those related to WC, WHR, WCadjBMI, or WHRadjBMI identified in Shungin et al. (10). Proxies were identified (r2 > 0.80) if a SNP was not directly genotyped.
Statistical Analysis
PS were calculated as number of effect alleles per SNP multiplied by the corresponding β weight from the combined ancestry results reported in Shungin et al. (10), summed and scaled across the 59 SNPs. Three separate PS were calculated using weights for associations with WCadjBMI in sex-combined, female, and male samples. Similarly, for WHRadjBMI, three additional PS were calculated using weights for associations with WHRadjBMI for application in females and males combined and the female and male subgroups.
Linear regression models were fit to analyze the association of the PS and individual SNPs with change in WCadjBMI and WHRadjBMI at 8–10 weeks and 1 year. The vast majority of participants in DiOGenes and NUGENOB were White, so there were no additional models by ancestry at 8–10 weeks. At 1 year, models were run in the two largest ancestry groups: White and African American. Age, biological sex (for the full sample), and baseline WCadjBMI or WHRadjBMI served as covariates. For LI trials with a low-contact control group (Look AHEAD, DPP, PREDIMED-Plus, and DPS), PS and individual SNP × treatment arm interaction terms were also tested.
Meta-analysis with fixed effects for each trial was used to combine the results across DiOGenes and NUGENOB for 8–10-week change in WCadjBMI and WHRadjBMI and Look AHEAD, DPP, DPS, DIETFITS, and PREDIMED-PLUS for 1-year change in WCadjBMI and WHRadjBMI. As the PS reflect primary hypotheses, a level of P = 0.05 was used to indicate statistical significance. False discovery rate (FDR) was used to account for multiple comparisons for tests of the individuals SNPs (31).
Data and Resource Availability
As the data and resource sharing differs by trial, we provide statements for each trial. Look AHEAD data are available on the National Institute of Diabetes and Digestive and Kidney Diseases repository. The genetic data are not available due to limitations in consent. DPP data are available on the National Institute of Diabetes and Digestive and Kidney Diseases repository. The genetic data are available on dbGaP. DIETFITS data are available on request to C.G. (cgardner@stanford.edu). Due to limitations in genetic consent, the PREDIMED-Plus data are not publicly available. Collaborations will be considered upon request. DPS data are not publicly available due to limitations in consent. DiOGenes data are available from the DiOGenes Steering group for any interested researcher who meets the criteria for access to confidential data and upon reasonable request. The NUGENOB data are available on request to the Project Steering Committee (e-mail committee chair T.H., torben.hansen@sund.ku.dk).
Results
Descriptive statistics of demographics, PS, and the adiposity variables for the data used in the present meta-analysis are provided in Supplementary Table 2A for trials contributing 8–10-week data (DiOGenes and NUGENOB) and Supplementary Table 2B for trials contributing 1-year data (Look AHEAD, DPP, DIETFITS, DPS, and PREDIMED-Plus). On average, participants across the trials were 35–60 years of age, and 55–75% were women. The European trials (DiOGenes, NUGENOB, DPS, and PREDIMED-Plus) were predominantly White; White and African American participants were included from the U.S. trials. Participants in all trials were on average in the obese range and tended to have clinically elevated WC.
Change in WCadjBMI
Eight to Ten Weeks
None of the PS or individual SNPs significantly predicted change in WCadjBMI (Table 1, Supplementary Fig. 1, and Supplementary Table 3).
Table 1.
PS associations with per-SD change in WCadjBMI at 8–10 weeks and year 1 in the LI arms
| Time | Subsample | k | β | SE | Lower limit | Upper limit | z | P value | I 2 |
|---|---|---|---|---|---|---|---|---|---|
| 8–10 weeks | |||||||||
| White participants | |||||||||
| Full sample | 2 | 0.002 | 0.004 | −0.005 | 0.010 | 0.550 | 0.582 | 0 | |
| Women | 2 | 0.001 | 0.005 | −0.008 | 0.011 | 0.236 | 0.814 | 0 | |
| Men | 2 | 0.008 | 0.006 | −0.008 | 0.020 | 1.350 | 0.177 | 0 | |
| 1 year | |||||||||
| White participants | |||||||||
| Full sample | 5 | 0.007 | 0.003 | 0.001 | 0.013 | 2.2261 | 0.026 | 0.178 | |
| Women | 5 | 0.006 | 0.004 | −0.002 | 0.015 | 1.483 | 0.138 | 0 | |
| Men | 5 | 0.010 | 0.004 | 0.002 | 0.019 | 2.533 | 0.011 | 0.171 | |
| African American participants | |||||||||
| Full sample | 3 | 0.006 | 0.009 | −0.011 | 0.024 | 0.707 | 0.479 | 0.226 | |
| Women | 3 | 0.026 | 0.011 | 0.005 | 0.047 | 2.393 | 0.016 | 0.753 | |
| Men | 2 | −0.005 | 0.0111 | −0.027 | 0.016 | −0.479 | 0.632 | 0 |
k, number of cohorts. P < 0.05 indicated in bold.
One Year
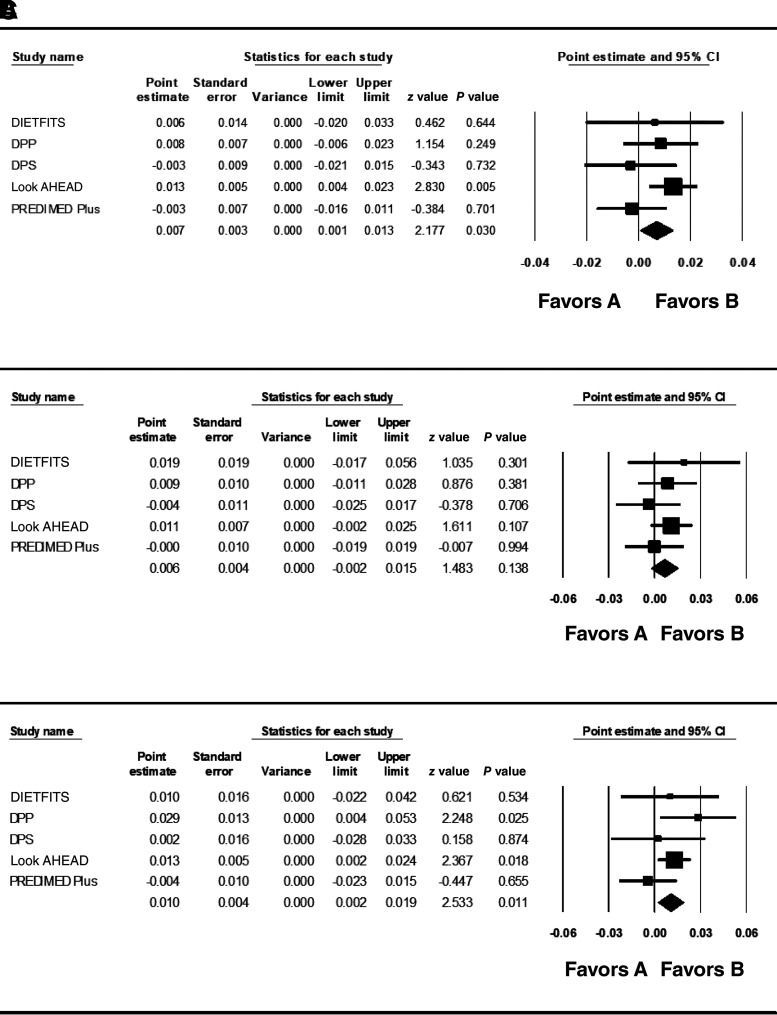
The PS weighted for WCadjBMI across men and women significantly predicted change in WCadjBMI in the White subgroup across the five clinical trials (Table 1 and Fig. 1A). The PS weighted in men also predicted change in WCadjBMI in the White male subset (Table 1 and Fig. 1C). Directionally consistent but nonsignificant results were seen in White women (Table 1 and Fig. 1B). In each case, the PS was associated with a higher WCadjBMI at 1 year, suggesting a poorer response to the LI. Taking the β of 0.007 (SE = 0.003 [95% CI 0.001, 0.013]) per SD higher WCadjBMI and the mean baseline (34.31 kg/m2) and mean 1-year reduction in BMI (−2.84 kg/m2) in White participants from DPP as an example, each SD of the PS contributed to a 0.20-cm (SE = 0.009) higher WC at 1 year in analyses combining men and women. For men under the same parameters, each SD of the PS contributed to a 0.28-cm (SE = 0.01) less change in WC.
Figure 1.
Meta-analysis of PS predicting 1-year change in WCadjBMI in all White participants (A), White women (B), and White men (C).
Interactions with the intervention and control arms were tested in Look AHEAD, DPP, DPS, and PREDIMED-Plus. In White men, there was an interaction of the PS with treatment arm at the level of borderline statistical significance (β = 0.011; SE = 0.005; P = 0.05 [95% CI −0.0002, 0.022]) (Supplementary Table 4). Results indicated that each SD change in the PS was associated with higher WCadjBMI at 1 year in the intervention arm (β = 0.010; SE = 0.004; P = 0.01 [95% CI 0.002, 0.019]) but nonsignificantly lower WCadjBMI in the control groups (β = −0.0015; SE = 0.004; P = 0.62 [95% CI −0.015, 0.006]). The interaction between intervention arm and PS in men and women combined and in women was not significant in White participants (P > 0.47; FDR q > 0.10) (Supplementary Table 4).
None of the individual SNPs was significantly associated with change in WCadjBMI in the LI arms (Supplementary Table 5) or in SNP × treatment arm interaction (Supplementary Table 4) after correction for multiple comparisons in White participants.
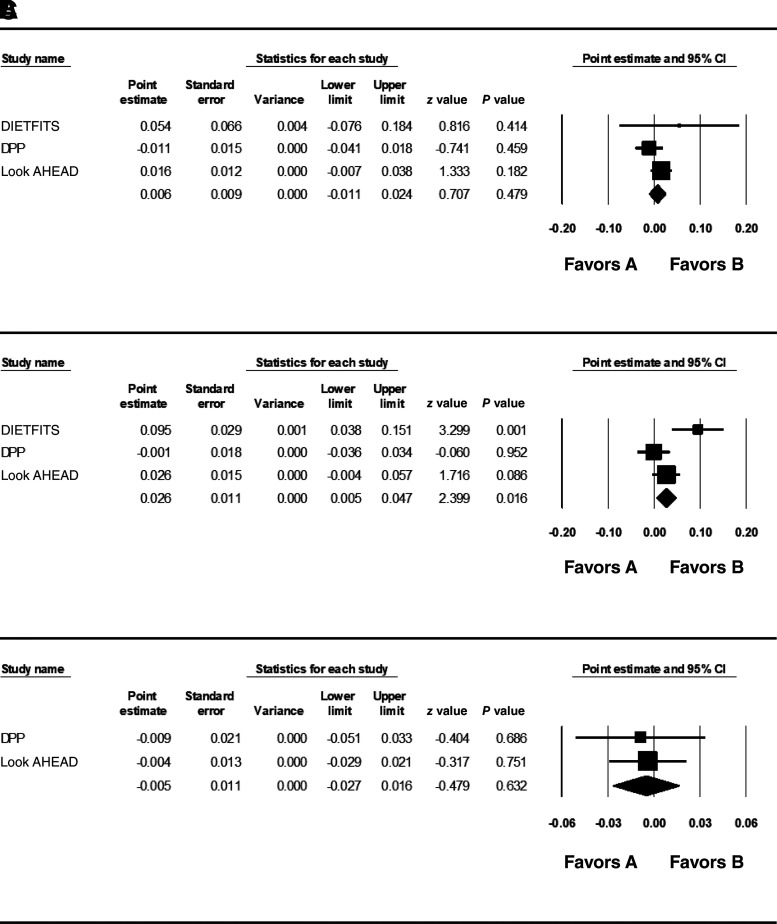
In African American participants, the PS weighted for WCadjBMI in women significantly predicted 1-year change in WCadjBMI in the LI arms in women. However, the heterogeneity score was 0.75, suggesting substantial difference in effect across studies (Table 1 and Fig. 2B). The effect was strongest in DIETFITS (β = 0.095; SE = 0.029; P = 0.001). In Look AHEAD (β = 0.026; SE = 0.015; P = 0.086) and DPP (β = −0.001; SE = 0.0018; P = 0.952), the effect size was small and not statistically significant. Thus, no clear pattern can be derived from the heterogeneous results. The PS weighted for men and women combined and the PS weighted for men were not significantly associated with change in WCadjBMI in the LI arm (Table 1 and Fig. 2A and C). No evidence for PS × treatment arm or SNP × treatment arm interaction was observed (Supplementary Table 6), and none of the individual SNPs predicted WCadjBMI in the lifestyle intervention arm after correction for multiple comparisons in African American participants (Supplementary Table 7).
Figure 2.
Meta-analysis of PS predicting 1-year change in WCadjBMI in all African American participants (A), African American women (B), and African American men (C).
Change in WHRadjBMI
Neither the PS nor any of the SNPs significantly predicted change in WHRadjBMI in the LI at 8–10 weeks (PS, P > 0.10; SNPs, FDR q > 0.10) or at 1 year in the three of five trials (DPP, DPS, and PREDIMED-Plus) that provided data on WHRadjBMI at 1 year (White participants: PS, P > 0.29; SNPs, FDR q > 0.10; African American participants: PS, P > 0.36; SNPs, FDR q > 0.10). In addition, the PS and SNPs did not significantly interact with treatment arm at 1 year in White (PS, P > 0.57; SNPs, FDR q > 0.10) or African American participants (PS, P > 0.39; SNPs, FDR q > 0.10).
Discussion
We identified associations between genetic factors and change in WCadjBMI during LI aimed at weight reduction. A weighted PS comprised of 59 SNPs previously associated cross-sectionally with WC, WHR, WCadjBMI, or WHRadjBMI in the literature (10) predicted less of a reduction in WCadjBMI over 1 year in White men and women combined, and in White men alone. In White men and women combined, and in White men, 1 SD of the PS accounted for ∼0.20–0.28 cm higher WC at year 1.
The PS did not predict change in WCadjBMI in African American participants overall or in African American men. In African American women, results were heterogenous (I2 = 0.75) with no clear pattern across trials. Lack of clear association may be attributable to smaller sample sizes or less representation of individuals with African ancestry in ancestry-combined results from Shungin et al. (10). Associations were also not seen for WCadjBMI at 8–10 weeks, potentially due to a need for a longer intervention for these effects to be manifested. Individual SNPs also were not significantly related to the WCadjBMI change at either 8–10 weeks or 1 year, potentially due to the smaller variance explained by individual SNPs relative to their additive effect. No significant associations were observed for change in WHRadjBMI at either 8–10 weeks or 1 year, potentially attributable to the smaller number of participants with hip data to calculate WHRadjBMI.
Overall, these results do indicate that some of the change in WC during LI is associated with polygenic risk, but the magnitude of genetic effects attributable to the 59 SNPs remains relatively small when compared with the impact of LI on WC at year 1. One-year WC change in Look AHEAD, DPP, DPS, and PREDIMED-Plus in the LI arms were a mean ± SD of −6.2 ± 10.2 cm (15), −6.6 ± 7.3 cm (18), −4.4 ± 5.2 cm SD (16), and −3.1 cm (95% CI −3.8 to −2.5) (17), respectively. With a 0.20 cm difference in WC per SD of the PS in White men and women combined, the difference between the 5th and 95th percentile is ∼0.80 cm. For White men, the difference of 0.28 cm WC per SD translates to a difference of 1.12 cm between the 5th and 95th percentile.
In precision medicine applications, genetics or genomics are used to improve diagnosis, guide pharmacological treatments, and identify contributing pathways to tailor interventions. In this study, the score comprised of 59 SNPs predicted change in WCadjBMI in evidence-based LI, but the association was small in comparison with the 3–6-cm mean WC changes observed in the contributing clinical trials. This is not sufficient to alter recommendations for LI as an evidence-based method to reduce WC. In addition, no single SNP significantly predicted change in WCadjBMI, suggesting that the combination of the variants was more predictive, as might be expected for a polygenic trait. Taken together, our results do not lend themselves to changes in clinical recommendations or identification of specific pathways contributing to change.
A major strength of this analysis is the inclusion of five effective LI trials with 1-year data and two effective lifestyle/diet interventions with 8–10-week data. Many of these trials represent the gold standard of LI in the field with well-described trials, some of the largest sample sizes, and published intervention materials. Several cohorts also included comparison groups, allowing us to explore PS and SNPs by intervention arm interactions.
Weaknesses of this study include the unavoidable heterogeneity of combining across clinical trials, both in terms of compositions of the cohorts and interventions applied, lack of sufficient data to explore PS × macronutrient composition interaction, relatively small sample sizes to examine associations within African Americans and other ancestry groups, and a lack of data on WHR in several of the clinical trials.
In summary, this meta-analysis across multiple LI clinical trials revealed that a PS comprised of 59 SNPs, previously associated with central adiposity, predicted less reduction in WCadjBMI over 1 year. These results indicate that genetic variants related to central adiposity associate with the change in WCadjBMI with LI, but the differences related to these PS are relatively small in comparison with the overall benefit of these interventions for reducing WC.
Article Information
Duality of Interest. J.C.F. has received speaking honoraria from Novo Nordisk, AstraZeneca, and Merck for research talks over which he had full control of content and consulting honoraria from AstraZeneca and Goldfinch Bio. P.W.F. has received consulting honoraria from Eli Lilly and Company and Novo Nordisk A/S; has received research grants from multiple pharmaceutical companies; is a consultant and stock owner in Zoe Global Ltd; and is currently the Scientific Director in Patient Care at the Novo Nordisk Foundation. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.M. conceptualized the paper, contributed to data curation, formal analysis and methodology, wrote the original draft, and contributed to review and editing. K.A.J. conceptualized the paper and contributed to data curation, methodology, formal analysis, and review and editing. Q.P. contributed to conceptualization, methodology, formal analysis, and review and editing. A.A. contributed to data curation and review and editing. M.R.C. contributed to formal analysis, review, and editing. D.C. contributed to data curation and to review and editing. L.M.L.C contributed to writing the original draft and review and editing. J.C.F. contributed to data curation and to review and editing. P.W.F. contributed to data curation and to review and editing. C.G. contributed to data curation and to review and editing. T.H. contributed to data curation and to review and editing. T.O.K. contributed to data curation and to review and editing. W.C.K. contributed to data curation and to review and editing. J.L. contributed to data curation and to review and editing. W.H.M.S. contributed to data curation and to review and editing. T.I.A.S. contributed to data curation and to review and editing. J.T. contributed to data curation and to review and editing. M.U. contributed to data curation and to review and editing. R.R.W. contributed to data curation and to review and editing. T.A.-C. contributed to conceptualization, review, and editing. J.M.M., K.A.J., and Q.P. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at the 81st Scientific Sessions of the American Diabetes Association, 25–29 June 2021.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.17890715.
References
- 1. Carmienke S, Freitag MH, Pischon T, et al. General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur J Clin Nutr 2013;67:573–585 [DOI] [PubMed] [Google Scholar]
- 2. Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol 2018;6:944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Global BMI Mortality Collaboration, Di Angelantonio E, Bhupathiraju S, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016;388:776–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc 2014;89:335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jacobs EJ, Newton CC, Wang Y, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med 2010;170:1293–1301 [DOI] [PubMed] [Google Scholar]
- 6. Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol 1997;145:614–619 [DOI] [PubMed] [Google Scholar]
- 7. Haffner SM, Mitchell BD, Hazuda HP, Stern MP. Greater influence of central distribution of adipose tissue on incidence of non-insulin-dependent diabetes in women than men. Am J Clin Nutr 1991;53:1312–1317 [DOI] [PubMed] [Google Scholar]
- 8. Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17:961–969 [DOI] [PubMed] [Google Scholar]
- 9. Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020;16:177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shungin D, Winkler TW, Croteau-Chonka DC, et al.; ADIPOGen Consortium; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GEFOS Consortium; GENIE Consortium; GLGC; ICBP; International Endogene Consortium; LifeLines Cohort Study; MAGIC Investigators; MuTHER Consortium; PAGE Consortium; ReproGen Consortium . New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pulit SL, Stoneman C, Morris AP, et al.; GIANT Consortium . Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet 2019;28:166–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shu X, Wu L, Khankari NK, et al.; Breast Cancer Association Consortium . Associations of obesity and circulating insulin and glucose with breast cancer risk: a Mendelian randomization analysis. Int J Epidemiol 2019;48:795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Censin JC, Peters SAE, Bovijn J, et al. Causal relationships between obesity and the leading causes of death in women and men. PLoS Genet 2019;15:e1008405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emdin CA, Khera AV, Natarajan P, et al. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA 2017;317:626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Look AHEAD Research Group; Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care 2007;30:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lindström J, Louheranta A, Mannelin M, et al.; Finnish Diabetes Prevention Study Group . The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–3236 [DOI] [PubMed] [Google Scholar]
- 17. Salas-Salvadó J, Díaz-López A, Ruiz-Canela M, et al.; PREDIMED-Plus investigators . Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-Plus Trial. Diabetes Care 2019;42:777–788 [DOI] [PubMed] [Google Scholar]
- 18. DeBoer MD, Filipp SL, Gurka MJ. Use of a metabolic syndrome severity Z score to track risk during treatment of prediabetes: an analysis of the Diabetes Prevention Program. Diabetes Care 2018;41:2421–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel KV, Bahnson JL, Gaussoin SA, et al.; Look AHEAD Research Group . Association of baseline and longitudinal changes in body domposition measures with risk of heart failure and myocardial infarction in type 2 diabetes: findings from the Look AHEAD Trial. Circulation 2020;142:2420–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olson KL, Neiberg RH, Espeland MA, et al.; Look AHEAD Research Group . Waist circumference change during intensive lifestyle intervention and cardiovascular morbidity and mortality in the Look AHEAD Trial. Obesity (Silver Spring) 2020;28:1902–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsen TM, Dalskov S, van Baak M, et al. The Diet, Obesity and Genes (Diogenes) Dietary Study in eight European countries - a comprehensive design for long-term intervention. Obes Rev 2010;11:76–91 [DOI] [PubMed] [Google Scholar]
- 22. Petersen M, Taylor MA, Saris WH, et al. Randomized, multi-center trial of two hypo-energetic diets in obese subjects: high- versus low-fat content. Int J Obes 2006;30:552–560 [DOI] [PubMed] [Google Scholar]
- 23. Ryan DH, Espeland MA, Foster GD, et al.; Look AHEAD Research Group . Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628 [DOI] [PubMed] [Google Scholar]
- 24. The Diabetes Prevention Program . The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanton MV, Robinson JL, Kirkpatrick SM, et al. DIETFITS study (diet intervention examining the factors interacting with treatment success) - study design and methods. Contemp Clin Trials 2017;53:151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martínez-González MA, Buil-Cosiales P, Corella D, et al.; PREDIMED-Plus Study Investigators . Cohort profile: design and methods of the PREDIMED-Plus randomized trial. Int J Epidemiol 2019;48:387–388o [DOI] [PubMed] [Google Scholar]
- 27. Sørensen TI, Boutin P, Taylor MA, et al.; NUGENOB Consortium . Genetic polymorphisms and weight loss in obesity: a randomised trial of hypo-energetic high- versus low-fat diets. PLoS Clin Trials 2006;1:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Look AHEAD Research Group; Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet 2003;361:598–604 [DOI] [PubMed] [Google Scholar]
- 30. Voight BF, Kang HM, Ding J, et al. The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet 2012;8:e1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300 [Google Scholar]