Abstract
Introduction
Children who receive prereferral rectal artesunate (RAS) require urgent referral to a health facility where appropriate treatment for severe malaria can be provided. However, the rapid improvement of a child’s condition after RAS administration may influence a caregiver’s decision to follow this recommendation. Currently, the evidence on the effect of RAS on referral completion is limited.
Methods
An observational study accompanied the roll-out of RAS in three malaria endemic settings in the Democratic Republic of the Congo (DRC), Nigeria and Uganda. Community health workers and primary health centres enrolled children under 5 years with suspected severe malaria before and after the roll-out of RAS. All children were followed up 28 days after enrolment to assess their treatment-seeking pathways.
Results
Referral completion was 67% (1408/2104) in DRC, 48% (287/600) in Nigeria and 58% (2170/3745) in Uganda. In DRC and Uganda, RAS users were less likely to complete referral than RAS non-users in the pre-roll-out phase (adjusted OR (aOR)=0.48, 95% CI 0.30 to 0.77 and aOR=0.72, 95% CI 0.58 to 0.88, respectively). Among children seeking care from a primary health centre in Nigeria, RAS users were less likely to complete referral compared with RAS non-users in the post-roll-out phase (aOR=0.18, 95% CI 0.05 to 0.71). In Uganda, among children who completed referral, RAS users were significantly more likely to complete referral on time than RAS non-users enrolled in the pre-roll-out phase (aOR=1.81, 95% CI 1.17 to 2.79).
Conclusions
The findings of this study raise legitimate concerns that the roll-out of RAS may lead to lower referral completion in children who were administered prereferral RAS. To ensure that community-based programmes are effectively implemented, barriers to referral completion need to be addressed at all levels. Alternative effective treatment options should be provided to children unable to complete referral.
Trial registrstion number
NCT03568344; ClinicalTrials.gov.
Keywords: child health, malaria, health policy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Prereferral rectal artesunate reduces case fatality in children with suspected severe malaria in the context of high referral completion.
Based on qualitative evidence, there are concerns that the administration of rectal artesunate may increase the risk of not completing referral.
WHAT THIS STUDY ADDS
There was a negative association between prereferral treatment with rectal artesunate and referral completion of children with signs of severe malaria.
The association of rectal artesunate administration with referral completion was context specific.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The implementation of prereferral rectal artesunate should be accompanied by a monitoring of referral completion in the target population.
Introduction
Rectal artesunate (RAS) is a potentially life-saving prereferral treatment for children presenting at the primary healthcare level with suspected severe malaria.1 Current guidelines require that children who received RAS be referred immediately to a health facility where comprehensive management of severe malaria can be provided.2 However, the rapid improvement of a child’s condition after the administration of RAS may result in children not being taken to a referral health facility (RHF), where appropriate treatment is available.3 4
According to WHO guidelines, appropriate postreferral treatment of severe malaria consists of an intramuscular or intravenous antimalarial for at least 24 hours followed by a full course of an oral artemisinin-based combination therapy (ACT) and accompanied by the management of clinical complications.5 Previous studies on RAS and referral defined referral completion as going to the nearest health facility, irrespective of the facility’s capacity to treat severe malaria.3 6–10 In the case of children first treated with RAS by a community health worker (CHW), this may be a primary health centre (PHC) that lacks the capacity to manage a severe malaria episode. In view of improving the case management of such children, more evidence is needed to understand the pathways by which children with suspected severe malaria reach a competent and capacitated healthcare provider and whether referral completion is impacted by the administration of RAS.
There is evidence that children who received prereferral treatment were less likely to complete referral than children without treatment prior to referral.11 12 However, this kind of evidence for RAS as a prereferral treatment is scarce. Most previous quantitative studies on RAS and referral completion did not compare RAS users versus non-RAS users,8–11 13 and, thus, did not estimate the potential effect of RAS administration on referral completion. Only one observational study tested for non-inferiority of referral completion among children receiving RAS compared with children not receiving RAS.6 The authors concluded non-inferiority because the predefined margin of 15% was not reached; however, referral completion in RAS users (84%) was lower than in non-RAS users (94%). In addition, the analysis did not control for other factors influencing referral completion. Factors that have previously been shown to influence referral completion are household dynamics and priorities, illness severity, the type of referring provider, the performance and result of diagnostic tests prior to referral, health workers’ communication skills, distance to the RHF, referral and treatment costs and the perceived quality of the RHF.3 6–8 11–16
The Community Access to Rectal Artesunate for Malaria (CARAMAL) project included an observational study accompanying the implementation of RAS in the Democratic Republic of the Congo (DRC), Nigeria and Uganda and is described in detail in a companion paper.17 The training provided to CHWs and staff of PHCs during the roll-out of RAS emphasised the need to refer severely sick children to an appropriate and recognised RHF capable of managing the child’s severe condition, rather than to the nearest or next-higher level provider. This manuscript aimed to assess referral completion of children with suspected severe malaria and its relationship with RAS implementation and administration, taking into consideration other factors influencing referral completion. The key study results are described by Hetzel et al18 and the treatment patterns in the RHFs are described by Signorell et al19
Methods
Study design
This observational study followed the implementation of prereferral RAS in three study areas in DRC, Nigeria and Uganda. Local health authorities in collaboration with UNICEF trained community-based providers on the use and administration of RAS. Community-based providers enrolled children under 5 years of age with fever and danger signs according to the national integrated community case management (iCCM, in the case of CHW) or integrated management of childhood illness (IMCI, in the case of PHC) guidelines. All eligible children were followed up 1 month after enrolment by dedicated research staff.
The study period covered approximately 10 months before the implementation of RAS (pre-roll-out: May 2018–February 2019) and 17 months thereafter (post-roll-out: March 2019–August 2020).
Study setting
The study was conducted in three districts in Uganda (Kole, Oyam and Kwania), three local government areas (LGA) in Adamawa State in Nigeria (Fufore, Song, Mayo-Belwa) and three health zones in the DRC (Kenge, Kingandu, Ipamu). The overall study population was 2.5 million of which 476 000 (19%) were children under 5 years. Further details are provided elsewhere.17
The public health system in the study areas consisted of several levels of community-based providers and at least one level of RHFs (table 1). Community-based providers implementing prereferral RAS included CHWs and PHCs. In the study health zones in DRC, CHWs were located in communities with no formal provider within a distance of 5 km. In the study of LGAs in Nigeria, CHWs were located in communities that were more than 5 km away from a public health facility, or the community was hard to reach due to bad road conditions or natural barriers like rivers or mountains. In the study districts in Uganda, there were two CHWs per village irrespective of the presence of other formal healthcare providers.
Table 1.
Local names and numbers of community health worker, primary health centres and referral health facilities in 2018, by country
| DRC | Nigeria | Uganda | ||||
| Name | N | Name | N | Name | N | |
| Community health worker | Site de Soins Communautaire (Community Care Site) | 42 | Community oriented resource person | 500 | Village health team | 5100 |
| Primary health centre |
Poste de Santé (Health Post) Centre de Santé (Health Centre) |
152 | Health post Primary health centre |
77 | Health centre II | 30 |
| Referral health facility |
Centre de Santé de Référence (Referral Health Centre) and Hôpital Général de Référence (General Referral Hospital) |
19 | Cottage hospital | 3 | Health centre III Health centre IV hospital |
20 |
DRC, Democratic Republic of the Congo.
According to national policies, community-based providers should refer severely ill children to the nearest higher level healthcare provider. In the case of CHWs, these are often PHCs (eg, in Uganda, a Village Health Team may refer a child to a Health Centre II). During RAS roll-out, community-based providers who were trained in the administration of prereferral RAS were instructed to refer children immediately to a designated RHF. The importance of speedy referral was emphasised in the training. Simultaneously, UNICEF implemented behaviour change communication campaigns that informed caregivers about the benefits of RAS and the importance of referral completion on billboards, posters and leaflets. Additionally, CHWs conducted home visits, community dialogues were held, and radio messages were aired. In Uganda and DRC, there were no interventions in place to support referral to an RHF. In Nigeria, an Emergency Transport System for severely ill children was introduced in July 2019, shortly after the implementation of RAS. The Emergency Transport System provided free transport to referral facilities.
Data collection
Local research partners established a patient surveillance system for the enrolment and follow-up of children with suspected severe malaria presenting to CHWs or PHCs in DRC and Nigeria and to CHWs in Uganda. Children were enrolled if they were under 5 years, had a history of fever and at least one danger sign for which RAS is indicated as per national iCCM and IMCI guidelines. On enrolment, community-based providers conducted a malaria rapid diagnostic test (mRDT) for study purposes. After referring the child to a higher level provider, the enrolling provider reported the case to the local study office where eligible children were recorded in a central case register. In Nigeria and Uganda, this contact happened via telephone. In DRC, community-based providers were regularly visited by CARAMAL research staff to record provisionally enrolled children in the central case register. Dedicated CARAMAL staff were stationed in RHFs in the study area to record the postreferral management of referred children who were admitted for treatment. CARAMAL research staff scheduled an interview 28 days after enrolment. Deceased children were followed up 2 months after enrolment to respect the mourning period. At the follow-up visit, caregivers or other knowledgeable family members provided information on signs and symptoms, treatment-seeking history, diagnosis and treatment during the child’s illness episode. The interviewer also recorded the geocoordinates of the home location of the child. Additional information on the child’s condition and administered treatment on enrolment was obtained from the enrolling provider. In Uganda and DRC, the reason for not administering RAS was collected from a subsample of children in the post-RAS phase.
Data were collected electronically on tablets with Open Data Kit (ODK) Collect (https://opendatakit.org/). During admission, CARAMAL research staff at RHFs recorded information on case management on paper forms before entering it into ODK Collect. The password-protected ODK Aggregate server was hosted at the Swiss Tropical and Public Health Institute in Switzerland.
Outcomes and explanatory variables
The primary outcome of this analysis was referral completion, defined as a child being brought to one of the designated RHFs at any stage during the treatment seeking process, after seeing a community-based provider, as reported by the caregiver or by CARAMAL staff stationed at the RHF. Secondary outcomes included going to any other public provider after seeing a community-based provider and going to a provider outside of the public health system after seeing a community-based provider. A further secondary outcome was timely referral completion defined as reaching an RHF on the same or next day after enrolment. The number of days between enrolment and reaching an RHF was either calculated as the difference between the enrolment date and the date of admission at an RHF or obtained from the treatment-seeking narrative as reported by the caregiver during follow-up.
The main exposures of interest were the RAS implementation phases (pre-roll-out vs post-roll-out) and prereferral RAS administration in the post-roll-out phase. To assess these effects, we grouped children into three study groups: (1) pre-RAS, (2) RAS non-users in the post-RAS phase and (3) RAS users in the post-RAS phase. We accounted for age and gender of the child and the interviewed caregiver and the child’s place of residence (health zone/LGA/district). The severity as perceived by the caregiver and the presence of a danger sign involving the central nervous system (CNS; convulsions, unusually sleepy or unconscious) were proxies for disease severity. Additional factors considered included the mRDT result at enrolment, the type of community-based provider (CHW vs PHC), the season, day of enrolment (workday vs weekend), enrolment during the COVID-19 pandemic (1 April 2020 or later), treatment-seeking delay between the onset of illness and going to the enrolling provider, the means of transport to enrolling provider, travel time between home and nearest RHF, and the administration of home treatment before presentation to enrolling provider.
To calculate the travel time between the home of the child and the nearest RHF, we used the Malaria Atlas Project friction surface 2015 with a 100 m × 100 m resolution.20 The calculation was done in RStudio21 using the method described by Bertozzi-Villa.22 Geolocations of RHFs were obtained from the CARAMAL Health Care Provider Surveys for RHFs within the study area and from Maina et al23 for RHFs surrounding the study area. All geolocations were verified using Google Maps24 and official government sources, where applicable.25 26
Statistical analysis
For each country, we used a logistic regression model to estimate the association of the implementation of RAS and RAS administration with referral completion. For children completing referral, we used logistic regression models to estimate the association between RAS implementation and administration and referral timeliness. All models included the enrolling PHC or CHW as random effects to account for clustering at that level. Exposure variables were selected based on rational grounds prior to analysis and included in the final model irrespective of their level of significance. Variables to test for interactions were chosen a priori. The interactions included in the final model were significant at the 5% level. We did not account for time trends other than the effects of the implementation of RAS, the rainy season and the COVID-19 pandemic. However, we conducted sensitivity analyses to test whether the effect of RAS implementation and administration on referral completion was sensitive to the effects of time. Observations with missing values for referral completion were excluded from the regression analysis. Statistical analyses were performed in Stata SE V.16.1.27
Ethics
The community-based providers informed caregivers about the CARAMAL study prior to enrolment and caregivers gave oral preconsent to be contacted for a follow-up interview. We obtained written consent from all caregivers of provisionally enrolled children either at the RHF or before the follow-up interview 28 days after enrolment.
Patient and public involvement
Patients and the public were not involved in the development of the research question and outcome measures, the design and conduct of the study.
Results
Study population
Between May 2018 and August 2020, community-based providers provisionally enrolled 8365 children (online supplemental file 1). The study team successfully followed up 7593 (91%) children and obtained informed consent. Of those, 6505 (78%) children fulfilled all inclusion criteria, of which 6449 had a known referral status (77%). The majority of included children were enrolled after the implementation of RAS (n=4396, 68%). Particularly in DRC, the sample receiving RAS in the post-RAS phase was substantially larger (N=1548) compared with the other two study groups (pre-RAS: N=368; post-RAS non-users: N=188) (table 2). In Nigeria, the numbers in the study groups were balanced. In Uganda, the number of children enrolled in the pre-RAS phase (N=1479) was comparable to the number of children receiving RAS in the post-RAS phase (N=1631); however, the number of children not receiving RAS in the post-RAS phase was substantially smaller (N=635).
Table 2.
Study population characteristics by country and study group
| Background characteristic | |||||||||
| DRC | Nigeria | Uganda | |||||||
| Pre-RAS | Post-RAS | Pre-RAS | Post-RAS | Pre-RAS | Post-RAS | ||||
| No RAS | RAS | No RAS | RAS | No RAS | RAS | ||||
| N | 368% | 188% | 1548% | 206% | 183% | 211% | 1479% | 635% | 1631% |
| Female | 47.0 | 45.7 | 46.8 | 38.8 | 35.5 | 43.1 | 46.0 | 48.2 | 46.9 |
| Age (years) | |||||||||
| 0 | 20.4 | 23.4 | 19.2 | 12.6 | 9.8 | 12.8 | 17.4 | 20.2 | 17.7 |
| 1 | 31.5 | 34.6 | 28.6 | 25.7 | 29.5 | 26.5 | 27.7 | 29.4 | 29.5 |
| 2 | 21.5 | 21.8 | 22.4 | 27.7 | 29.0 | 28.4 | 23.9 | 22.8 | 24.0 |
| 3 | 12.2 | 10.6 | 15.7 | 21.8 | 16.9 | 20.4 | 18.7 | 18.6 | 18.4 |
| 4 | 14.4 | 9.6 | 14.1 | 12.1 | 14.8 | 11.8 | 12.3 | 9.0 | 10.4 |
| Study area (DRC/Nigeria/Uganda) | |||||||||
| Ipamu/Mayo-Belwa/Kole | 22.6 | 23.9 | 36.4 | 27.7 | 49.2 | 49.3 | 65.2 | 60.6 | 24.5 |
| Kenge/Fufore/Oyam | 42.7 | 37.2 | 34.8 | 57.3 | 37.7 | 27.5 | 16.5 | 28.5 | 34.7 |
| Kingandu/Song/Kwania | 34.8 | 38.8 | 28.7 | 15.0 | 13.1 | 23.2 | 18.3 | 10.9 | 40.8 |
| Danger signs | |||||||||
| Unusually sleepy or unconscious | 44.0 | 28.2 | 23.8 | 68.0 | 66.7 | 61.6 | 65.0 | 87.6 | 91.7 |
| Not able to drink or feed | 61.1 | 71.8 | 47.9 | 70.4 | 60.7 | 54.5 | 62.5 | 82.5 | 78.1 |
| Vomiting everything | 14.1 | 10.6 | 26.0 | 82.5 | 71.6 | 56.9 | 78.3 | 72.1 | 65.2 |
| Convulsions | 56.0 | 45.7 | 61.7 | 55.3 | 64.5 | 82.9 | 39.8 | 25.5 | 52.0 |
| CNS involvement* | 70.9 | 59.0 | 68.1 | 81.1 | 79.8 | 89.1 | 78.6 | 90.9 | 96.9 |
| Positive malaria test at enrolment | 82.3 | 92.0 | 98.7 | 95.1 | 95.1 | 93.8 | 97.4 | 98.3 | 99.4 |
| Enrolment location | |||||||||
| Community health worker | 6.0 | 2.1 | 4.5 | 71.8 | 41.5 | 43.1 | 100.0 | 100.0 | 100.0 |
| Primary health centre | 94.0 | 97.9 | 95.5 | 28.2 | 58.5 | 56.9 | 0.0 | 0.0 | 0.0 |
| Enrolled during rainy season† | 90.5 | 39.9 | 47.6 | 64.6 | 78.7 | 80.1 | 61.7 | 91.2 | 52.9 |
| Enrolled on a workday | 78.8 | 68.6 | 74.8 | 78.2 | 85.8 | 82.9 | 74.9 | 76.7 | 73.0 |
| Enrolled during COVID-19 pandemic | 0.0 | 8.0 | 29.1 | 0.0 | 13.1 | 44.1 | 0.0 | 9.3 | 22.3 |
| Delay to enrolling provider | |||||||||
| 0–1 days | 26.1 | 27.7 | 33.5 | 33.0 | 31.1 | 34.1 | 48.5 | 56.7 | 64.8 |
| >1 day | 60.1 | 68.1 | 63.8 | 47.1 | 57.4 | 59.2 | 49.8 | 42.0 | 34.4 |
| Missing | 13.9 | 4.3 | 2.7 | 19.9 | 11.5 | 6.6 | 1.7 | 1.3 | 0.8 |
| Transport to enrolling provider | |||||||||
| No vehicle | 72.6 | 79.3 | 82.4 | 56.8 | 45.4 | 47.9 | 91.8 | 91.8 | 93.3 |
| Vehicle | 12.5 | 15.4 | 15.0 | 25.7 | 43.2 | 46.0 | 7.7 | 7.2 | 6.1 |
| Missing | 14.9 | 5.3 | 2.6 | 17.5 | 11.5 | 6.2 | 0.5 | 0.9 | 0.6 |
| Time to referral health facility (min) | |||||||||
| 0–<15 | 34.0 | 39.9 | 39.1 | 18.0 | 18.0 | 16.1 | 59.6 | 57.8 | 45.9 |
| 15–<30 | 17.7 | 13.3 | 15.0 | 12.1 | 15.3 | 16.1 | 34.0 | 37.6 | 43.8 |
| 30–<60 | 20.9 | 11.2 | 15.4 | 19.9 | 26.8 | 28.0 | 5.0 | 4.6 | 10.2 |
| ≥60 | 19.3 | 6.9 | 9.3 | 49.5 | 37.2 | 36.5 | 0.1 | 0.0 | 0.0 |
| Missing | 8.2 | 28.7 | 21.2 | 0.5 | 2.7 | 3.3 | 1.2 | 0.0 | 0.0 |
| Child perceived fatally ill | 29.9 | 31.9 | 24.2 | 29.6 | 22.4 | 28.0 | 43.3 | 43.3 | 45.5 |
| Missing | 0.3 | 0.5 | 1.0 | 0.0 | 1.1 | 1.4 | 0.5 | 0.0 | 0.2 |
| Home treatment | 65.2 | 76.1 | 59.9 | 24.8 | 47.5 | 39.3 | 15.2 | 13.9 | 9.9 |
| Missing | 0.0 | 0.0 | 0.0 | 0.0 | 1.1 | 0.9 | 0.0 | 0.0 | 0.0 |
*Convulsions, unusually sleepy or unconscious.
†DRC: October–April; Nigeria: May–October; Uganda: April–October.
CNS, central nervous system; RAS, rectal artesunate.
bmjgh-2021-008346supp001.pdf (226KB, pdf)
In Uganda, the reason for not administering RAS was recorded for 300 children in the post-RAS phase. The single most important reason was stock-out of RAS (87%). In some cases, the CHWs did not administer RAS because they kept the suppository for more severe cases (9%). In DRC, where most children received RAS in the post-RAS phase, the subsample was too small (N=18) to make valid assumptions about the reasons for not administering RAS.
Within each country, the age and gender distribution were similar among children enrolled in the pre-RAS phase, RAS users and non-users in the post-RAS phase. In Nigeria, there were fewer children under 1 year than in the other countries. Danger signs involving the CNS were most common in Uganda followed by Nigeria and DRC. In all countries, more than 90% of eligible children tested positive for malaria at enrolment. In DRC, eligible children were almost exclusively enrolled by PHCs (95%), while in Uganda, all children were enrolled by CHWs. In Nigeria, a higher proportion of children was enrolled by CHWs in the pre-RAS phase (72%) compared with the post-RAS phase (42%). Enrolment in PHCs started later because of a strike by PHC health workers at the beginning of the study. In the post-RAS phase, between 19% (Uganda) and 30% (Nigeria) of the children were enrolled during the COVID-19 pandemic. In all countries, the proportion of children receiving RAS was higher during the COVID-19 pandemic.
Referral completion
In DRC, overall, 1408 (67%) children completed referral to a designated RHF. Few children went to another public provider (6%) or to any other provider (3%). In Nigeria, 287 (48%) children completed referral to a designated RHF. An additional 21% of the children went to another public provider and only 4% of the children went to a non-public provider. In Uganda, 2170 (58%) of the children completed referral to the designated RHFs. Going to another public provider was infrequent (9%), but 29% of the children in Uganda were brought to providers outside of the public health system.
In all countries, referral completion to an RHF was slightly lower among RAS users compared with RAS non-users in the post-RAS phase (figure 1, table 3 and table 4). In DRC and Uganda, referral completion in the post-RAS phase was comparable to referral completion in the pre-RAS phase. Meanwhile, in Nigeria, referral completion increased from the pre-RAS to the post-RAS phase. The difference between the pre-RAS and post-RAS phase was mainly driven by PHC enrolments being substantially more likely to complete referral to an RHF than CHW enrolments, in combination with an increase in the number of PHC enrolments in the post-RAS phase (figure 2).
Figure 1.
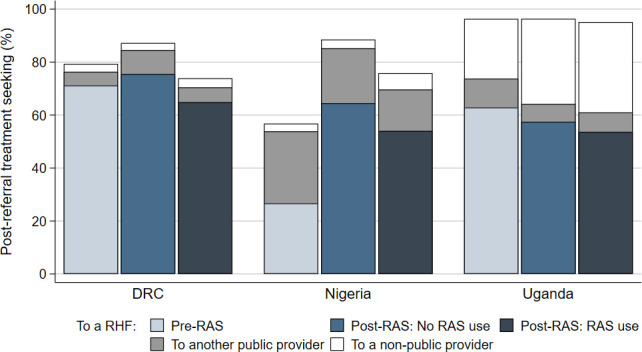
Post-referral treatment seeking from a referral health facility (RHF), from other public providers and from non-public providers, for children enrolled before the implementation of rectal artesunate (pre-RAS), and after the implementation of RAS (post-RAS) for RAS non-users and RAS users, by country.
Table 3.
Estimated associations between selected factors and referral completion in DRC and Uganda
| DRC | Uganda | |||||||||
| N§ | Referral completion (%) | Adjusted OR* | 95% CI | P value | N§ | Referral completion (%) | Adjusted OR* | 95% CI | P value | |
| All | 2104 | 66.9 | 3745 | 57.9 | ||||||
| Study group | ||||||||||
| Pre-RAS | 368 | 71.2 | Ref. | 1479 | 62.9 | Ref. | ||||
| Post-RAS | ||||||||||
| No RAS use | 188 | 75.5 | 0.34 | 0.18 to 0.66 | 0.001 | 635 | 57.5 | 0.80 | 0.63 to 1.01 | 0.06 |
| RAS use | 1548 | 64.9 | 0.48 | 0.30 to 0.77 | 0.002 | 1631 | 53.6 | 0.72 | 0.58 to 0.88 | 0.002 |
| Enrolment location | ||||||||||
| CHW | 96 | 43.8 | Ref. | 3745 | 57.9 | |||||
| PHC | 2008 | 68.0 | 4.85 | 1.22 to 19.25 | 0.02 | 0 | NA | NA | ||
| CNS danger sign† | ||||||||||
| No | 678 | 78.5 | Ref. | 425 | 62.8 | Ref. | ||||
| Yes | 1426 | 61.4 | 0.58 | 0.41 to 0.82 | 0.002 | 3320 | 57.3 | 0.80 | 0.61 to 1.04 | 0.09 |
| Enrolled during rainy season‡ | ||||||||||
| No | 959 | 69.3 | Ref. | 1392 | 55.5 | Ref. | ||||
| Yes | 1145 | 64.9 | 0.77 | 0.56 to 1.07 | 0.12 | 2353 | 59.4 | 1.15 | 0.97 to 1.38 | 0.11 |
| Enrolled on a workday | ||||||||||
| No | 527 | 69.3 | Ref. | 959 | 53.8 | Ref. | ||||
| Yes | 1577 | 66.1 | 0.91 | 0.65 to 1.29 | 0.60 | 2786 | 59.4 | 1.19 | 1.00 to 1.41 | 0.05 |
| Enrolled during COVID-19 pandemic | ||||||||||
| No | 1638 | 66.7 | Ref. | 3323 | 57.8 | Ref. | ||||
| Yes | 466 | 67.8 | 1.15 | 0.78 to 1.70 | 0.48 | 422 | 58.8 | 0.90 | 0.69 to 1.19 | 0.48 |
| Delay to enrolling provider | ||||||||||
| 0–1 days | 667 | 60.3 | Ref. | 2134 | 56.8 | Ref. | ||||
| >1 day | 1336 | 68.5 | 1.07 | 0.77 to 1.49 | 0.69 | 1565 | 59.1 | 1.14 | 0.97 to 1.34 | 0.12 |
| Missing | 101 | 90.1 | 3.33 | 0.86 to 12.83 | 0.08 | 46 | 71.7 | 1.11 | 0.43 to 2.86 | 0.83 |
| Transport to enrolling provider | ||||||||||
| No vehicle | 1691 | 63.0 | Ref. | 3462 | 57.8 | Ref. | ||||
| Vehicle | 307 | 81.8 | 1.08 | 0.66 to 1.79 | 0.75 | 260 | 57.7 | 1.06 | 0.78 to 1.42 | 0.72 |
| Missing | 106 | 86.8 | 4.80 | 1.37 to 16.84 | 0.01 | 23 | 78.3 | 3.31 | 0.78 to 14.06 | 0.11 |
| Time to referral health facility (min) | ||||||||||
| 0–<15 | 806 | 82.9 | Ref. | 1998 | 67.5 | Ref. | ||||
| 15–<30 | 322 | 72.0 | 1.12 | 0.65 to 1.95 | 0.68 | 1457 | 47.5 | 0.72 | 0.58 to 0.89 | 0.003 |
| 30–<60 | 336 | 67.9 | 0.80 | 0.46 to 1.40 | 0.44 | 270 | 42.6 | 0.55 | 0.39 to 0.79 | 0.001 |
| ≥60 | 228 | 35.1 | 0.46 | 0.24 to 0.89 | 0.02 | 2 | 0.0 | NA | ||
| Missing | 412 | 48.5 | 0.87 | 0.52 to 1.46 | 0.60 | 18 | 77.8 | 1.88 | 0.53 to 6.67 | 0.33 |
| Perceived severity | ||||||||||
| Not fatal | 1542 | 63.5 | Ref. | 2078 | 56.8 | Ref. | ||||
| Fatal | 545 | 75.8 | 1.86 | 1.28 to 2.71 | 0.001 | 1657 | 59.4 | 1.11 | 0.94 to 1.30 | 0.21 |
| Missing | 17 | 94.1 | 16.14 | 0.61 to 429.60 | 0.10 | 10 | 50.0 | 0.80 | 0.20 to 3.19 | 0.75 |
| Home treatment | ||||||||||
| No | 793 | 56.1 | Ref. | 3271 | 57.4 | Ref. | ||||
| Yes | 1311 | 73.5 | 1.43 | 1.03 to 1.99 | 0.03 | 474 | 62.0 | 1.10 | 0.87 to 1.39 | 0.41 |
| Missing | 0 | NA | NA | 0 | NA | NA | ||||
*OR additionally adjusted for child sex, child age, caregiver sex, caregiver age, location of residence (health zone, district) and malaria test result.
†Danger signs involving the CNS: convulsions, unusually sleepy or unconscious.
‡DRC: October–April; Uganda: April–October.
§Denominator.
CHW, community health worker; CNS, central nervous system; DRC, Democratic Republic of the Congo; PHC, primary health centre; RAS, rectal artesunate.
Table 4.
Estimated associations between selected factors and referral completion in Nigeria
| Nigeria | |||||
| N* | Referral completion (%) | Adjusted OR** | 95% CI | P value | |
| All | 600 | 47.8 | |||
| Study group by enrolment location | |||||
| CHW | |||||
| Pre-RAS | 148 | 6.1 | Ref. | ||
| Post-RAS | |||||
| No RAS use | 76 | 21.1 | 3.97 | 1.07 to 14.75 | 0.04 |
| RAS use | 91 | 26.4 | 9.95 | 2.71 to 36.58 | <0.001 |
| PHC† | |||||
| Pre-RAS | 58 | 79.3 | Ref. | ||
| Post-RAS | |||||
| No RAS use | 107 | 95.3 | 35.09 | 6.52 to 188.75 | <0.001 |
| RAS use | 120 | 75.0 | 6.45 | 1.62 to 25.67 | 0.01 |
| Enrolment location | |||||
| CHW | 315 | 15.6 | Ref. | ||
| PHC† | 285 | 83.5 | 19.79 | 2.97 to 131.71 | 0.002 |
| CNS involvement‡ | |||||
| No | 99 | 33.3 | Ref. | ||
| Yes | 501 | 50.7 | 0.48 | 0.17 to 1.38 | 0.17 |
| Enrolled during rainy season§ | |||||
| No | 154 | 50.6 | Ref. | ||
| Yes | 446 | 46.9 | 0.70 | 0.29 to 1.67 | 0.42 |
| Enrolled on a workday | |||||
| No | 107 | 30.8 | Ref. | ||
| Yes | 493 | 51.5 | 0.74 | 0.31 to 1.76 | 0.50 |
| Enrolled during COVID-19 pandemic | |||||
| No | 483 | 49.5 | Ref. | ||
| Yes | 117 | 41.0 | 0.09 | 0.03 to 0.26 | <0.001 |
| Delay to enrolling provider | |||||
| 0–1 days | 197 | 45.7 | Ref. | ||
| >1 day | 327 | 47.1 | 1.64 | 0.77 to 3.52 | 0.20 |
| Missing | 76 | 56.6 | 3.53 | 1.03 to 12.09 | 0.04 |
| Transport to enrolling provider | |||||
| No vehicle/missing¶ | 371 | 33.7 | Ref. | ||
| Vehicle | 229 | 70.7 | 0.82 | 0.32 to 2.10 | 0.68 |
| Time to referral health facility (min) | |||||
| 0–<15 | 104 | 81.7 | Ref. | ||
| 15–<30 | 87 | 71.3 | 0.48 | 0.13 to 1.73 | 0.26 |
| 30–<60 | 149 | 55.7 | 0.23 | 0.07 to 0.77 | 0.02 |
| ≥60 | 247 | 19.4 | 0.06 | 0.02 to 0.22 | <0.001 |
| Missing | 13 | 69.2 | 0.13 | 0.01 to 1.84 | 0.13 |
| Perceived severity | |||||
| Not fatal | 434 | 48.8 | Ref. | ||
| Fatal | 161 | 44.7 | 0.63 | 0.30 to 1.32 | 0.23 |
| Missing | 5 | 60.0 | 1.23 | 0.01 to 153.00 | 0.93 |
| Home treatment | |||||
| No/missing¶ | 379 | 43.8 | Ref. | ||
| Yes | 221 | 54.8 | 1.08 | 0.54 to 2.16 | 0.82 |
*OR additionally adjusted for child sex, child age, caregiver sex, caregiver age, location of residence (LGA) and malaria test result.
†Adjusted for LGA. OR shown for Mayo-Belwa. ORs for Fufore and Song are higher.
‡Danger signs involving the CNS: convulsions, unusually sleepy or unconscious.
§May–October.
¶Observations with missing values added to reference category because no meaningful OR could be computed due to the data structure (missing values in other covariates).
**Denominator.
CHW, community health worker; CNS, central nervous system; PHC, primary health centre; RAS, rectal artesunate.
Figure 2.
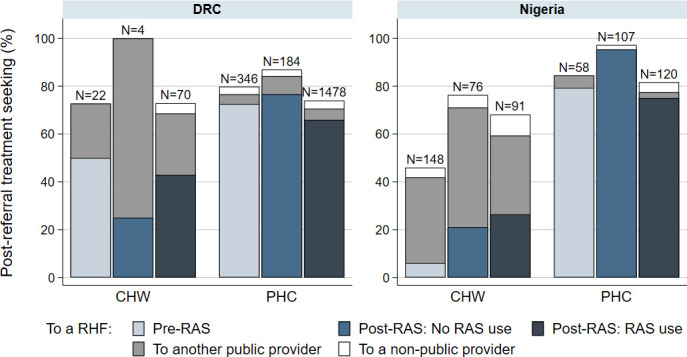
Post-referral treatment seeking from a referral health facility (RHF), from other public providers and from non-public providers for children enrolled before the implementation of rectal artesunate (pre-RAS), and after the implementation of RAS (post-RAS) for RAS non-users and RAS users, by enrolment location, in DRC and Nigeria. CHW, community health worker; DRC, Democratic Republic of the Congo; PHC, primary health centre.
In both DRC and Nigeria, going to any other provider than an RHF was uncommon for PHC enrolments (figure 2); but seemed more common for CHW enrolments who frequently went to a public provider other than a designated RHF. In Uganda, children rarely went to another public provider but tended instead to go to a private provider, compensating the lower referral completion to an RHF in the two post-RAS study groups (figure 1).
In DRC and Uganda, referral completion was lower in the post-RAS phase compared with the pre-RAS phase after adjusting for other factors, irrespective of whether children had received RAS (table 3). The opposite occurred in Nigeria, where referral completion in the post-RAS phase irrespective of RAS use was higher compared with the pre-RAS phase (table 4).
When taking RAS non-users in the post-RAS phase, as a reference, the odds of completing referral did not significantly differ between children not receiving RAS and children receiving RAS in DRC (adjusted OR (aOR)=1.39, 95% CI 0.82 to 2.35) and Uganda (aOR=0.90, 95% CI 0.70 to 1.16). In Nigeria, the same was true for children enrolled by a CHW (aOR=2.51, 95% CI 0.76 to 8.24); however, among children enrolled by a PHC, those who had received RAS were significantly less likely to complete referral than those not receiving RAS in the post-RAS phase (aOR=0.18, 95% CI 0.05 to 0.71).
Besides RAS implementation and RAS administration, we found other factors significantly associated with referral completion. In all countries, increasing travel time to the RHF had a negative effect on referral completion. In DRC and Nigeria, children were more likely to complete referral if they were referred by a PHC compared with a CHW (not applicable in Uganda). Other factors that had a positive effect on referral completion included being perceived fatally ill by the caregiver (DRC), having received home treatment (DRC) or being enrolled on a workday (Uganda). Factors with a negative effect on referral completion included having a CNS danger sign (DRC) or being enrolled during the COVID-19 pandemic (Nigeria).
The adjusted ORs did not differ substantially from the unadjusted estimates except for the effect of the malaria test result in DRC and being enrolled by a PHC in Nigeria. Unadjusted estimates are provided in online supplemental file 2.
The sensitivity analyses showed that the effects of RAS implementation and administration were sensitive to assumptions about the underlying time trends in DRC and Uganda. If a linear time trend for the outcome variable was additionally included, then in DRC, there was no significant difference in referral completion between the pre-RAS and post-RAS phases if the model accounted for time in months. In Uganda, referral completion significantly increased with the implementation of RAS but decreased at a higher rate thereafter. In Nigeria, the results did not change if the model was adjusted for time in trimesters. However, the data were not sufficient to estimate time trends and the contributions of time and RAS could not be distinguished well.
Referral timeliness
Of the 3865 children that completed referral to an RHF, data on the timeliness of referral completion weere available for 3598 children (93%) (online supplemental file 1). Timely referral completion to an RHF on the same or next day after seeing a community-based provider was 76% in DRC, 86% in Nigeria and 92% in Uganda. In all countries, timely referral was highest among children receiving RAS in the post-RAS phase; however, the differences between study groups were rather small (figure 3). After adjusting for other factors, children in Uganda receiving RAS in the post-RAS phase were significantly more likely to complete referral on time than children in the pre-RAS phase (OR=1.81, 95% CI 1.17 to 2.79). Other comparisons between study groups were not significant in any of the countries. Complete tables with denominators and regression results are presented in online supplemental file 3.
Figure 3.
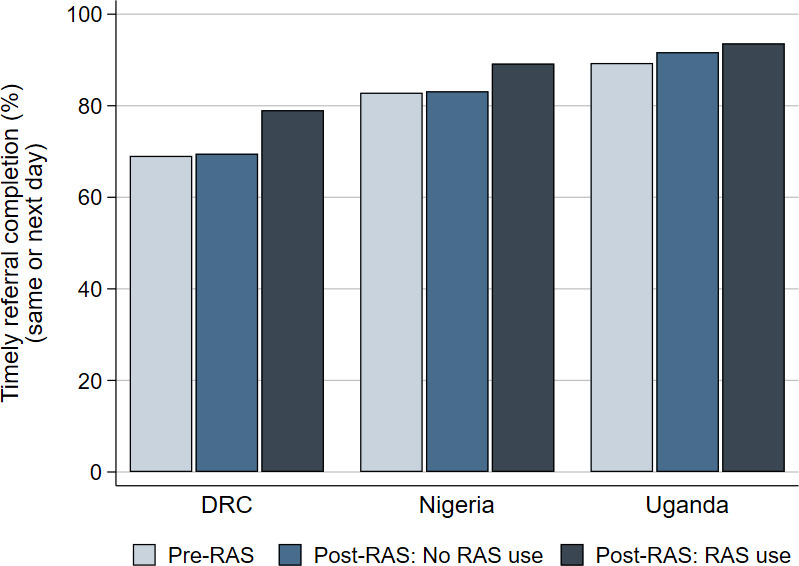
Timely referral completion on the same or next day after referral by a community-based provider, from all patients completing referral to a referral health facility, for children enrolled before the implementation of rectal artesunate (pre-RAS), and after the implementation of RAS (post-RAS) for RAS non-users and RAS users, by country. DRC, Democratic Republic of the Congo.
Discussion
After the administration of prereferral RAS, current guidelines recommend referral completion to a health facility where intramuscular or intravenous treatment is available.5 Findings from previous studies on RAS and referral completion were mostly reassuring; however, the effect of RAS on referral completion was either not adjusted for other factors6 or did not compare RAS users to non-users.8–11 13 Additionally, none of the studies took into consideration that the nearest health facility might not have the capacity of administering parenteral antimalarial treatment. The CARAMAL Project for the first time provides adjusted estimates of the effect of RAS on referral completion to an RHF at which, according to national policy, appropriate postreferral treatment is available. Postreferral treatment with an injectable antimalarial followed by a full course of ACT ensures that children are effectively treated for severe malaria, and RAS (and parenteral artemisinin) is not applied as a monotherapy, thereby reducing the risk of the development and selection of artemisinin-resistant parasites.5
In the context of the large-scale implementation of RAS, our study found a negative association between RAS and referral completion. In DRC and Uganda, referral completion was lower in the post-roll-out phase compared with the pre-roll-out phase. In Nigeria, the opposite was the case. However, in Nigeria, children who were administered RAS in a PHC were less likely to complete referral to an RHF than children who did not receive RAS. Referral completion by children attending a PHC in Nigeria and DRC was consistently higher when compared with the referral completion of children attending a CHW (not applicable in Uganda). In all countries, children living a greater distance from an RHF (measured in the time it would take to travel to the facility) were significantly less likely to complete referral than those living in the vicinity of the facility. In all countries, the majority of children who completed referral to an RHF did so on the same or next day after being referred.
The findings of this study raise legitimate concerns that the roll-out of RAS may lead to lower referral completion in children who were administered prereferral RAS. This was most notable among children enrolled in PHCs in Nigeria, and also the results from DRC may suggest a negative association between RAS and referral completion. In DRC, the comparatively small number of RAS non-users limited the validity of the comparison between RAS users and non-users in the post-roll-out phase. Therefore, the negative association between RAS implementation and referral completion may indicate that children who received RAS were at higher risk of not completing referral; even though other causal links cannot be excluded. The reason for a decrease in referral completion after RAS use is most likely the rapid improvement of children after RAS administration, a result of the fast reduction of the parasite to blood concentration and the drug’s antipyretic effect.28–31 Considering that treatment seeking is often delayed due to lack of transport and money,32–34 the child’s condition may have improved in the meantime. In such a situation, a caregiver is likely to balance between the referral recommendation and priorities at home, in addition to expenses for transport and those that would be incurred at the RHF.3 Obviously, such a practice raises the concern that children may not receive the appropriate postreferral treatment with potentially fatal consequences.
Measures to improve referral completion must take into consideration the actual capacity of RHFs to provide appropriate case management for severe malaria. As opposed to previous studies, this study considered referral to be completed only if the patient arrived at an RHF with the capacity to manage severe malaria cases. However, an analysis of the quality of care at RHFs in the CARAMAL study areas found that the treatment of children with severe malaria was often inadequate.19 Meanwhile, some children sought postreferral treatment from a lower level public or from a non-public provider. Considering that referral completion did not improve the health outcome of children enrolled in the CARAMAL study in DRC and Uganda,18 sufficient treatment may have been provided by non-RHF providers.35 It is also possible that less severely sick children recover faster and are hence less likely to be brought to an RHF after prereferral RAS treatment. Therefore, children with suspected severe malaria first attending a community-based provider may not always require treatment at the level of RHFs. However, recognising such children with a more moderate form of severe malaria remains a challenge.
The finding that a substantial proportion of children (33%–52%) did not complete referral emphasises the need to address referral-related barriers at all levels: for example, sensitising caregivers, properly training community-based healthcare providers, facilitating access to and increasing trust in RHFs. Such a package of supportive interventions accompanying the roll-out of RAS in Zambia has previously been shown to reduce the mortality of children with severe malaria.36 In a trial conducted by Gomes et al,1 RAS had a protective effect in the context of high completion of referral (though not necessarily to a RHF in the African sites). In the CARAMAL study in Nigeria, the implementation of an Emergency Transport System most likely increased referral completion, and referral completion significantly improved the health outcomes of children with suspected severe malaria in this country. The available evidence strongly indicates that community-based programmes should always be accompanied by measures strengthening referral.
Irrespective of the effort to strengthen referral, it is important to acknowledge that some caregivers to children may delay or not complete referral. Therefore, the training materials and referral guidelines for community-based providers need to emphasise the importance of a close follow-up of severely sick children, if necessary at their home. If referral cannot be completed or is refused by the caregiver, the treatment with prereferral drugs should be continued. Such a recommendation already exists for RAS until oral treatment with an ACT is tolerated.2 Similarly, the WHO recommends that CHWs continue administering amoxicillin to children with pneumonia with chest in-drawing if referral is not feasible.37 Sufficient stocks of prereferral drugs are, therefore, essential for providing adequate care to children unable to complete referral.
Referral completion to an RHF was a problem particularly among CHW enrolments in DRC and Nigeria. Unlike in Uganda, CHWs in these two countries are placed in especially hard-to-reach areas. In our analyses, we accounted for difficulties in geographical access to an RHF (availability of transport and travel time to RHF), but we may have missed additional barriers to accessing an RHF. Another explanation could be that children attending a PHC are more severely ill than children attending a CHW.38 Caregivers may be more likely to make increased efforts to reach the first provider as well as to complete referral if the child is more severely ill. Meanwhile, irrespective of the reasons, an active follow-up of children at home seems to be particularly important in the most hard-to-reach places where referral completion is the least likely. As community programmes continue to be the preferred approach to extend health services to remote communities, the challenges associated with these hard-to-reach places need to be acknowledged in referral and treatment guidelines and the promotion of best practices.
Even though treatment-seeking practices including referral are highly contextual, some recommendations based on the results of this study can be generalised to other settings, that is, programmes implementing RAS need to consider the potential effects on referral completion. More generally, community-based programmes should be supported by measures facilitating referral completion and provide a back-up option for those children who fail to complete referral. Alternative treatment options are particularly important in hard-to-reach places.
This study has several strengths. First, it covered three different contexts with varying intensities of malaria transmission, access to healthcare and differences in the implementation of iCCM/IMCI policies.17 Second, the study was community based and enrolled a large number of children with severe febrile illness from remote communities. Large community-based studies in far-to-reach places are mostly cross-sectional surveys that rarely capture severe illness episodes because of their low incidence and always exclude children who are deceased, resulting in a lack of understanding of severe illnesses at community level.39 Third, the study achieved a high follow-up rate, thereby reducing the risk of selection bias.
This study comes with several limitations. First, the low enrolment numbers of children not receiving RAS in the post-roll-out phase did not allow a clear conclusion about the association between RAS administration and referral completion in DRC. Second, the enrolment strategy in Nigeria and Uganda may have introduced selection bias. The notification of enrolments from the enrolling provider to the local study office depended on a contact via mobile phone. Thus, the study may have excluded systematically children in the most remote places because of unstable network coverage. It is likely that these children would have also been the least likely to complete referral leading to an overestimation of referral completion in our study. Third, the observational design and the retrospective data collection 28 days after enrolment did not allow for direct causal inferences.
Conclusion
Providing prompt and appropriate healthcare to severely sick children in remote communities remains a challenge. Children in hard-to-reach places are the least likely to complete referral after seeing a community-based provider. In addition, referral completion may further be negatively affected by the administration of RAS. To ensure that community-based programmes are effectively implemented, barriers to referral completion need to be addressed at all levels. Alternative effective treatment options should be provided to children unable to complete referral.
Acknowledgments
The authors would like to express their sincere thanks to the children and their caregivers who agreed to participate in this study; the health workers and local and national health authorities who provided their support; the study teams of the School of Public Health in Kinshasa (DRC), Akena Associates (Nigeria), and Makerere University School of Public Health (Uganda); and the colleagues of the local CHAI and UNICEF offices. We greatly appreciate Aurelio DiPasquale’s support with the ODK software and Robert Canavan’s editorial support.
Footnotes
Handling editor: Sanni Yaya
Twitter: @NiniBrunner, @Jokitje, @AitaSignorell, @GDelvento, @lipangalala
Contributors: CL, CB, MWH, AS and VBdL conceptualised and designed the study. NCB, MWH, EO, PAw, JO, AT, AS, AR, CB and CL developed the methodology. EO, PAw, JO, AT, NCB, J-CK, BA, AK, CO, OY, PAt, JK, GT, IA led and supervised the data collection. NC, TV, HGN, JMC and VB provided project management and coordination support. JO, PAw, AS, NCB, BA, AK, CO, OY, PAt, JK, GT, IA, GD and TTL curated the data and contributed to data analysis. NCB and AR led the data analysis. NCB wrote the manuscript. MWH is responsible for the overall content as a guarantor. All authors contributed to data interpretation and approved the final draft of the manuscript.
Funding: This study was funded by Unitaid (grant reference XM-DAC-30010-CHAIRAS). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Individual de-identified participant data that underlie the results reported in this article are available at zenodo.org upon reasonable request (DOI: 10.5281/zenodo.5570278).
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by the Research Ethics Review Committee of the World Health Organization (WHO ERC, Number ERC.0003008), the Ethics Committee of the University of Kinshasa School of Public Health (Number 012/2018), the Health Research Ethics Committee of the Adamawa State Ministry of Health (S/MoH/1131/I), the National Health Research Ethics Committee of Nigeria (NHREC/01/01/2007-05/05/2018), the Higher Degrees, Research and Ethics Committee of the Makerere University School of Public Health (Number 548), the Uganda National Council for Science and Technology (UNCST, Number SS 4534), and the Scientific and Ethical Review Committee of CHAI (Number 112, 21 November 2017). Participants gave informed consent to participate in the study before taking part.
References
- 1.Gomes MF, Faiz MA, Gyapong JO, et al. Pre-referral rectal artesunate to prevent death and disability in severe malaria: a placebo-controlled trial. Lancet 2009;373:557–66. 10.1016/S0140-6736(08)61734-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Malaria Programme . Rectal artesunate for pre-referral treatment of severe malaria. Geneva: World Health Organization; 2017. [Google Scholar]
- 3.Simba DO, Kakoko DC, Warsame M, et al. Understanding caretakers' dilemma in deciding whether or not to adhere with referral advice after pre-referral treatment with rectal artesunate. Malar J 2010;9:123. 10.1186/1475-2875-9-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomes M, Ribeiro I, Warsame M, et al. Rectal artemisinins for malaria: a review of efficacy and safety from individual patient data in clinical studies. BMC Infect Dis 2008;8:39. 10.1186/1471-2334-8-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Guidelines for the treatment of malaria - 3rd edition. Geneva: World Health Organization; 2015. [Google Scholar]
- 6.Mvumbi PM, Musau J, Faye O, et al. Adherence to the referral advice after introduction of rectal artesunate for pre-referral treatment of severe malaria at the community level: a noninferiority trial in the Democratic Republic of the Congo. Malar J 2019;18:438. 10.1186/s12936-019-3074-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strachan CE, Nuwa A, Muhangi D, et al. Community understanding of the concept of pre-referral treatment and how this impacts on referral related decision-making following the provision of rectal artesunate: a qualitative study in Western Uganda. BMC Health Serv Res 2018;18:470. 10.1186/s12913-018-3209-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simba DO, Warsame M, Kimbute O, et al. Factors influencing adherence to referral advice following pre-referral treatment with artesunate suppositories in children in rural Tanzania. Trop Med Int Health 2009;14:775–83. 10.1111/j.1365-3156.2009.02299.x [DOI] [PubMed] [Google Scholar]
- 9.Siribié M, Ajayi IO, Nsungwa-Sabiiti J, et al. Compliance with referral advice after treatment with prereferral rectal artesunate: a study in 3 sub-Saharan African countries. Clin Infect Dis 2016;63:S283–9. 10.1093/cid/ciw627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ajayi IO, Nsungwa-Sabiiti J, Siribié M, et al. Feasibility of malaria diagnosis and management in Burkina Faso, Nigeria, and Uganda: a community-based observational study. Clin Infect Dis 2016;63:S245–55. 10.1093/cid/ciw622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lal S, Ndyomugenyi R, Paintain L, et al. Caregivers' compliance with referral advice: evidence from two studies introducing mRDTs into community case management of malaria in Uganda. BMC Health Serv Res 2018;18:317. 10.1186/s12913-018-3124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanyonjo A, Bagorogoza B, Kasteng F, et al. Estimating the cost of referral and willingness to pay for referral to higher-level health facilities: a case series study from an integrated community case management programme in Uganda. BMC Health Serv Res 2015;15:347. 10.1186/s12913-015-1019-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warsame M, Gyapong M, Mpeka B, et al. Pre-referral rectal artesunate treatment by community-based treatment providers in Ghana, Guinea-Bissau, Tanzania, and Uganda (study 18): a cluster-randomized trial. Clin Infect Dis 2016;63:S312–21. 10.1093/cid/ciw631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Zoysa I, Bhandari N, Akhtari N, et al. Careseeking for illness in young infants in an urban slum in India. Soc Sci Med 1998;47:2101–11. 10.1016/s0277-9536(98)00275-5 [DOI] [PubMed] [Google Scholar]
- 15.Kalter HD, Salgado R, Moulton LH, et al. Factors constraining adherence to referral advice for severely ill children managed by the integrated management of childhood illness approach in Imbabura Province, Ecuador. Acta Paediatr 2003;92:103–10. 10.1111/j.1651-2227.2003.tb00478.x [DOI] [PubMed] [Google Scholar]
- 16.Thomson A, Khogali M, de Smet M, et al. Low referral completion of rapid diagnostic test-negative patients in community-based treatment of malaria in Sierra Leone. Malar J 2011;10:94. 10.1186/1475-2875-10-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lengeler C, Burri C, Awor P, et al. Community access to rectal artesunate for malaria (CARAMAL): a large-scale observational implementation study in the Democratic Republic of the Congo, Nigeria and Uganda. medRxiv 2021. 10.1101/2021.12.10.21266567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hetzel MW, Okitawutshu J, Tshefu A, et al. Effectiveness of rectal artesunate as pre-referral treatment for severe malaria in children. medRxiv 2021. 10.1101/2021.09.24.21263966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signorell A, Awor P, Okitawutshu J, et al. Health worker compliance with severe malaria treatment guidelines in the context of implementing pre-referral rectal artesunate: an operational study in three high burden countries. medRxiv 2021. 10.1101/2021.11.26.21266917 [DOI] [Google Scholar]
- 20.Weiss DJ, Nelson A, Gibson HS, et al. A global map of travel time to cities to assess inequalities in accessibility in 2015. Nature 2018;553:333–6. 10.1038/nature25181 [DOI] [PubMed] [Google Scholar]
- 21.RStudio: integrated development for R [program]. Boston, MA: RStudio, PBC 2020.
- 22.Bertozzi-Villa A. Mapping travel times with malariaAtlas and friction surfaces, 2018. Available: https://medium.com/@abertozz/mapping-travel-times-with-malariaatlas-and-friction-surfaces-f4960f584f08 [Accessed 19 May 2021].
- 23.Maina J, Ouma PO, Macharia PM, et al. A spatial database of health facilities managed by the public health sector in sub Saharan Africa. Sci Data 2019;6:134. 10.1038/s41597-019-0142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Google . [Google Maps referral health facility locations in the Democratic Republic of the Congo, Nigeria, and Uganda]. Available: https://www.google.com/maps
- 25.Federal Ministry of Health Nigeria . NIGERIA health facility registry. Available: https://hfr.health.gov.ng/facilities/hospitals-list
- 26.Ministry of Health Uganda . National health facility master list 2018 Kampala: Ministry of Health Uganda, Division of Health Information, 2018. Available: http://library.health.go.ug/publications/health-facility-inventory/national-health-facility-master-facility-list-2018
- 27.Stata Statistical Software: Release 16 [program]. College Station, TX: StataCorp LLC 2019.
- 28.Awad MI, Alkadru AMY, Behrens RH, et al. Descriptive study on the efficacy and safety of artesunate suppository in combination with other antimalarials in the treatment of severe malaria in Sudan. Am J Trop Med Hyg 2003;68:153–8. 10.4269/ajtmh.2003.68.153 [DOI] [PubMed] [Google Scholar]
- 29.Barnes KI, Mwenechanya J, Tembo M, et al. Efficacy of rectal artesunate compared with parenteral quinine in initial treatment of moderately severe malaria in African children and adults: a randomised study. Lancet 2004;363:1598–605. 10.1016/S0140-6736(04)16203-X [DOI] [PubMed] [Google Scholar]
- 30.Krishna S, Planche T, Agbenyega T, et al. Bioavailability and preliminary clinical efficacy of intrarectal artesunate in Ghanaian children with moderate malaria. Antimicrob Agents Chemother 2001;45:509–16. 10.1128/AAC.45.2.509-516.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson JA, Agbenyega T, Barnes KI, et al. Population pharmacokinetics of artesunate and dihydroartemisinin following intra-rectal dosing of artesunate in malaria patients. PLoS Med 2006;3:e444. 10.1371/journal.pmed.0030444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dillip A, Alba S, Mshana C, et al. Acceptability--a neglected dimension of access to health care: findings from a study on childhood convulsions in rural Tanzania. BMC Health Serv Res 2012;12:113. 10.1186/1472-6963-12-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hildenwall H, Tomson G, Kaija J, et al. "I never had the money for blood testing" - caretakers' experiences of care-seeking for fatal childhood fevers in rural Uganda - a mixed methods study. BMC Int Health Hum Rights 2008;8:12. 10.1186/1472-698X-8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desmond NA, Nyirenda D, Dube Q, et al. Recognising and treatment seeking for acute bacterial meningitis in adults and children in resource-poor settings: a qualitative study. PLoS One 2013;8:e68163. 10.1371/journal.pone.0068163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunner NC, Karim A, Athieno P, et al. Starting at the community: treatment seeking pathways of children with suspected severe malaria in Uganda. medRxiv 2021. 10.1101/2021.12.09.21267055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green C, Quigley P, Kureya T, et al. Use of rectal artesunate for severe malaria at the community level, Zambia. Bull World Health Organ 2019;97:810–7. 10.2471/BLT.19.231506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization . Revised who classification and treatment of pneumonia in children at health facilities: evidence summary. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 38.Lee TT, Omoluabi E, Ayodeji K, et al. Treatment-Seeking for children with suspected severe malaria attending community health workers and primary health centres in Adamawa state, Nigeria. medRxiv 2021. 10.1101/2021.12.01.21267130 [DOI] [Google Scholar]
- 39.Brunner NC, Awor P, Hetzel MW. Definitions of severity in treatment seeking studies of febrile illness in children in low and middle income countries: a scoping review. Int J Public Health 2021;66:634000. 10.3389/ijph.2021.634000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-008346supp001.pdf (226KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Individual de-identified participant data that underlie the results reported in this article are available at zenodo.org upon reasonable request (DOI: 10.5281/zenodo.5570278).


