Abstract
Objective
To evaluate the effectiveness and safety of Qingjin Yiqi granules (QJYQ) on post‐COVID‐19 condition (PCC).
Method
Patients who met the inclusion criteria were randomly assigned to two groups, the QJYQ group received QJYQ combined with standard rehabilitation treatments (SRTs) and the control group only received SRTs. The treatment course was 14 days. The primary outcomes were modified Medical Research Council (mMRC) scale and Borg scale, while the secondary outcomes included symptoms score and 6‐minute walking distance (6MWD). The safety outcome was the incidence of adverse events.
Results
A total of 388 patients with PCC were enrolled and randomly assigned to the QJYQ group (n = 194) and the control group (n = 194). Compared to the controls, the mMRC scale was improved in the QJYQ group, which was better than that of the control group [β (95%CI): –0.626 (–1.101, –0.151), p = 0.010]. A significant improvement in Borg scale was also observed in the QJYQ group compared to the control group [β (95%CI): –0.395(–0.744, –0.046), p = 0.026]. There was no statistically significant difference in symptoms score and 6MWD between the two groups (p = 0.293, p = 0.724). No treatment‐related adverse events were observed in either group.
Conclusions
QJYQ can bring benefits to patients with PCC, mainly in the improvement of breathlessness and fatigue.
Keywords: COVID‐19, post‐COVID‐19 condition, Qingjin Yiqi granules, randomized clinical trial, rehabilitation treatment
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) is an acute respiratory tract disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), 1 , 2 which is a Public Health Emergency of International Concern, posing a serious risk to human health, daily life and global economy. 3 Till January 5, 2022, there were 293.75 million confirmed cases of COVID‐19 and 5.45 million deaths. 4 There were approximately more than 200 million patients with post‐COVID‐19 condition (PCC), 5 , 6 of which over 90% suffered from persistent symptoms, including fatigue (53.1%−80%), breathlessness (43.4%−53%), anxiety (14.6%−23%), and insomnia (26%−30.8%), 7 ‐ 11 substantially reducing the quality of life. 12 , 13
For about two years, global researchers have made great achievements in vaccines and drugs, which are powerful weapons for fighting against COVID‐19. 14 , 15 However, for patients with PCC, there was lack of new treatment. In China, there were several guidelines for the rehabilitation of patients with PCC, 16 , 17 where respiratory training as well as traditional Chinese therapies including herbal medicine, acupuncture, traditional exercise (e.g., Baduanjin) were recommended. 18
Qingjin Yiqi granules (QJYQ) is a new prescription for patients with PCC, which comprises by 16 herbals: Renshen (Ginseng Radix Et Rhizoma), Maidong (Ophiopogonis Radix), Wuweizi (Schisandrae Chinensis Fructus), Fuling (Poria), Banxia (Pinelliae Rhizoma), Xuanshen (Scrophulariae Radix), Cangzhu (Atractylodis Rhizoma), Chenpi (Citri Reticulatae Pericarpium), Gancao (Glycyrrhizae Radix Et Rhizoma), Chaihu (Bupleuri Radix), Shengma (Cimicifugae Rhizoma), Yiyiren (Coicis Semen), Huangqin (Scutellariae Radix), Mabiancao (Verbenae Herba), Lugen (Phragmitis Rhizoma), and Danzhuye (Lophatheri Herba). These herbs are initially extracted with water, followed by concentration and spray‐drying to powder, after which excipients are added with the final mixture pelletized by dry granulation method. Each package contains 10 g, equivalent to 52 g of the crude drug. QJYQ has been recommended by the Rehabilitation Guidelines of Integrated Medicine for COVID‐19 Patients in Hebei and Tianjin, and has been widely used in clinical practice. 16 In order to provide more clinical evidence on the effectiveness and safety of QJYQ, a randomized clinical trial was carried out.
2. METHODS
2.1. Study design
This study was a randomized, open‐label clinical trial to evaluate the effectiveness and safety of QJYQ for patients with PCC. The study was done in Shijiazhuang (Hebei, China).
Ethical approval was granted by the Medical Ethics Committee of Tianjin University of Traditional Chinese Medicine (TJUTCM‐EC20210001). Orally informed consent was obtained from all patients or their legal representatives. The protocol of this study was registered in Chinese Clinical Trail Registry (ChiCTR2100042822).
2.2. Participants
Participants were recruited from Shijiazhuang People's Hospital and Shijiazhuang Fifth Hospital, who were hospitalized at the two hospitals during the treatment period. Patients who met the discharge criteria were transformed into the rehabilitation ward, where the patients were candidates for this study. Eligible participants were randomly divided into the QJYQ group and the control group in a 1:1 ratio.
2.3. Eligibility criteria
Eligible participants were mild, ordinary, or severe COVID‐19 patients who met the discharge criteria according to the Guidelines for the Diagnosis and Treatment of COVID‐19 (trial version 8): normal temperature for more than 3 days; significant improvements in respiratory symptoms; substantial enhancements of acute exudative lesions on chest radiography; two consecutive negative results of RT‐PCR tests with at least a 1‐day interval between tests. 19 Exclusion criteria were as follows: chronic lung diseases (e.g., chronic obstructive pulmonary disease, interstitial lung disease); previous history of pulmonary surgical intervention; severe clinical condition (e.g., heart failure, stroke); severe chronic disease (e.g., liver cirrhosis, malignant tumors); allergic constitution or allergic to QJYQ; pregnant or breast feeding women.
2.4. Interventions
All participants received standard rehabilitation treatments (SRTs), which consisted of respiratory training (Lip breathing training, 20 minutes each time, 3 times daily; abdominal breathing training was performed at a rate of 7 breaths per minute for 20 minutes, twice a day; respiratory rhythm training, 20 minutes each time, 3 times per day); and Baduanjin exercise, twice a day. 20 Participants in the QJYQ group took QJYQ orally with a dose of 10 g twice daily for 14 days. Any other herbal medicines were prohibited in either group.
2.5. Outcomes
The primary outcomes were the modified Medical Research Council (mMRC) scale and the Borg scale. The mMRC was used to access breathlessness 21 , 22 and the Borg scale was employed to evaluate perceived exertion and fatigue. 23 Outcomes were detected and assessed at baseline, 7 days, and 14 days after randomization.
The secondary outcomes were the 6‐minute walking distance (6MWD) and the symptoms score. 24 The symptoms score was the sum of each symptom score, which covered cough, expectoration, pharynx ministry unwell, fatigue, breathlessness, chest distress, chest pain, body pain, palpitation, dysphoria, headache, dizziness, insomnia, hair loss, hidrosis, decreased appetite, constipation, diarrhea, smell disorder, and taste disorder. The degree of each symptoms varied from 0 to 3, 0 = no symptom, 1 = mild, 2 = ordinary, 3 = severe.
The safety outcome was treatment‐related adverse events. Treatment‐related adverse events involved symptoms and abnormal liver and renal function that were counted as “normal to abnormal” or “abnormal increase”. 25 ‐ 27 The correlation between adverse events and treatment was assessed by clinical investigators.
2.6. Statistical analysis
Statistical analysis was performed by R software, following the intention to treat (ITT) principle with the mean imputation applied for missing data. Exploratory subgroup analyses of each efficacy outcomes were performed according to gender, age, and comorbidities.
Continuous variables (e.g., age, height, weight) were expressed as mean ± standard error of the mean (SEM), which were compared by the Student's t‐test (normally distributed variables) or the Mann–Whitney test (variables without normal distribution). Categorical variables (e.g., gender, symptoms) were presented as counts and percentages, which were compared by the chi‐square test. The comparison of efficacy between two groups was conducted by generalized estimating equation (GEE) analyses with the results displayed as β and 95% confidence interval (CI). Statistical tests were two‐sided, and the significance level was set at α = 0.05.
3. RESULTS
3.1. Participants and baseline characteristics
From January to March 2021, a total of 388 participants (194 per group) were admitted and randomly distributed into two groups. Altogether four (1.03%) participants dropped out, with one participant (0.52%) leaving the QJYQ group due to the taste of QJYQ and three participants (1.55%) withdrawing from the control group owing to their poor compliance. Mean imputation was implemented for the data missing caused by those dropouts. Eventually, all 388 participants were included in the ITT analysis (Figure 1).
FIGURE 1.
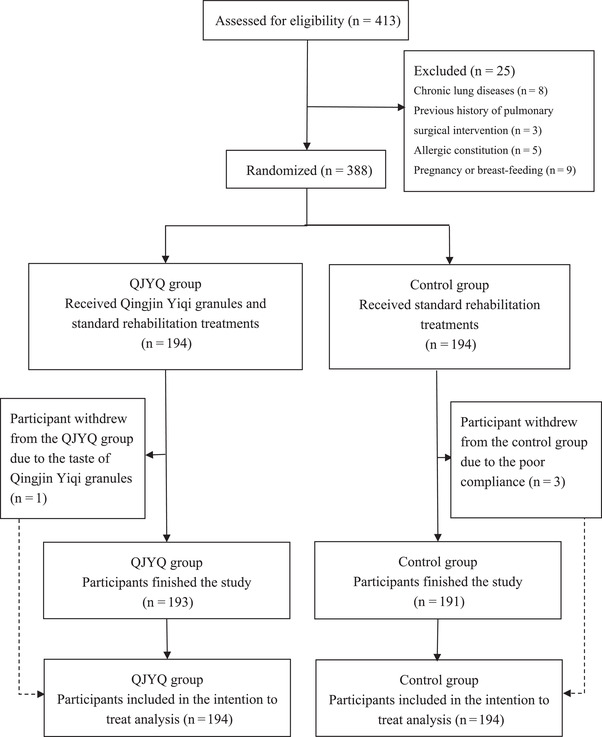
Flowchart of study
The common symptoms at baseline were breathlessness and fatigue (both were 115 times, 29.64%), chest distress (94 times, 24.23%), cough (71 times, 18.30%), insomnia (70 times, 18.04%), pharynx ministry unwell (56 times, 14.43%), and expectoration (48 times, 12.37%). There was no statistically significant difference between the two groups in the baseline characteristics (Tables 1 and 2).
TABLE 1.
Demographic and clinical characteristics
| QJYQ group | Control group | ||
|---|---|---|---|
| Variables | (n = 194) | (n = 194) | p Value |
| Age (year) | 47.96 ± 1.36 | 44.82 ± 1.17 | 0.050 |
| Gender (male/female) | 72/122 | 77/117 | 0.602 |
| Height (cm) | 163.34 ± 0.54 | 164.30 ± 0.53 | 0.231 |
| Weight (kg) | 68.43 ± 1.05 | 66.79 ± 0.93 | 0.185 |
| Temperature (℃) | 36.38 ± 0.02 | 36.40 ± 0.03 | 0.672 |
| Systolic pressure (mmHg) | 132.69 ± 1.31 | 130.39 ± 1.19 | 0.362 |
| Diastolic pressure (mmHg) | 86.93 ± 1.25 | 85.14 ± 0.92 | 0.421 |
| Respiration rate (bpm) | 19.21 ± 0.53 | 19.20 ± 0.37 | 0.054 |
| Heart rate (bpm) | 81.24 ± 0.93 | 82.04 ± 0.90 | 0.734 |
| Oxygen saturation (%) | 98.14 ± 0.09 | 98.14 ± 0.08 | 0.754 |
| White blood cell count (109/L) | 6.30 ± 0.09 | 6.42 ± 0.12 | 0.971 |
| Neutrophil count (109/L) | 5.82 ± 0.63 | 5.25 ± 0.48 | 0.390 |
| Lymphocyte count (109/L) | 2.78 ± 0.23 | 2.58 ± 0.22 | 0.133 |
| Hematocrit (%) | 39.46 ± 1.25 | 37.12 ± 0.45 | 0.136 |
| Hemoglobin (g/L) | 130.82 ± 0.92 | 129.72 ± 0.91 | 0.156 |
| Blood platelet (109/L) | 232.34 ± 3.38 | 233.30 ± 3.63 | 0.942 |
Note: Data are mean ± SEM.
QJYQ = Qingjin Yiqi granules.
TABLE 2.
Persistent symptoms
| Total | QJYQ group | Control group | ||
|---|---|---|---|---|
| Variables | (n = 388) | (n = 194) | (n = 194) | p Value |
| Breathlessness | 115 (29.64) | 61 (31.44) | 54 (27.84) | 0.436 |
| Fatigue | 115 (29.64) | 59 (30.41) | 56 (28.87) | 0.739 |
| Chest distress | 94 (24.23) | 46 (23.71) | 48 (24.74) | 0.813 |
| Cough | 71 (18.30) | 35 (18.04) | 36 (18.56) | 0.896 |
| Insomnia | 70 (18.04) | 36 (18.56) | 34 (17.53) | 0.792 |
| Pharynx ministry unwell | 56 (14.43) | 27 (13.92) | 29 (14.95) | 0.773 |
| Expectoration | 48 (12.37) | 27 (13.92) | 21 (10.82) | 0.355 |
| Hair loss | 30 (7.73) | 17 (8.76) | 13 (6.70) | 0.447 |
| Palpitation | 18 (4.64) | 10 (5.15) | 8 (4.12) | 0.629 |
| Constipation | 17 (4.38) | 9 (4.64) | 8 (4.12) | 0.804 |
| Decreased appetite | 16 (4.12) | 8 (4.12) | 8 (4.12) | 1 |
| Dizziness | 12 (3.09) | 6 (3.09) | 6 (3.09) | 1 |
| Hidrosis | 12 (3.09) | 7 (3.61) | 5 (2.58) | 0.558 |
| Body pain | 9 (2.32) | 6 (3.09) | 3 (1.55) | 0.312 |
| Dysphoria | 8 (2.06) | 3 (1.55) | 5 (2.58) | 0.475 |
| Headache | 8 (2.06) | 5 (2.58) | 3 (1.55) | 0.475 |
| Smell disorder | 8 (2.06) | 6 (3.09) | 2 (1.03) | 0.153 |
| Diarrhea | 7 (1.80) | 5 (2.58) | 2 (1.03) | 0.253 |
| Taste disorder | 4 (1.03) | 3 (1.55) | 1 (0.52) | 0.315 |
| Chest pain | 3 (0.77) | 2 (1.03) | 1 (0.52) | 0.562 |
Note: Data are n (%).
QJYQ = Qingjin Yiqi granules.
3.2. Effectiveness
Participants in the QJYQ group exhibited remarkable improvements on both primary outcomes compared with the control group (Table 3). The mMRC scale was illustrated in Figure 2A. The score of mMRC scale was 1.29 ± 0.05 in the QJYQ group and 1.27 ± 0.05 in the control group at baseline. After 7 days of treatment, the score was 0.65 ± 0.04 in the QJYQ group and 0.80 ± 0.05 in the control group. After 14 days of treatment, the score was 0.20 ± 0.03 in the QJYQ group and 0.34 ± 0.04 in the control group. The GEE estimated difference of the mMRC scale was [β (95%CI) = −0.626 (−1.101, −0.151); p = 0.010].
TABLE 3.
Primary outcomes
| QJYQ group | Control group | ||||
|---|---|---|---|---|---|
| Outcomes | Time | (n = 194) | (n = 194) | β (95%CI) | p Value |
| mMRC scale | Baseline | 1.29 ± 0.05 | 1.27 ± 0.05 | −0.626 (−1.101, −0.151) | 0.010 |
| 7 days | 0.65 ± 0.04 | 0.80 ± 0.05 | |||
| 14 days | 0.20 ± 0.03 | 0.34 ± 0.04 | |||
| Borg scale | Baseline | 10.55 ± 0.16 | 10.51 ± 0.13 | –0.395 (−0.744, −0.046) | 0.026 |
| 7 days | 9.29 ± 0.12 | 9.51 ± 0.11 | |||
| 14 days | 7.89 ± 0.09 | 8.37 ± 0.10 |
Note: Data are mean ± SEM.
QJYQ = Qingjin Yiqi granules; mMRC = modified Medical Research Council.
FIGURE 2.
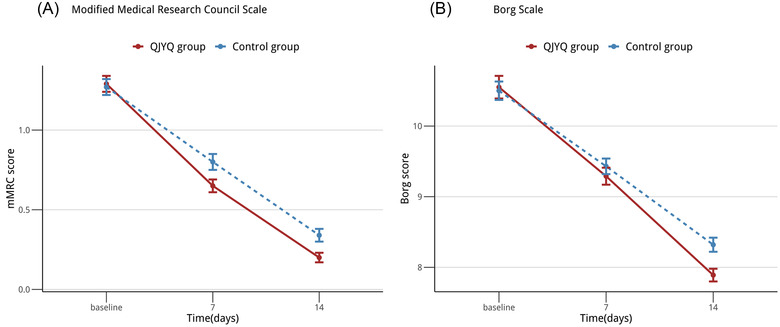
Line chart: primary outcomes. (A) The changes of modified Medical Research Council (mMRC) scale , from baseline to 14 days. (B) The changes of Borg scale, from baseline to 14 days. All data were expressed as mean ± standard error of the mean; n = 194 per group
The Borg scale was depicted in Figure 2B. At baseline, the score of Borg scale was 10.55 ± 0.16 in the QJYQ group and 10.51 ± 0.13 in the control group. After 7 days of treatment, the score decreased to 9.29 ± 0.12 and 9.51 ± 0.11, respectively. The score, after 14 days of treatment, appeared to be 7.89 ± 0.09 in the QJYQ group and 8.37 ± 0.10 in the control group. The GEE estimated difference of the Borg score was [β (95%CI) = −0.395 (−0.744, −0.046); p = 0.026].
The improvements of the symptoms score and the 6MWD were similar in the QJYQ group and the control group (Figure 3) with the GEE estimated difference of the symptoms score being [β (95%CI) = 0.235 (−0.204, 0.674); p = 0.293] and that of the 6MWD being [β (95%CI) = 0.006 (−0.029, 0.041); p = 0.724] (Table 4).
FIGURE 3.
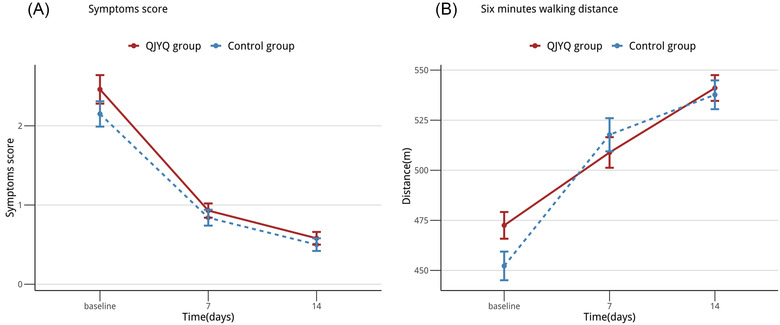
Line chart: secondary outcomes. (A) The changes of symptoms score, from baseline to 14 days. (B) The changes of 6‐minute walking distance (6MWD), from baseline to 14 days. All data were expressed as means ± standard error of the mean; n = 194 per group
TABLE 4.
Secondary outcomes
| Outcomes | Time | QJYQ group (n = 194) | Control group (n = 194) | β (95%CI) | p Value |
|---|---|---|---|---|---|
| Symptoms score | Baseline | 2.46 ± 0.18 | 2.15 ± 0.16 | 0.235 (−0.204, 0.674) | 0.293 |
| 7 days | 0.93 ± 0.09 | 0.84 ± 0.10 | |||
| 14 days | 0.58 ± 0.08 | 0.50 ± 0.08 | |||
| 6MWD | Baseline | 472.50 ± 6.67 | 452.23 ± 7.15 | 0.006 (−0.029, 0.041) | 0.724 |
| 7 days | 508.88 ± 7.64 | 517.74 ± 8.27 | |||
| 14 days | 541.07 ± 6.43 | 537.68 ± 7.18 |
Note: Data are mean ± SEM.
QJYQ = Qingjin Yiqi granules; 6MWD = 6‐minute walking distance.
The subgroup analysis of mMRC scale showed that the QJYQ group performed better than the control group [β (95% CI) = −1.021 (−1.988, 0.053); p = 0.039] in the subgroup of males. Data showed that QJYQ can markedly decrease the mMRC score in the subgroup of patients younger than 65 years old [β (95% CI) = −0.725 (−1.261, −0.189); p = 0.008]. Besides, in the subgroup of patients without comorbidities, the QJYQ group was superior to the control group [β (95%CI) = −0.754 (−1.384, −0.125); p = 0.019]. As for other subgroups, no statistically significant difference between the QJYQ group and the control group was witnessed (Figure 4).
FIGURE 4.
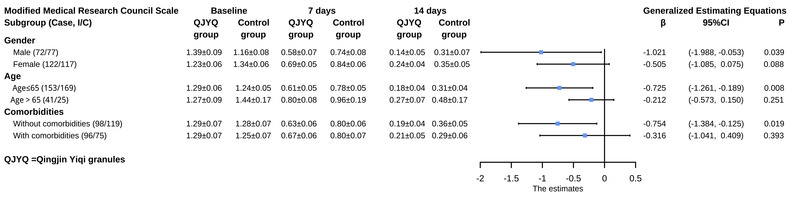
Subgroup analyses of modified Medical Research Council (mMRC) scale. Subgroup analyses were performed on the basis of gender, age, and comorbidities. Data of mMRC scale at baseline, 7 days, and 14 days after randomization were expressed as mean ± standard error of the mean; the effect estimates were represented as β and 95% confidence interval
The subgroup analysis of Borg scale disclosed that the performance of the QJYQ group was greater than that of the control group in the subgroup of males [β (95%CI) = −0.765 (−1.331, −0.198); p = 0.008). In addition, a remarkable reduction of the Borg score in the QJYQ group was observed in the subgroup of patients younger than 65 years old [β (95%CI) = −0.446 (−0.826, −0.066); p = 0.021]. In terms of other subgroups, there was no statistically significant difference between the QJYQ group and the control group (Figure 5).
FIGURE 5.
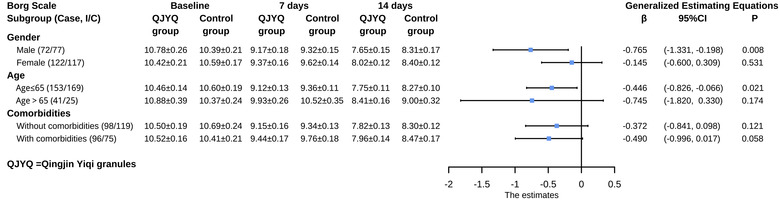
Subgroup analyses of Borg scale. Subgroup analyses were performed on the basis of gender, age, and comorbidities. Data of Borg scale at baseline, 7 days, and 14 days after randomization are expressed as mean ± standard error of the mean; the effect estimates were represented as β and 95% confidence interval
In the subgroup analyses of symptoms score and 6MWD, neither the QJYQ group nor the control group showed statistically significant differences in all subgroups (Figures 6 and 7).
FIGURE 6.
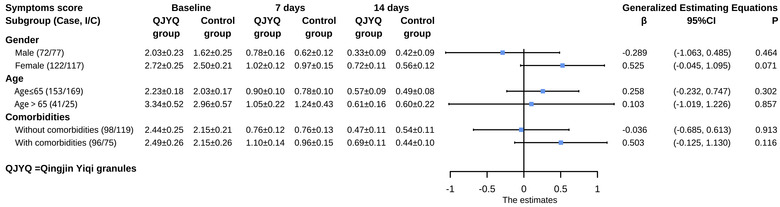
Subgroup analyses of symptoms score. Subgroup analyses were performed on the basis of gender, age, and comorbidities. Data of symptoms score at baseline, 7 days, and 14 days after randomization were expressed as mean ± standard error of the mean; the effect estimates were represented as β and 95% confidence interval
FIGURE 7.
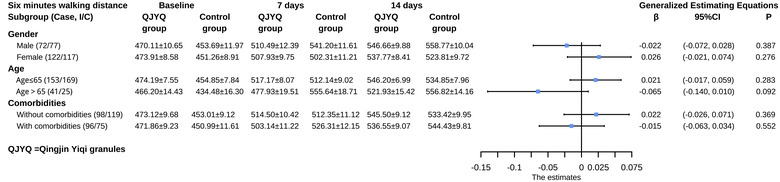
Subgroup analyses of 6‐minute walking distance (6MWD). Subgroup analyses were performed on the basis of gender, age, and comorbidities. Data of 6MWD at baseline, 7 days, and 14 days after randomization are expressed as mean ± standard error of the mean; the effect estimates were represented as β and 95% confidence interval
3.3. Safety
There was no death or serious adverse event that occurred in either group. Besides, no “abnormal increase” of liver and renal function and no “normal to abnormal” of total bilirubin (TBIL), creatinine (Cr) and blood urea nitrogen (BUN) was observed in both groups. It was found that a total of 19 participants experienced “normal to abnormal” of alanine transaminase (ALT), 9 of them came from the QJYQ group and 10 of them belonged to the control group. Twelve participants were observed to undergo “normal to abnormal” of aspartate aminotransferase (AST), with 4 from the QJYQ group and 8 from the control group. None of the abnormalities in ALT and AST was considered to be related to QJYQ and SRTs by the assessment of clinical investigators.
4. DISCUSSION
The results of this study provided evidence that QJYQ could bring benefit to patients with PCC. In this study, data showed the QJYQ group was superior to the control group in mMRC scale and Borg scale, improving the symptoms of breathlessness and fatigue of patients with PCC. No adverse event related to QJYQ was recorded.
In terms of symptoms score and 6MWD, there was no significant difference between the two groups. The participants in this study, whose conditions were predominantly mild instead of serious. Therefore, the symptoms score and 6MWD in this study were close to normal at baseline. SRTs incorporate respiratory training and Baduanjinhad been proven to exert the effects of improving the symptoms and pulmonary function by prior studies. 28 ‐ 30 So, it was difficult to detect the specific add‐on effect of QJYQ, especially in a small sample clinical trial.
In this study, common symptoms among participants were breathlessness (29.64%), fatigue (29.64%), chest distress (24.23%), cough (18.30%), and insomnia (18.04%). The percentages of other symptoms were under 15%. Compared with other studies, some similarities and differences were found.
First, the types of symptoms observed in this study were similar to those reported in other studies. Swapna Mandal et al. conducted a study in Britain from April to June 2020 with 384 patients and found that the most common symptoms in patients with PCC included fatigue (69%), breathlessness (53%), and cough (34%). 8 Moreover, Chaolin Huang et al. reported a cohort study conducted in China from January to May 2020 with 1733 patients, in which fatigue (63%) and insomnia (26%) were the most prevalent symptoms. 9
Second, the proportion of symptoms in this study was notably lower than that in studies from other countries. Aleksander Och et al. reported a study carried out in Europe from October 2020 to February 2021, including 79 patients, with 60.76% fatigue and more than 20% of 12 kinds of symptoms. 31 Similarly, Greg Vanichkachorn et al. published a cohort study undertaken in America from June to December 2020, which included 100 patients, with 80% fatigue, 49% breathlessness, and more than 82% limitation of normal function. 11
Third, the symptoms ratio of this study was less than studies conducted in China at the early stage of COVID‐19 pandemic. Lixue Huang et al. performed a study in China from January to May 2020, in which 1230 patients attended and the ratio of fatigue and insomnia were 52% and 27%, respectively. 32 A higher symptoms ratio was reported in Chaolin Huang's study, with 63% fatigue and 26% insomnia. 9
The reasons for the low symptoms ratios in this study can be summarized as follows: (1) mutations in SARS‐CoV‐2 occurred over the last 2 years with different variants causing the different ratio of symptoms; 33 , 34 (2) the differences in geographic distribution and climate among each study, also influenced the symptoms; (3) the difference interventions for patients with acute COVID‐19 condition is an important factor, which can influence the symptom ratio. TCM was rarely used in other countries, and the utilization rate of TCM in the early stage of COVID‐19 pandemic was insufficient in China. All the patients included in this study received TCM during the acute period of COVID‐19, which was an important factor in the reduction of symptoms.
There were several limitations in this study. First, it was an open‐label study without placebo control, rather than the double‐ blind trial, which might cause selection bias and detection bias. Second, add on design was used based on ethical considerations. As a consequence, the real efficacy of QJYQ might be underestimated. Third, the treatment course was no more than 14 days. So, the data of this study could not find the long‐time effectiveness of QJYQ. Trials with long‐time follow‐up were warranted.
5. CONCLUSIONS
The results of this study provided evidence that QJYQ is a safe Chinese herbal medicine which can bring benefits to patients with PCC. Large sample, strictly designed, randomized clinical trials are still needed to provide more high‐quality evidence for the long‐term effect of QJYQ for PCC.
ACKNOWLEDGMENTS
This work is supported by National Natural Science Foundation of China: (82004505), (82074583); National Key Research and Development Program of China: (2021YFC0863200).
Pang W, Yang F, Zhao Y, et al. Qingjin Yiqi granules for post‐COVID‐19 condition: a randomized clinical trial. J Evid Based Med. 2022;15:30–38. 10.1111/jebm.12465
Wentai Pang, Fengwen Yang, and Yubin Zhao contributed equally to this work.
Contributor Information
Boli Zhang, Email: zhangbolipr@163.com.
Junhua Zhang, Email: zjhtcm@foxmail.com.
REFERENCES
- 1. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324(8), 782–793. Aug 25. [DOI] [PubMed] [Google Scholar]
- 2. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020. Apr;5(4), 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . 2019‐nCoV outbreak is an emergency of international concern. https://www.euro.who.int/en/health‐topics/health‐emergencies/international‐health‐regulations/news/news/2020/2/2019‐ncov‐outbreak‐is‐an‐emergency‐of‐international‐concern; 2020 (accessed 20 December 2021)
- 4. World Health Organization . WHO Coronavirus (COVID‐19) Dashboard. https://covid19.who.int/; 2022 (accessed 5 January 2022)
- 5. World Health Organization . A clinical case definition of post COVID‐19 condition by a Delphi consensus. https://apps.who.int/iris/handle/10665/345824; 2021 (accessed 20 December 2021)
- 6. Cortinovis M, Perico N, Remuzzi G. Long‐term follow‐up of recovered patients with COVID‐19. Lancet. 2021;397(10270), 173–175. Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carfì A, Bernabei R, Landi F. Gemelli against COVID‐19 post‐acute care study group. Persistent symptoms in patients after acute COVID‐19. JAMA. 2020;324(6), 603–605. Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mandal S, Barnett J, Brill SE, et al.; ARC Study Group. ‘Long‐COVID’: a cross‐sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID‐19. Thorax. 2021;76(4), 396–398. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270), 220–232. Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garrigues E, Janvier P, Kherabi Y, et al. Post‐discharge persistent symptoms and health‐related quality of life after hospitalization for COVID‐19. J Infect. 2020;81(6), e4–e6. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vanichkachorn G, Newcomb R, Cowl CT, et al. Post‐COVID‐19 syndrome (long haul syndrome): description of a multidisciplinary clinic at Mayo Clinic and characteristics of the initial patient cohort. Mayo Clin Proc. 2021;96(7), 1782–1791. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhan Y, Zhu Y, Wang S, et al. SARS‐CoV‐2 immunity and functional recovery of COVID‐19 patients 1‐year after infection. Signal Transduct Target Ther. 2021;6(1), 368. Oct 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID‐19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open. 2021;4(9), e2127403. Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. García‐Montero C, Fraile‐Martínez O, Bravo C, et al. An updated review of SARS‐CoV‐2 vaccines and the importance of effective vaccination programs in pandemic times. Vaccines (Basel). 2021;9(5), 433. Apr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeyanathan M, Afkhami S, Smaill F, et al. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol. 2020;20(10), 615–632. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Health Commission of Hebei Province . Hebei administration of traditional Chinese medicine, rehabilitation guidelines of integrated medicine for COVID‐19 patients in Hebei Province. http://health.hebei.com.cn/system/2021/02/04/100592970.shtml; 2021 (accessed 21 December 2021)
- 17. National Health Commission of the People's Republic of China . Rehabilitation guidelines for discharged COVID‐19 patients (Trial Version). http://www.nhc.gov.cn/xcs/zhengcwj/202003/d4558d2cc35e44d5b9adba7c911e0b4c.shtml; 2020 (accessed 21 December 2021)
- 18. Lyu M, Fan G, Xiao G, et al. Traditional Chinese medicine in COVID‐19. Acta Pharm Sin B. 2021;11(11), 3337–3363. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Health Commission of the People's Republic of China . Guidelines for the diagnosis and treatment of novel coronavirus (2019‐nCoV) infection (Trial Version 8). http://www.nhc.gov.cn/yzygj/s7653p/202008/0a7bdf12bd4b46e5bd28ca7f9a7f5e5a/files/a449a3e2e2c94d9a856d5faea2ff0f94; 2020 (accessed 21 December 2021)
- 20. Zhou J, Yu Y, Cao B, et al. Characteristic of clinical studies on Baduanjin during 2000–2019: a comprehensive review. Evid Based Complement Alternat Med. 2020;2020:4783915. Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Torres JP, Pinto‐Plata V, Ingenito E, et al. Power of outcome measurements to detect clinically significant changes in pulmonary rehabilitation of patients with COPD. Chest. 2002;121(4), 1092–1098. Apr. [DOI] [PubMed] [Google Scholar]
- 22. Hu WP, Wu XD, Li ZZ, Zhang J. Preventive effects of Qingfei Yihuo capsules on air pollution associated exacerbation of chronic obstructive pulmonary disease: a single‐blind, randomized, controlled trial. Chin J Integr Med. 2020;26(11), 806–811. Nov. [DOI] [PubMed] [Google Scholar]
- 23. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5), 377–381. [PubMed] [Google Scholar]
- 24. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166(1), 111–117. [DOI] [PubMed] [Google Scholar]
- 25. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non‐ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40(6), 1321–1326. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ng JH, Hirsch JS, Hazzan A, et al. Northwell nephrology COVID‐19 research consortium. Outcomes among patients hospitalized with COVID‐19 and acute kidney injury. Am J Kidney Dis. 2021;77(2), 204–215.e1. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Delanaye P, Huart J, Bouquegneau A, Jouret F. Long‐term effects of COVID‐19 on kidney function. Lancet. 2021;397(10287), 1807. May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim BS, Kim JH, Park SH, Seo HS, Lee HS, Lee MM. Effect of a respiratory training program using wind instruments on cardiopulmonary function, endurance, and quality of life of elderly women. Med Sci Monit. 2018;24:5271–5278. Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hallage T, Krause MP, Haile L, et al. The effects of 12 weeks of step aerobics training on functional fitness of elderly women. J Strength Cond Res. 2010;24(8), 2261–2266. Aug. [DOI] [PubMed] [Google Scholar]
- 30. Chen DM, Yu WC, Hung HF, Tsai JC, Wu HY, Chiou AF. The effects of Baduanjin exercise on fatigue and quality of life in patients with heart failure: a randomized controlled trial. Eur J Cardiovasc Nurs. 2018;17(5), 456–466. Jun. [DOI] [PubMed] [Google Scholar]
- 31. Och A, Tylicki P, Polewska K, et al. Persistent post‐COVID‐19 syndrome in hemodialyzed patients—a longitudinal cohort study from the north of Poland. J Clin Med. 2021;10(19), 4451. Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang L, Yao Q, Gu X, et al. 1‐year outcomes in hospital survivors with COVID‐19: a longitudinal cohort study. Lancet. 2021;398(10302), 747–758. Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ong SWX, Chiew CJ, Ang LW, et al. Clinical and virological features of SARS‐CoV‐2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta). Clin Infect Dis. 2021:ciab721. Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loconsole D, Centrone F, Morcavallo C, et al. Changing features of COVID‐19: characteristics of infections with the SARS‐CoV‐2 Delta (B.1.617.2) and Alpha (B.1.1.7) variants in Southern Italy. Vaccines (Basel). 2021;9(11), 1354. Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]


