Editor,
Vaccination is a major tool in the efforts towards ending the coronavirus disease 2019 (COVID‐19) pandemic. Cutaneous reactions associated with the COVID‐19 vaccination have been reported. Fixed drug eruption (FDE) is a very rare cutaneous complication of vaccines and of COVID‐19 vaccines in particular. Here, we describe a case of generalized bullous fixed drug eruption (GBFDE) that developed after ChAdOx1 nCoV‐19 (AstraZeneca‐Oxford) vaccination. To date, only three cases of FDE in the COVID‐19‐vaccinated patients have been reported. 1 , 2 , 3 The three previously published cases concerned two cases of mRNA vaccines and so far only one previous case of an adenoviral vector‐based vaccine.
A 41‐year‐old healthy woman developed a well‐defined pruritic, erythematous, painful plaque on her left shoulder three days following the first dose of ChAdOx1 nCoV‐19 vaccine (Fig. 1a). There was no history of any other drug intake prior to or at the time of appearance of the lesion. The patient had no known allergies to medications, or vaccines, and reported no insect bites, trauma or burns to the area. Four days later, new lesions continued to appear at distant sites. Therefore, the patient was hospitalized. Clinical examination showed a bullous erythematous plaque surrounding the injection site on the upper left arm with a 8 × 5 cm diameter. Two other lesions were noted at the upper and lower limbs. Systemic symptoms were absent. There was no evidence of mucosal involvement. She was tested negative for COVID‐19. A diagnosis of GBFDE was suspected, and a diagnostic punch biopsy was performed. The biopsy from the lesion revealed epidermal necrosis leading to bullae formation with superficial and deep perivascular mixed infiltrate of lymphocytes, neutrophils and eosinophils, in addition to dermal melanophages (Fig. 1b). Direct and indirect immunofluorescence were negative. The diagnosis of GBFDE was made. Potent topical corticosteroids and hydroxyzine were initiated. The resolution was noted few days later leaving residual, postinflammatory hyperpigmentation. No new lesions had appeared after three months, but residual pigmented macules persisted. Patch tests could be suggested in the investigation of FDE, because it is a safe and valuable procedure. Unfortunately, in our case, the patient was not willing to be tested.
Figure 1.
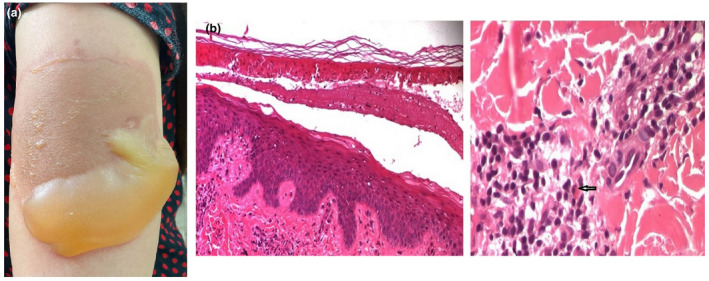
(a) A well‐defined bullous erythematous plaque. (b) Epidermal necrosis leading to bullae formation (haematoxylin‐eosin, original magnification ×100) with superficial and deep perivascular mixed infiltrate of lymphocytes, neutrophils and eosinophils (arrow), in addition to dermal melanophages (haematoxylin‐eosin, original magnification ×400).
ChAdOx1 nCoV‐19 vaccine‐associated cutaneous reactions include local injection‐site reactions, generalized urticaria, maculopapular reactions, delayed large local reactions and Covid toes. 4 Singular cases of cellulitis, psoriasis, rosacea, vitiligo, Raynaud phenomenon and symmetrical drug‐related intertriginous have been also reported. The incidence of adverse cutaneous reactions was significantly higher in females and those in the younger age groups. FDE has not been reported in clinical trials. Only one previous case of GBFDE associated with ChAdOx1 vaccine has been described in the literature. 3 The latter was reported in a 74‐year‐old Thai male who presented with a rash appearing 25 hours after receiving the first dose of ChAdOx1 nCoV‐19 vaccine.
The precise triggering mechanisms are still unknown, and it is unclear which component of the vaccine (virotopes and excipients) may cause the FDE. Vaccine‐induced cutaneous hypersensitivity reactions are thought to be due to antigens present in the vaccine being expressed on the surface of keratinocytes, leading to a T‐lymphocyte immune response against the cells of the epidermis and ultimately cell death and detachment at the dermoepidermal junction. 5
ChAdOx1 nCoV‐19 vaccine contains the excipient polysorbate 80, which is thought to be implicated in the pathogenesis of allergic reactions such as urticaria and anaphylactoid eruptions. 6 GBFDE seems to be a result of a hypersensitivity reaction to the ChAdOx1 nCoV‐19 virotopes rather than to the excipient polysorbate 80. Actually, our patient had already tolerated drugs containing this excipient.
ChAdOx1 nCoV‐19 is well tolerated in most people. It has also been shown to be so effective that the benefits of receiving the vaccination outweigh the risk of developing mild and self‐limiting cutaneous adverse reactions such as FDE.
Conflicts of interest
None.
Funding sources
None.
Acknowledgement
The patient in this manuscript has given written informed consent to the publication of her case details.
Data availability statement
The data used for this study are available from the corresponding author at reasonable request.
References
- 1. Mintoff D, Pisani D, Betts A, Scerri L. SARS‐CoV‐2 mRNA vaccine‐associated fixed drug eruption. J Eur Acad Dermatol Venereol 2021; 35: e560–e563. [DOI] [PubMed] [Google Scholar]
- 2. Kong J, Cuevas‐Castillo F, Nassar M et al. Bullous drug eruption after second dose of mRNA‐1273 (Moderna) COVID‐19 vaccine: case report. J Infect Public Health 2021; 14: 1392–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wantavornprasert K, Noppakun N, Klaewsongkram J, Rerknimitr P. Generalized bullous fixed drug eruption after Oxford‐AstraZeneca (ChAdOx1 nCoV‐19) vaccination. Clin Exp Dermatol 2022; 47: 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Loon A, Mortelmans D, Siozopoulou V et al. A first case of 'Covid toes' from a viral vector‐based COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2022; 36: e400–e402. [DOI] [PubMed] [Google Scholar]
- 5. Lavery MJ, Nawimana S, Parslew R, Stewart L. A flare of pre‐existing erythema multiforme following BNT162b2 (Pfizer‐BioNTech) COVID‐19 vaccine. Clin Exp Dermatol 2021; 46: 1325–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim MA, Lee YW, Kim SR et al. COVID‐19 Vaccine‐associated anaphylaxis and allergic reactions: consensus statements of the KAAACI Urticaria/Angioedema/Anaphylaxis Working Group. Allergy Asthma Immunol Res 2021; 13: 526–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this study are available from the corresponding author at reasonable request.


