Abstract
Pesticides remain one of the most effective ways of controlling agricultural and public health insects, but much is still unknown regarding how these compounds reach their targets. Specifically, the role of ABC transporters in pesticide absorption and excretion is poorly understood, especially compared to the detailed knowledge about mammalian systems. Here, we present a comprehensive characterization of pesticide transporters in the model insect Drosophila melanogaster. An RNAi screen was performed, which knocked down individual ABCs in specific epithelial tissues and examined the subsequent changes in sensitivity to the pesticides spinosad and fipronil. This implicated a novel ABC drug transporter, CG4562, in spinosad transport, but also highlighted the P-glycoprotein orthologue Mdr65 as the most impactful ABC in terms of chemoprotection. Further characterization of the P-glycoprotein family was performed via transgenic overexpression and immunolocalization, finding that Mdr49 and Mdr50 play enigmatic roles in pesticide toxicology perhaps determined by their different subcellular localizations within the midgut. Lastly, transgenic Drosophila lines expressing P-glycoprotein from the major malaria vector Anopheles gambiae were used to establish a system for in vivo characterization of this transporter in non-model insects. This study provides the basis for establishing Drosophila as a model for toxicology research on drug transporters.
Keywords: pesticides, transporter, p-glycoprotein, Drosophila
1. Introduction
Small molecule pesticides (often referred to as synthetic or chemical pesticides) have been the most versatile method of control for both agricultural and public health insect pests. However, there is an urgent need for new compounds due to the emergence of pesticide resistance and unacceptably high deleterious effects on off-target species. One obstacle in the design of new pesticides is bioavailability, the proportion of a drug (e.g. pesticide) that can cross epithelial barriers and reach its target within the body. Although a variety of different factors determine a drug's ability to cross these epithelia [1], transporters are particularly interesting from a genetic perspective. By translocating molecules across cell membranes, they can either decrease or increase bioavailability depending on their localization and direction of transport. Such drug transporters have been extensively studied in the context of pharmaceutical development; regulatory agencies have specified guidelines for transporter-conscious drug design [2], and companies have been founded which focus exclusively on drug transporter services (https://www.solvobiotech.com/). However, the role of these transporters in pesticide toxicology has so far been understudied.
Of particular interest is the ATP-binding cassette (ABC) superfamily. Each functioning ABC transporter is composed of two conserved nucleotide-binding domains (NBDs) and two transmembrane domains (TMDs) involved in substrate recognition [3]. Individual transporters are classified into families (A–H) based on homology, which can suggest function but does not allow prediction of which specific substrates are transported by members of a given family. Selected members of the A, B, C and G families are known to transport xenobiotics in mammalian systems [4]. Perhaps the best-studied transporter is P-glycoprotein (aka ABCB1, P-gp), from the B family. This protein was first identified in a cancer-resistant mammalian cell line [5] and has been found to act as a polyspecific drug transporter across a range of different taxa.
Much work on ABCs in insects has been focused on their role in detoxifying pesticides and plant secondary metabolites [6]. Most of these studies have been indirect, implicating pesticide transporters via their upregulation in resistant populations or following pesticide exposure. While some transporters have been functionally characterized [7–9], and while a great deal more has been done regarding the role of ABC transporters as receptors in resistance to Bacillus thuringiensis [10], the diversity of proteins that directly transport small molecule pesticides is poorly understood. Like other taxa, P-glycoprotein has been the most studied drug transporter in insects. A family-wide expansion of P-gp was previously found in Lepidoptera (moths and butterflies), which may underpin their extreme polyphagy [11]. CRISPR-Cas9 removal and in vitro studies have also highlighted their conserved role as drug transporters in this order [12,13]. However, a more detailed characterization of P-gp in vivo is lacking.
The study of ABC transporters like P-gp is often difficult in vitro despite a wide variety of assays being available [14]. More direct methods of transport such as monolayer cell assays and in vivo mice models are more accurate but are relatively expensive and thus not available to all laboratories. The fruit fly Drosophila melanogaster represents a potentially powerful system to study ABC transporters in vivo at least with respect to toxins such as pesticides [15]. Apart from its advantages as a genetically tractable model organism, it also has physiologically well-characterized epithelial tissues such as the midgut [16], Malpighian tubules [17] and blood–brain-barrier (BBB; [18]). Recent studies have also considered the four P-glycoprotein paralogues in Drosophila. Mdr65 was localized to the BBB and shown to efflux fluorescent dyes [19]. Deletion of this gene along with the tubule predominant Mdr49 and midgut predominant Mdr50 significantly increased sensitivity to a variety of pesticides [20–23]. Strikingly, removal of Mdr49 and Mdr50 displayed opposite pesticide responses (more tolerant or more sensitive) depending on which pesticide was used. Similar results were found when RNAi was used to target these two genes [23]. This apparent paradox does not have a proven explanation but may be rooted in the localization of these transporters within tissues and cells. Such differences in localization among the well-studied ABCC (MRP) transporters in mammals have explained such opposing effects [24]. However, these questions have so far not been investigated in insects.
Here we present a series of experiments that sought to use D. melanogaster to (i) identify novel pesticide transporters, (ii) characterize their effect on pesticide toxicology in vivo and (iii) establish Drosophila as a heterologous pesticide transporter model.
2. Results
(a) . An in vivo RNAi screen identifies putative pesticide transporting ABCs
Of the 56 total ABC transporters in D. melanogaster, 37 belong to the B, C or G subfamilies that have been previously implicated as drug transporters in mammalian systems. For our study we further only considered transporters they had an expression level of greater than five RPKM in a given tissue on the FlyAtlas expression database or were present above that threshold in a separate midgut transcriptome [25,26]. This narrowed the list to 34 transporter genes and 70 combinations of transporter tissue combinations, 50 of which were tested in this study (electronic supplementary material, table S1).
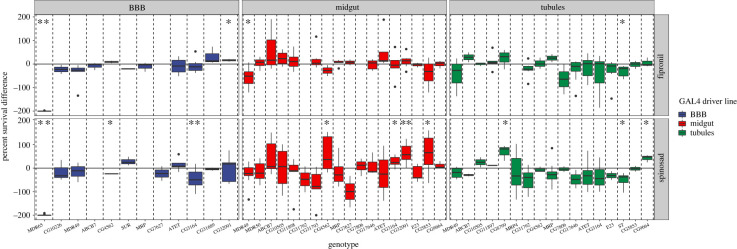
An RNAi screen was performed whereby genetic knockdown (KD) of individual transporters was performed in a single tissue and subsequent changes in insecticide tolerance were assessed. KD of several transporters in a single epithelium yielded differences in sensitivity compared to their respective control genotypes, while the majority showed either no difference or were inconsistent (figure 1; electronic supplementary material, tables S2 and S3). Most notably, KD of Mdr65 substantially increased the sensitivity of both spinosad and fipronil (figure 1). Another closely related gene Mdr49 increased sensitivity to fipronil when inhibited in the midgut. To a far lesser extent KD of the CG32091 (ABCG family), CG3164 (ABCG family) and CG4562 (ABCC family) in the midgut tissue increased tolerance to spinosad. Furthermore, KD of CG8799 (ABCC family), and CG9664 (ABCG family) in the tubules increased tolerance to spinosad, while knockdown of the Scarlet gene (ABCG family) increased sensitivity to both compounds. However, the variation inherent in bioassays and RNAi preclude firm conclusions from being drawn from only this data alone.
Figure 1.
ABC Transporter RNAi screen. A knockdown of ABC transporters was performed in the BBB (left), midgut (middle) and Malpighian tubules (right), followed by a bioassay on either spinosad (bottom) or fipronil (top). Vertical dashed lines distinguish the different ABC transporters targeted by RNAi. Horizontal solid lines signify how the genetically matched control line behaved, while boxes reflect the percentage difference between the knockdown and control. In other words, the further from the vertical line a box is the more susceptible (lower) or resistant (higher) the knockdown was. Any transportertissue combination with a p-value lower than 0.05 was highlighted with a significance star. Combinations which yielded a corresponding q-value (false discovery rate) of below 0.05 were marked with two stars. (Online version in colour.)
(b) . CRISPR-Cas9 of uncharacterized ABC transporter increases spinosad tolerance
CG4562, CG3164 and CG32091 were selected for knockout (KO) using CRISPR-Cas9 as they showed the most consistent differences in the RNAi toxicology screen. A KO line for CG3164 was unable to be established as it did not homozygose from the balancer stock, suggesting the gene plays an essential role. This is similar to the direct orthologue in Tribolium [27]. KO of CG32091 and CG4562 were successful. Of these, only CG4562 showed a decrease in spinosad sensitivity which mimicked the RNAi phenotype (figure 2; electronic supplementary material, table S4). This suggests that CG4562 plays a role in spinosad transport in D. melanogaster, but the magnitude of this change was far less than that observed for Mdr65 knockdown.
Figure 2.
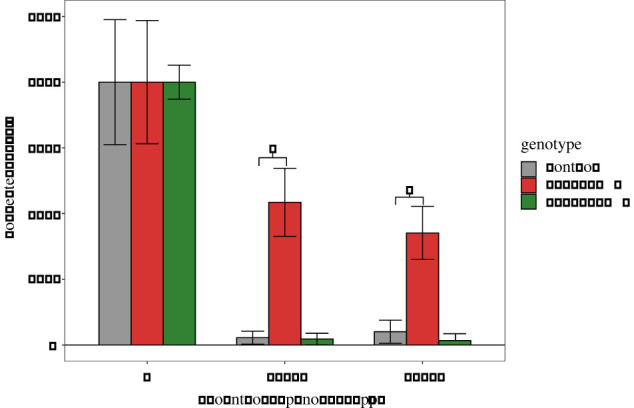
CRISPR-KO of candidate drug transporters. Bioassay data from the KO of CG32091 and CG4562 is shown. The genetic control background (Nanos-Cas9) and the CG32091 showed a strong response to spinosad, indicated by a low survival proportion on the y-axis. Removal of CG4562 showed a higher proportion of survival across all doses of spinosad (x-axis). Error bars represent 95% confidence intervals generated by Abbot's correction. (Online version in colour.)
(c) . Overexpression of P-glycoprotein in Drosophila
The knockdown of Mdr65 at the BBB yielded the highest magnitude change observed in the RNAi screen and drastically increased sensitivity to both spinosad and fipronil (figure 1). Further, CRISPR-Cas9 based KO of Mdr65 and its paralogues Mdr49 and Mdr50 indicates that each of the genes has a role in insecticide response [20]. We thus sought to expand upon these findings using transgenic UAS responder lines containing the ORFs of Mdr49, Mdr50 and Mdr65. Overexpression of Mdr65 at the BBB yielded slightly increased but statistically insignificant tolerance to spinosad and showed no difference against fipronil in contrast to the striking increase in susceptibility observed with RNAi in this tissue (electronic supplementary material, figure S1A,B). Insignificant differences in pesticide toxicity were observed when Mdr49 or Mdr50 were driven in the tubules or the midgut respectively (electronic supplementary material, figure S1C-F; and table S5).
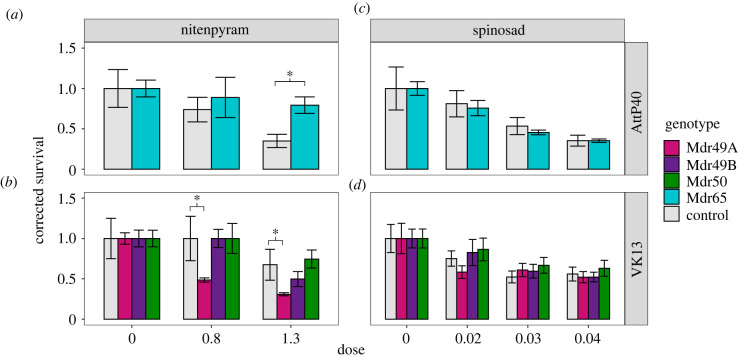
Transgenic expression of these P-gp genes with a strong ubiquitous driver yielded a larger effect. Both Mdr49A and Mdr65 increased sensitivity to spinosad while Mdr50 overexpression lines were slightly more resistant (figure 3b,d; electronic supplementary material, table S5). With the pesticide nitenpyram ubiquitous expression of both Mdr65 and Mdr50 caused resistance while overexpression of Mdr49 caused increased sensitivity (figure 3a,c). Overexpression of Mdr49B had limited effect. These results agree with previous findings [20,23], demonstrating that global deletion or expression of genes can have variable effects on the direction of toxicological change (more susceptible or resistant) depending on the gene and pesticide under investigation.
Figure 3.
Global overexpression of P-gp paralogues. Bioassay data are shown for the pesticides spinosad (c,d) and nitenpyram (a,b), the latter chosen due to the difficulty of scoring fipronil toxicology in this genetic background. As the Mdr65 overexpression genotype was generated in a distinct background it was considered separately (a,b), while all other overexpression constructs were analysed jointly (c,d). In each panel the y-axis represents survival while the x-axis signifies the dose of the pesticide under investigation. (Online version in colour.)
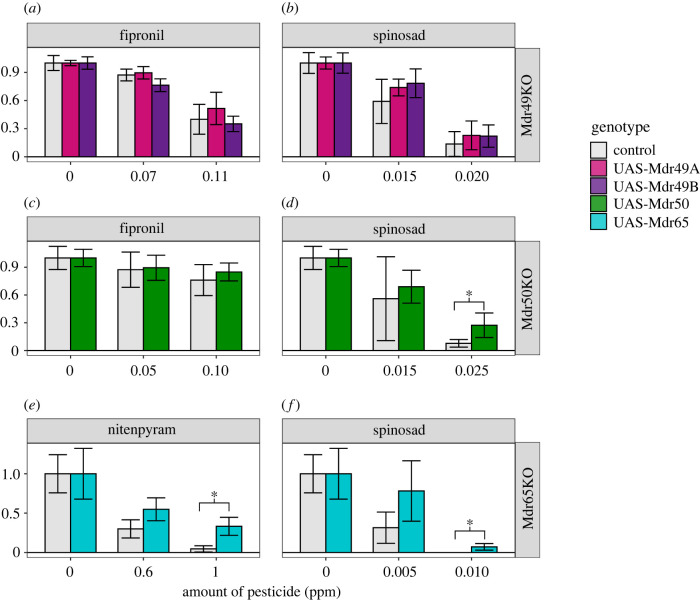
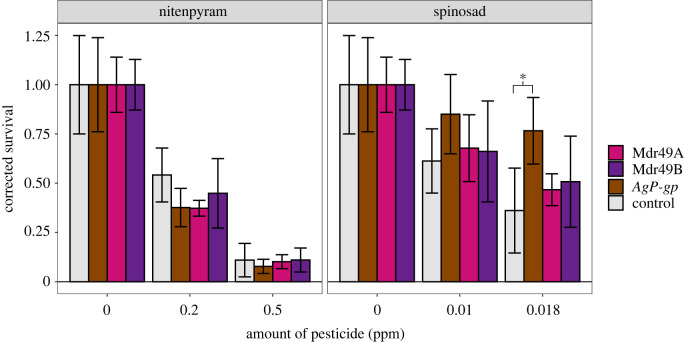
Rescue experiments were performed whereby we replaced an endogenous copy of P-gp with itself in a specific tissue. The expression of Mdr49A or Mdr49B in the Malpighian tubules of the Mdr49KO background did not have any measurable effect on the toxicology of spinosad or fipronil (figure 4a,b; electronic supplementary material, table S7). Likewise, the reintroduction of Mdr50 into the midgut increased survival but only very subtly; the effect was observed only at one dose (figure 4c,d). A much clearer effect was seen with the rescue of Mdr65. Significantly higher survival was observed in both pesticides when a functional copy of Mdr65 was added into the Mdr65KO background specifically at the BBB (figure 4e,f).
Figure 4.
Genetic rescue of Drosophila knockouts. Genetic rescue of the three knockouts Mdr49KO, Mdr50KO and Mdr65KO was tested for their pesticide toxicological response. Spinosad (b,d,f), fipronil (a,c), and nitenpyram (e) were used against different genotypes, with the latter being chosen due to the difficulty of scoring fipronil toxicology in this genetic background. In each panel the x-axis represents pesticide dose and the y-axis represents survival corrected with abbots correction. Error bars represent 95% confidence intervals generated by Abbot's correction. (Online version in colour.)
(d) . Immunolocalization of endogenous P-gp
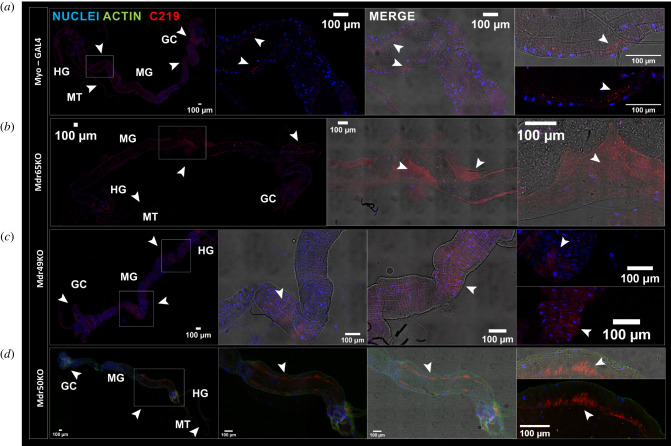
Given that overexpression of P-glycoprotein orthologues in Drosophila yielded qualitatively different phenotypes depending on the insecticide and gene in question, we sought to localize these proteins with immunofluorescence. The C219 antibody was used, which targets an epitope common to all P-glycoprotein paralogues found in Drosophila, and a genotype with a wild-type P-gp gene (Myo-GAL4) was used in comparison with all three P-gp KOs. No staining was found in the fat body in any genotype under investigation, suggesting that this tissue does not contain notable P-gp expression (electronic supplementary material, figure S2). The dissected central nervous system showed strong staining in all genotypes except Mdr65KO, confirming that the MDR65 protein is the predominant P-gp orthologue at the BBB (electronic supplementary material, figure S3). In the Malpighian tubule, removal of Mdr49 had a drastic effect and abolished almost all expression especially in the lumen (electronic supplementary material, figure S4C′). The remaining non-luminal P-gp is presumably due to expression of Mdr50 or Mdr65 present in small quantities in the tubule.
Lastly, the midgut was examined which provided the most complicated yet interesting tissue under study. In wild type and Mdr65KO flies, P-glycoprotein was found localized primarily to a region of the posterior midgut (defined as R5 by [28]), which matches transcriptomic data for these genes (figure 5a,b). There were also lower amounts of expression detectable in the anterior midgut near the gastric caeca. However, the MDR50 and MDR49 proteins appeared to show differing subcellular localizations. While MDR49 (inferred from the Mdr50KO genotype) showed an apical specific phenotype (figure 5d), the MDR50 (inferred from the Mdr49KO genotype) had a more diffuse localization (figure 5c). While we cannot say whether the MDR50 protein is restricted to the basolateral side of the midgut, these experiments strongly suggest that different paralogues differ by their subcellular localization despite residing in the same tissue.
Figure 5.
Immunolocalization of P-glycoprotein paralogues. Immunostaining is shown for (a) the control genotype (Myo), (b) Mdr65KO, (c) Mdr49KO and (d) Mdr50KO. For each row, zoomed out images of the whole midgut are shown along with zoomed images based depending on where fluorescence was detected. Brightfields are shown along with confocal images and merges for each column. In each image, DAPI stained nuclei are in blue, phalloidin stained actin is in green and the C219 antibody localization (P-gp) is in red.
(e) . Transgenic expression of P-glycoprotein from a malaria vector
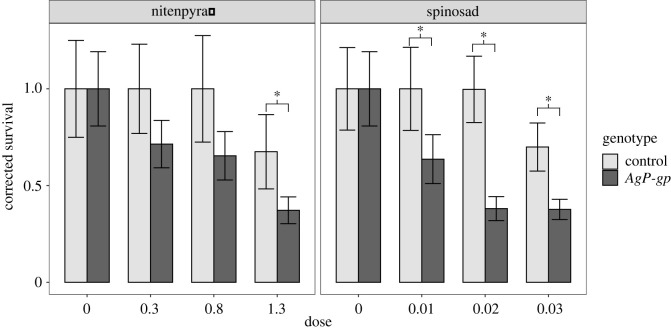
We lastly sought to extend our findings to P-glycoproteins from pest species such as the malarial vector Anopheles gambiae. Strong, ubiquitous expression of AgP-gp (AGAP005639) in Drosophila resulted in increases in sensitivity to both nitenpyram and spinosad with effect sizes on par with or greater than the endogenous Drosophila proteins (figure 6; electronic supplementary material, table S6). Given the larger effect sizes observed when rescuing the Mdr65KO at the blood brain barrier, AgP-gp was also expressed specifically at the blood brain barrier in an Mdr65KO background which yielded a significantly higher tolerance to the pesticide spinosad, but not fipronil (figure 7; electronic supplementary material, table S8). The expression of Mdr49A or Mdr49B did not yield any effect in this system suggesting that different paralogues may not be active in this tissue.
Figure 6.
Global overexpression of P-glycoprotein in A. gambaie. The single P-glycoprotein orthologue from Anopheles gambiae was transgenically expressed in D. melanogaster under the control of a strong ubiquitous promoter (Actin-GAL4). These transgenic flies (dark grey) were then compared with their matched controls (light grey) using a larval toxicology assay against nitenpyram (left) or spinosad (right). In each panel the x-axis represents pesticide dose and the y-axis represents survival corrected with abbots correction. Error bars represent 95% confidence intervals generated by Abbot's correction.
Figure 7.
Genetic rescue of Drosophila Mdr65KO with AgP-gp. Three different UAS-P-gp constructs expressing Mdr49A (Pink), Mdr49B (Purple) and AgP-gp (dark grey) were expressed in a Mdr65KO background and tested on the pesticides nitenpyram (left) and spinosad (right). In each panel the x-axis represents pesticide dose, and the y-axis represents survival corrected with abbots correction. Error bars represent 95% confidence intervals generated by Abbot's correction. Although there is no established statistical test for Abbot's correction non-overlapping 95% confidence intervals are considered a very conservative estimate and significance stars were included in this graph reflecting this.
3. Discussion
The emergence of resistance and unacceptably high externalities posed by the current generation of pesticides necessitates the development of novel, safe and effective compounds for insect control. Bioavailability presents an obstacle to rational pesticide design, and this is worsened by a poor understanding of insect drug transporters in vivo. Here, we develop the Drosophila model system for transporter-mediated toxicology by (i) characterizing the diversity of pesticide transporters in Drosophila, (ii) providing a more in-depth characterization of P-glycoprotein and (iii) heterologously expressing an orthologous mosquito transporter in D. melanogaster.
(a) . The diversity of ABCs involved in pesticide transport
One of the goals of this study was to identify which ABCs were involved in pesticide transport, and a toxicology screen of RNAi lines was performed to address this. Identification of the previously known Mdr65 gene suggested that the assay could detect true positives and the screen further implicated an ABC transporter encoded by CG4562 in spinosad transport in the midgut (figure 1). This was confirmed by CRISPR-Cas9 KO, which phenocopied the RNAi knockdown (figure 2). CG4562 belongs to the ABCC family, several members of which have been implicated in drug transport in humans [29], and in insects such as the human body louse [30], red flour beetle [8] and even D. melanogaster [31]. Furthermore, ABCC proteins such as ABCC2 have been widely implicated in resistance to Bt toxins, but this is thought to be due to their role as a receptor protein rather than a drug transporter [32]. Unlike several other ABCC drug transporters identified in insects, removal of CG4562 increases tolerance rather than increasing sensitivity. This runs counter to the narrative of ABCs as ‘resistance’ genes and highlights a more complex role for transporters in influencing the distribution of compounds around the body. Similar findings in mammalian ABCC genes have shown that transporters localizing to the basolateral side of tissues like the intestine have the effect of pumping compound into the body [24]. While CG4562 was not localized in this study, we hypothesize it to be present at the basolateral membrane and thus pump toxin out of the midgut cell into the haemolymph. However, it is also possible that CG4562 would act as an importer. While such importers have not been identified in insects previously, the unexpected identification of such importers in plants [33] suggests that ABC importers could exist in insects.
However, several limitations of the assay may have skewed a bias of the RNAi screen towards false negatives. For example, Mdr50 was not detected in the RNAi screen despite CRISPR-Cas9 KO yielding clear effects [20]. This may stem from considering each tissue in isolation or may also represent a limitation of the strength of the knockdown achieved in this study, either of which could mask subtle effect sizes. Future studies could make use of the next generation of genetic tools in Drosophila such as somatic CRISPR for tissue-specific removal or simply making heritable mutations in a larger range of transporters [34] which would likely elicit larger effects. Combinatorial knockouts or knockdowns may also be an option as synergistic action has been previously associated with toxicology in both mammals [35] and insects [36]. Furthermore, this study did not address other transporters which may be involved in drug uptake. In humans, a widespread role for transporters from the Solute carrier (SLC) superfamily in drug uptake has been demonstrated [29,37], but these have been severely understudied in insects. Recent genomic [38] and functional [39] work on these SLC transporters suggests that they may play a key role in drug uptake in humans.
(b) . P-glycoprotein plays a predominant role in insecticide toxicology
Knockdown of the previously known Mdr65 gene at the blood–brain barrier created an effect size that was an order of magnitude higher than for any other transporter, suggesting a predominant role for this protein in pesticide toxicology. Such a role would match the situation in mammals where P-glycoprotein has by far the largest effect in most tissues [4]. Interestingly, P-glycoprotein has only been rarely associated with insecticide resistance, defined as the abnormally high levels of tolerance to pesticides causing control failures in field settings. Selected studies have shown P-gp's role in resistance [40], but this mechanism does not appear to be widespread among insects compared to other methods like target site mutations or upregulation of drug-metabolizing enzymes [41]. Indeed, in the current study only strong ubiquitous expression was able to generate changes in toxicology and these were modest compared to the effect of removing the gene (figure 1, electronic supplementary material, figure S1). P-glycoprotein thus appears to play a strong role in the toxicology of pesticides, but increased expression may have only a limited effect on pesticide resistance. Interestingly, an opposite role is played by some cytochrome P450 s; overexpression of Cyp6g1 confers insecticide resistance, while removal has a limited effect [42].
Strikingly, the ubiquitous expression of Mdr49 or Mdr50 caused opposite changes in toxicology (increased tolerance or increased sensitivity) depending on both the pesticide and transgene, agreeing with previous CRISPR-Cas9 data [20,23]. These differences may be due to changes in localization, and immunostaining at the cellular level indicated distinct patterns of MDR49 and MDR50 localization in the midgut and Malpighian tubules (figure 5). However, it is currently unclear how any potential localization changes cause the observed disparities in toxicology among pesticides. Another hypothesis is that a compensatory mechanism exists whereby the removal of one transporter impacts another as was seen for organic anion transport in the tubule [43]. There may also be restrictions as to which tissue a given paralogue can act. Ubiquitous expression of different paralogues yielded different effects (figure 3), and only certain paralogues (Mdr65 and AgP-gp) were shown to be functional in the BBB rescue system (figure 7). Lastly, it is possible and perhaps likely that these transporters are dynamically expressed depending on life stage, nutritional state or environmental stress. Indeed, many examples of the dynamic regulation of detoxification genes exist in response to diet [44], and life-stage Drosophila transcriptomic data from databases such as FlyAtlas show that genes like Mdr49 and CG32091 differ substantially in tissues like the fat body during development [26]. Differences between transcript levels and protein levels are also likely. Future work will be needed to address these paradoxes, which promise to have important implications for toxicokinetics and for basic research on how transporter genes function.
(c) . Drosophila as a model system for ABC mediated toxicology
The characterization of the P-gp orthologue from A. gambiae showed that it was able to influence the toxicological profile of the pesticide spinosad when expressed both ubiquitously and in a tissue specific Mdr65 rescue system at the BBB (figure 6 and 8). Although this system no doubt requires optimization, the successful heterologous expression of a pest transporter in vivo opens the door for the study and comparison of these genes in the future. Such a heterologous system would be useful to complement endogenous in vivo data generated, for example, by using CRISPR-Cas9 to remove a gene under investigation in a pest insect such as a mosquito. So far it has been challenging to find suitable expression systems for drug transporters and Drosophila represents an unexplored alternative. Side by side expression of transporters from pest species (e.g. mosquitoes, agricultural pests) with non-target organisms (e.g. humans, bees), in a Drosophila like system could provide the basis for pest selective insecticides in the future. Such heterologous expression in Drosophila has been used to previously characterize pesticide target proteins [45], and given the challenging interpretation of in vitro transporter data [14], Drosophila may provide a welcome alternative.
4. Material and methods
(a) . Previously generated Drosophila genotypes
Several driver genotypes were used in this study which expressed GAL4 in defined epithelial tissues. The Mex-Gal4 line was previously reported [46] and drove expression specifically in the midgut. Similarly, the Myo1A GAL4 driver (Myo-GAL4; 67057) was used as an alternative to Mex-G4 in the P-gp rescue experiments as it also drives expression in the midgut enterocytes. The Urate_Oxidase-GAL4 (UO-GAL4) localized expression specifically in the Malpighian tubules [47]. Lastly, a previously published driver which localized GAL4 to the subperineural glial cells of the BBB was used (SPG-GAL4) [48]. Strong ubiquitous expression was also achieved with Actin-GAL4 (Bloomington no. 3953).
Publicly available UAS-RNAi lines were obtained from the transgenic RNAi project (Trip) or the Vienna Drosophila RNAi Centre (VDRC) and contained RNA interference sequences targeting an individual ABC transporter under the control of a UAS promoter (electronic supplementary material, table S1) [49,50]. Crossing of any one of these flies to a GAL4 would express the RNAi construct and knock down the specified ABC in the specified tissue.
(b) . Generation of CRISPR-Cas9 knockout genotypes
Selected genes identified in the RNAi screen were confirmed by removing the majority of the gene from the genome using CRISPR-Cas9. Pairs of sgRNAs for each were first designed via the CRISPR optimal target finder [51]. These were selected so that one sgRNA targeted a sequence at the 5′ and 3′ end of the gene. Each sgRNA was cloned into the Bsbi site in the pU6 vector (Addgene no. 45946) by annealing overlapping custom primers (electronic supplementary material, table S9). If the target gene was on the 2nd chromosome, pU6-sgRNA plasmids were injected into the fly line carrying a Nanos-Cas9 construct on the 3rd chromosome (Bloomington no. 78782). The opposite was true for genes on the 3rd chromosome which were injected into flies with Cas9 expression on the 2nd chromosome (Bloomington no. 78781). Surviving larvae were then crossed to a balancer line derived from the appropriate Nanos-Cas9 line to maintain the same genetic background but without the Nanos-Cas9 construct. Offspring were individually screened for positive CRISPR events and crossed to the same balancer line one more time before being homozygosed. In either case the chromosome carrying Cas9 was crossed out so that all deletions were eventually in identical backgrounds apart from the ABC transporter being removed.
(c) . Transgenic expression genotypes
Transgenic lines containing an open reading frame (ORF) of a given transporter regulated by a GAL4 inducible UAS promoter were created. ORFs were first amplified with gene specific primers and cloned into the pGEM-T-easy shuttle vector. The fragments were then digested with flanking NotI sites, subcloned into the NotI site of the pUASt-AttB vector [52], and injected into the AttP40 (Bloomington no. 25709) or the VK13 (Bloomington no. 24864) D. melanogaster lines harbouring AttP sites and an endogenous source of φC31 integrase. This strategy was used to introduce Mdr49A, Mdr49B, Mdr50, Mdr65 and the P-gp derived from the VK7 strain of Anopheles gambiae (AgP-gp). Sequences of all open reading frames were included in electronic supplementary material, file S1.
In addition to expression in ‘wild type’ backgrounds, transgenic expression of these P-gp genes was performed in P-gp KO backgrounds [20] in order to observe any complementation (rescue) of toxicology phenotypes derived from their removal. In order to match known endogenous expression patterns, Mdr49 was expressed specifically in the Malpighian tubules, Mdr50 specifically in the midgut, and Mdr65 specifically in the BBB. For Mdr49 and Mdr50, stable lines were made carrying (i) the deletion, (ii) the UAS-transgene and (iii) the GAL4. As the KO and transgenic GAL4 cassette were on the same chromosome, recombination was used to generate flies carrying both alleles. Each of the two previously characterized Mdr49 isoforms (A and B; [21] were considered separately. For Mdr65, the transgene and the BBB-GAL4 cassette (on the 2nd chromosome) were both independently crossed into the Mdr65KO background separately and finally crossed together to make the rescue line. For the heterologous BBB rescue system, the UAS-Pgp construct was inserted into the VK13 landing site (3rd chromosome), recombined with the Mdr65KO allele (also 3rd chromosome), and crossed into a background with BBB-GAL4 on the 2nd. Due to genetic incompatibilities irrespective of the genes under investigation, BBB-GAL4 was unable to be homozygosed with the Mdr65KO 3rd chromosome, so all rescue lines were maintained and assayed over a 2nd chromosome CyO balancer.
(d) . Insecticide bioassays
Fipronil (CAS# 120068-37-3) and Spinosad (CAS# 168316-95-8) were both obtained as analytical standards from Sigma. Insecticide bioassays were performed as described previously [20]. First instar larvae were obtained by collecting between 150–200 females and 100 males of defined genotypes and allowing them to lay eggs on cherry juice agar plates for 24 h. After this period, the plates were changed, and the eggs washed onto a fine mesh. Eggs were then transferred onto fresh cherry juice agar plates and left undisturbed for an additional 24 h which generated a population of 1st instar larvae. 50 larvae were transferred to standard media dosed with a defined concentration of insecticide. Survival was scored after 16 days by counting the number of total pupae in the vial. Pupae were considered rather than adults due to the difficulty in scoring partially emerged adults brought about by the fipronil treatment. For genotypes carrying a CyO balancer, 100 larvae were picked in each vial and total numbers of straight wing flies were counted as there were no significant differences among the number of CyO emerging individuals (data not shown).
(e) . Data analysis
Raw mortality data was then analysed in the R statistical environment. The majority of mortality data was analysed using Abbot's formula to correct for background mortality with appropriate confidence intervals [53]. As no established statistical test exists for this correction, a conservative criterion of non-overlapping 95% confidence intervals was used to denote significance as recommended previously [53]. For the high throughput RNAi screen a different approach was used whereby percentage differences between knockdown and control lines were calculated using the formula
for all doses used in the experiment. A t-test was then used to measure whether these % differences were significantly different from the null hypothesis of no change (a value of 0). In order to correct for multiple testing, a false discovery test was also implemented using the ‘p. adjust’ function in R with the ‘false discovery rate’ method. All experiments were repeated at least twice, and each dose consisted of at least four replicates.
(f) . Immunolocalization of P-glycoprotein
In order to analyse protein localization of P-gp, immunostaining was performed as described previously using the C219 antibody [19], with some modifications. In brief, third instar larvae were dissected in SF-900 II insect medium (SFM, cat. no. 10902-096, Gibco) enriched with penicillin-streptomycin mix (50×, cat. no. P4458, Sigma-Aldrich) and placed on ice. Following dissections, the tissues were incubated in 4% formaldehyde solution (Methanol free, Thermo Scientific, cat. no. 28906) for 60 min and then in 3% Blocking Solution (BS, 3% BSA, 0.1% Triton in PBS1X) overnight at 4°C. Tissues were incubated with mouse anti-P-glycoprotein C219 antibody at 3.6 µg ml−1 (Invitrogen, cat no. MA1-26528) overnight at 4°C. On the third day, the tissues were incubated with Alexa Fluor 555-conjugated anti-mouse IgG secondary antibody (Invitrogen) (1 : 1000 in BS) for 1.5 h at RT. After BS washes, actin filaments were stained with Alexa Fluor 488-conjugated phalloidin (1 : 50 in BS for 30 min at RT, Invitrogen Thermo Scientific, A12379) and nuclei were stained with DAPI (1 : 100 in BS for 20 min at RT, AppliChem, cat. no. A1001). Tissues were then gently rinsed twice with PBS1X, prior to mounting in Vectashield (Vector Laboratories, H-1000-10) on SuperFrost+ slides. Imaging was conducted using a Leica TCS SP8 confocal laser scanning microscope unit, equipped with an inverted microscope (DMI6000 CS), a high-end Scientific fluorescence CCD camera (Leica DFC365FX) and a set of the appropriate high-quality lasers, housed in the Institute of Molecular Biology and Biotechnology (IMBB) Microscope Facility of Foundation for Research & Technology-Hellas (FORTH).
Acknowledgements
The authors would like to thank Ioannis Livdaras for performing Drosophila microinjections.
Contributor Information
Shane Denecke, Email: sdenecke@upenn.edu.
John Vontas, Email: vontas@imbb.forth.gr.
Data accessibility
All data are included within the article and electronic supplementary material [54].
Authors' contributions
S.D.: conceptualization, data curation, formal analysis, supervision, visualization, writing—original draft; H.N.B.L.: conceptualization, data curation, formal analysis, investigation, methodology, visualization; V.K.: investigation, methodology; M.K.: investigation; R.S.: investigation; S.H.: investigation; K.V.: funding acquisition, writing—review and editing; R.N.: conceptualization, funding acquisition, writing—review and editing; P.B.: conceptualization, writing—review and editing; S.G.: conceptualization, funding acquisition, writing—review and editing; J.V.: conceptualization, funding acquisition, resources, supervision, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
This work was funded as a component of a joint collaboration between Bayer CropScience and the Institute of Molecular Biology and Biotechnology (Greece). S.D. and H.N.B.L. were funded with this collaboration. K.V., R.N. and S.G. are employees of Bayer CropScience.
Funding
This work was funded as a component of a joint collaboration between Bayer CropScience and the Institute of Molecular Biology and Biotechnology (Greece). S.D. and H.N.B.L. were funded with this collaboration.
References
- 1.Yang NJ, Hinner MJ. 2015. Getting across the cell membrane: an overview for small molecules, peptides, and proteins, pp. 29-53. New York, NY: Humana Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maeda K, Sugiyama Y. 2013. Transporter biology in drug approval: regulatory aspects. Mol. Aspects Med. 34, 711-718. ( 10.1016/j.mam.2012.10.012) [DOI] [PubMed] [Google Scholar]
- 3.Zolnerciks JK, Andress EJ, Nicolaou M, Linton KJ. 2011. Structure of ABC transporters. In Essays In biochemistry, vol. 50 (ed. Sharom FJ), pp. 43-61. London, UK: Portland Press. [DOI] [PubMed] [Google Scholar]
- 4.Giacomini KM, et al. 2010. Membrane transporters in drug development. Nat. Rev. Drug Discov. 9, 215-236. ( 10.1038/nrd3028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juliano RL, Ling V. 1976. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta - Biomembr. 455, 152-162. ( 10.1016/0005-2736(76)90160-7) [DOI] [PubMed] [Google Scholar]
- 6.Dermauw W, Van Leeuwen T. 2014. The ABC gene family in arthropods: comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 45, 89-110. ( 10.1016/j.ibmb.2013.11.001) [DOI] [PubMed] [Google Scholar]
- 7.Chahine S, O'Donnell MJ. 2009. Physiological and molecular characterization of methotrexate transport by Malpighian tubules of adult Drosophila melanogaster. J. Insect Physiol. 55, 927-935. ( 10.1016/j.jinsphys.2009.06.005) [DOI] [PubMed] [Google Scholar]
- 8.Rösner J, Merzendorfer H. 2020. Transcriptional plasticity of different ABC transporter genes from Tribolium castaneum contributes to diflubenzuron resistance. Insect Biochem. Mol. Biol. 116, 103282. ( 10.1016/J.IBMB.2019.103282) [DOI] [PubMed] [Google Scholar]
- 9.Tarnay JN, et al. 2004. The dMRP/CG6214 gene of Drosophila is evolutionarily and functionally related to the human multidrug resistance-associated protein family. Insect Mol. Biol. 13, 539-548. ( 10.1111/j.0962-1075.2004.00512.x) [DOI] [PubMed] [Google Scholar]
- 10.Franz L, Raming K, Nauen R. 2022. Recombinant expression of ABCC2 variants confirms the importance of mutations in extracellular loop 4 for Cry1F resistance in fall armyworm. Toxins 14, 157. ( 10.3390/TOXINS14020157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denecke S, Rankić I, Driva O, Kalsi M, Luong NBH, Buer B, Nauen R, Geibel S, Vontas J. 2021. Comparative and functional genomics of the ABC transporter superfamily across arthropods. BMC Genom. 22, 1-13. ( 10.1186/S12864-021-07861-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aurade R, Jayalakshmi SK, Sreeramulu K. 2006. Stimulatory effect of insecticides on partially purified P-glycoprotein ATPase from the resistant pest Helicoverpa armigera. Biochem. Cell Biol. 84, 1045-1050. ( 10.1139/o06-194) [DOI] [PubMed] [Google Scholar]
- 13.Zuo Y, Huang J-L, Wang J, Feng Y, Han T-T, Wu Y-D, Yang Y-H. 2017. Knockout of a P-glycoprotein gene increases susceptibility to abamectin and emamectin benzoate in Spodoptera exigua. Insect Mol. Biol. 27, 36-45. ( 10.1111/imb.12338) [DOI] [PubMed] [Google Scholar]
- 14.Bentz J, et al. 2013. Variability in P-glycoprotein inhibitory potency (IC50) using various in vitro experimental systems: implications for universal digoxin drug-drug interaction risk assessment decision criteria. Drug Metab. Dispos. 41, 1347-1366. ( 10.1124/DMD.112.050500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott JG, Buchon N. 2019. Drosophila melanogaster as a powerful tool for studying insect toxicology. Pestic. Biochem. Physiol. 161, 95-103. ( 10.1016/J.PESTBP.2019.09.006) [DOI] [PubMed] [Google Scholar]
- 16.Denecke S, Swevers L, Douris V, Vontas J. 2018. How do oral insecticidal compounds cross the insect midgut epithelium? Insect Biochem. Mol. Biol. 103, 22-35. ( 10.1016/J.IBMB.2018.10.005) [DOI] [PubMed] [Google Scholar]
- 17.Davies S-A, Cabrero P, Marley R, Corrales GM, Ghimire S, Dornan AJ, Dow JAT. 2019. Epithelial function in the Drosophila malpighian tubule: an in vivo renal model, pp. 203-221. New York, NY: Humana Press. [DOI] [PubMed] [Google Scholar]
- 18.Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. 2008. Organization and function of the blood brain barrier in Drosophila. J. Neurosci. 28, 587-597. ( 10.1523/JNEUROSCI.4367-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. 2009. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J. Neurosci. 29, 3538-3550. ( 10.1523/JNEUROSCI.5564-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denecke S, Fusetto R, Batterham P. 2017. Describing the role of Drosophila melanogaster ABC transporters in insecticide biology using CRISPR-Cas9 knockouts. Insect Biochem. Mol. Biol. 91, 1-9. ( 10.1016/j.ibmb.2017.09.017) [DOI] [PubMed] [Google Scholar]
- 21.Seong KM, Sun W, Clark JM, Pittendrigh BR. 2016. Splice form variant and amino acid changes in MDR49 confers DDT resistance in transgenic Drosophila. Sci. Rep. 6, 23355. ( 10.1038/srep23355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seong KM, Pittendrigh B. 2020. Molecular mechanism of ABC transporter Mdr49A associated with a positive cross-resistance in transgenic Drosophila. Korean J. Appl. Entomol. 59, 341-348. ( 10.5656/KSAE.2020.10.0.044) [DOI] [Google Scholar]
- 23.Sun H, Buchon N, Scott JG. 2017. Mdr65 decreases toxicity of multiple insecticides in Drosophila melanogaster. Insect Biochem. Mol. Biol. 89, 11-16. ( 10.1016/j.ibmb.2017.08.002) [DOI] [PubMed] [Google Scholar]
- 24.Ming X, Thakker DR. 2010. Role of basolateral efflux transporter MRP4 in the intestinal absorption of the antiviral drug adefovir dipivoxil. Biochem. Pharmacol. 79, 455-462. ( 10.1016/J.BCP.2009.08.029) [DOI] [PubMed] [Google Scholar]
- 25.Harrop TWR, Pearce SL, Daborn PJ, Batterham P. 2014. Whole-genome expression analysis in the third instar larval midgut of Drosophila melanogaster. G3 (Bethesda). 4, 2197-2205. ( 10.1534/g3.114.013870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leader DP, Krause SA, Pandit A, Davies SA, Dow JAT. 2018. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res. 46, D809-D815. ( 10.1093/nar/gkx976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broehan G, Kroeger T, Lorenzen M, Merzendorfer H. 2013. Functional analysis of the ATP-binding cassette (ABC) transporter gene family of Tribolium castaneum. BMC Genom. 14, 6. ( 10.1186/1471-2164-14-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchon N, Osman D, David FPA, Fang HY, Boquete J-P, Deplancke B, Lemaitre B. 2013. Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 3, 1755. ( 10.1016/j.celrep.2013.05.019) [DOI] [PubMed] [Google Scholar]
- 29.Estudante M, Soveral G, Morais JG, Benet LZ. 2016. Insights into solute carriers: physiological functions and implications in disease and pharmacokinetics. MedChemComm 7, 1462-1478. ( 10.1039/C6MD00188B) [DOI] [Google Scholar]
- 30.Kim JH, Gellatly KJ, Lueke B, Kohler M, Nauen R, Murenzi E, Yoon KS, Clark JM. 2018. Detoxification of ivermectin by ATP binding cassette transporter C4 and cytochrome P450 monooxygenase 6CJ1 in the human body louse, Pediculus humanus humanus. Insect Mol. Biol. 27, 73-82. ( 10.1111/imb.12348) [DOI] [PubMed] [Google Scholar]
- 31.Yeh JT-H, Nam K, Yeh JT-H, Perrimon N. 2017. eUnaG: a new ligand-inducible fluorescent reporter to detect drug transporter activity in live cells. Sci. Rep. 7, 41619. ( 10.1038/srep41619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jurat-Fuentes JL, Heckel DG, Ferré J. 2021. Mechanisms of resistance to insecticidal proteins from Bacillus thuringiensis. Annu. Rev. Entomol. 66, 121-140. ( 10.1146/ANNUREV-ENTO-052620-073348) [DOI] [PubMed] [Google Scholar]
- 33.Theodoulou FL, Kerr ID. 2015. ABC transporter research: going strong 40 years on. Biochem. Soc. Trans. 43, 1033-1040. ( 10.1042/BST20150139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Port F, et al. 2020. A large-scale resource for tissue-specific CRISPR mutagenesis in Drosophila. Elife 9, e53865. ( 10.7554/ELIFE.53865) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Waterschoot RAB, Schinkel AH.. 2011. A critical analysis of the interplay between cytochrome P450 3A and P-glycoprotein: recent insights from knockout and transgenic mice. Pharmacol. Rev. 63, 390-410. ( 10.1124/pr.110.002584) [DOI] [PubMed] [Google Scholar]
- 36.Samantsidis G-R, Panteleri R, Denecke S, Kounadi S, Christou I, Nauen R, Douris V, Vontas J. 2020. ‘What I cannot create, I do not understand’: functionally validated synergism of metabolic and target site insecticide resistance. Proc. R. Soc. B 287, 20200838. ( 10.1098/rspb.2020.0838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girardi E, et al. 2020. A widespread role for SLC transmembrane transporters in resistance to cytotoxic drugs. Nat. Chem. Biol. 16, 1-10. ( 10.1038/s41589-020-0483-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denecke SM, Driva O, Luong HNB, Ioannidis P, Linka M, Nauen R, Geibel S, Vontas J. 2020. The identification and evolutionary trends of the solute carrier superfamily in arthropods. Genome Biol. Evol. 12, 1429-1439. ( 10.1093/gbe/evaa153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rösner J, Tietmeyer J, Merzendorfer H. 2021. Organic anion-transporting polypeptides are involved in the elimination of insecticides from the red flour beetle, Tribolium castaneum. J. Pest Sci. 94, 1427-1437. ( 10.1007/s10340-020-01317-4) [DOI] [Google Scholar]
- 40.Lanning CL, Fine RL, Corcoran JJ, Ayad HM, Rose RL, Abou-Donia MB. 1996. Tobacco budworm P-glycoprotein: biochemical characterization and its involvement in pesticide resistance. Biochim. Biophys. Acta - Gen. Subj. 1291, 155-162. ( 10.1016/0304-4165(96)00060-8) [DOI] [PubMed] [Google Scholar]
- 41.Porretta D, Epis S, Mastrantonio V, Ferrari M, Bellini R, Favia G, Urbanelli S. 2016. How heterogeneous is the involvement of ABC transporters against insecticides? Acta Trop. 157, 131-135. ( 10.1016/j.actatropica.2016.02.002) [DOI] [PubMed] [Google Scholar]
- 42.Denecke S, Fusetto R, Martelli F, Giang A, Battlay P, Fournier-Level A, O’ Hair RA, Batterham P. 2017. Multiple P450 s and variation in neuronal genes underpins the response to the insecticide imidacloprid in a population of Drosophila melanogaster. Sci. Rep. 7, 11338. ( 10.1038/s41598-017-11092-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chahine S, Seabrooke S, O'Donnell MJ. 2012. Effects of genetic knock-down of organic anion transporter genes on secretion of fluorescent organic ions by Malpighian tubules of Drosophila melanogaster. Arch. Insect Biochem. Physiol. 81, 228-240. ( 10.1002/arch.21066) [DOI] [PubMed] [Google Scholar]
- 44.Bretschneider A, Heckel DG, Vogel H. 2016. Know your ABCs: characterization and gene expression dynamics of ABC transporters in the polyphagous herbivore Helicoverpa armigera. Insect Biochem. Mol. Biol. 72, 1-9. ( 10.1016/j.ibmb.2016.03.001) [DOI] [PubMed] [Google Scholar]
- 45.Perry T, Somers J, Yang YT, Batterham P. 2015. Expression of insect α6-like nicotinic acetylcholine receptors in Drosophila melanogaster highlights a high level of conservation of the receptor:spinosyn interaction. Insect Biochem. Mol. Biol. 64, 106-115. ( 10.1016/j.ibmb.2015.01.017) [DOI] [PubMed] [Google Scholar]
- 46.Phillips MD, Thomas GH. 2006. Brush border spectrin is required for early endosome recycling in Drosophila. J. Cell Sci. 119, 1361-1370. ( 10.1242/jcs.02839) [DOI] [PubMed] [Google Scholar]
- 47.Terhzaz S, Finlayson AJ, Stirrat L, Yang JL, Tricoire H, Woods DJ, Dow JAT, Davies SA. 2010. Cell-specific inositol 1,4,5 trisphosphate 3-kinase mediates epithelial cell apoptosis in response to oxidative stress in Drosophila. Cell. Signal. 22, 737-748. ( 10.1016/j.cellsig.2009.12.009) [DOI] [PubMed] [Google Scholar]
- 48.Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. 2005. GPCR signaling is required for blood-brain barrier formation in Drosophila. Cell 123, 133-144. ( 10.1016/J.CELL.2005.08.037) [DOI] [PubMed] [Google Scholar]
- 49.Dietzl G, et al. 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151-156. ( 10.1038/nature05954) [DOI] [PubMed] [Google Scholar]
- 50.Perkins LA, et al. 2015. The transgenic RNAi project at harvard medical school: resources and validation. Genetics 201, 843-852. ( 10.1534/genetics.115.180208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, Oconnor-Giles KM. 2014. Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 196, 961-971. ( 10.1534/genetics.113.160713) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. 2007. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl Acad. Sci. USA 104, 3312-3317. ( 10.1073/pnas.0611511104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenheim JA, Hoy MA. 1989. Confidence intervals for the Abbott's formula correction of bioassay data for control response. J. Econ. Entomol. 82, 331-335. ( 10.1093/jee/82.2.331) [DOI] [Google Scholar]
- 54.Denecke S, et al. 2022. Characterization of a novel pesticide transporter and P-glycoprotein orthologues in Drosophila melanogaster. FigShare. ( 10.6084/m9.figshare.c.5973580) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included within the article and electronic supplementary material [54].