Abstract
Introduction
Cancer-related fatigue (CRF) is one of the most common and debilitating adverse effects of cancer and its treatment reported by cancer survivors. Physical activity, psychological interventions and management of concurrent symptoms have been shown to be effective in alleviating CRF. This pilot randomised controlled trial (RCT) will determine the feasibility of a telehealth CRF clinic intervention (T-CRF) to implement evidence-based strategies and assess the impact of the intervention on CRF and other clinical factors in comparison to usual care.
Methods and analysis
A parallel-arm (intervention vs usual care) pilot RCT will be conducted at the Princess Alexandra Hospital in Queensland, Australia. Sixty cancer survivors aged 18 years and over, who report moderate or severe fatigue on the Brief Fatigue Inventory and meet other study criteria will be recruited. Participants will be randomised (1:1) to receive the T-CRF intervention or usual care (ie, specialist-led care, with a fatigue information booklet). The intervention is a 24-week programme of three telehealth nurse-led consultations and a personalised CRF management plan. The primary objective of this pilot RCT is to determine intervention feasibility, with a secondary objective to determine preliminary clinical efficacy. Feasibility outcomes include the identification of recruitment methods; recruitment rate and uptake; attrition; adherence; fidelity; apathy; and intervention functionality, acceptability and satisfaction. Clinical and resource use outcomes include cancer survivor fatigue, symptom burden, level of physical activity, productivity loss, hospital resource utilisation and carer’s fatigue and productivity loss. Descriptive statistics will be used to report on feasibility and process-related elements additional to clinical and resource outcomes.
Ethics and dissemination
This trial is prospectively registered (ACTRN12620001334998). The study protocol has been approved by the Metro South Health and Hospital Services Human Research Ethics Committee (MSHHS HREC/2020/QMS/63495). Findings will be disseminated through peer-reviewed publications, national and international conferences and seminars or workshops.
Trial registration number
Australian New Zealand Clinical Trials Registry ID: ACTRN12620001334998; Pre-results. Trial Version: Version 1.1. Last updated 10 December 2020.
Keywords: Telemedicine, ONCOLOGY, COMPLEMENTARY MEDICINE
Strengths and limitations of this study.
This randomised controlled trial (RCT) assesses a ‘telehealth cancer-related fatigue clinic’ intervention embedded in the community setting as distinct from the clinical setting.
This feasibility pilot RCT study will provide data for an adequately powered effectiveness trial.
This study design will enable individualised treatment flexibility and compare interventions in a real-world community setting to realistically inform clinical and community practice directly.
This study is not powered to examine intervention efficacy and does not assess regionality.
Due to the nature of the intervention, blinding of the participants and treatment providers (cancer nurses and intervention physiotherapist) will not be possible.
Introduction
Background
Cancer-related fatigue (CRF) is one of the most common and debilitating adverse effects experienced by cancer survivors during and after cancer treatment,1 2 with two in three cancer survivors reporting some level of fatigue, and one in three cases assessed as severe.2 CRF differs to ‘normal’ fatigue as it cannot not be relieved through rest and sleep, and is defined as ‘a distressing persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning’.1 While the exact mechanisms of CRF are unknown, its influence on the quality of life and functional capacity of cancer survivors is well established. CRF has long lasting negative impacts on the physical, mental, emotional and social well-being of people with cancer,3–9 often resulting in general weakness, diminished concentration or attention, emotional instability and decreased motivation or interest to engage in usual activities.1 10 CRF can also adversely affect the ability to return to work and engage in meaningful social relationships and leisure activities; negatively affecting cancer survivors’ mental health and quality of life.11 12 Moreover, CRF can influence a cancer survivor’s willingness to commence or continue with their cancer treatment, or their willingness and ability to attend follow-up appointments, potentially influencing treatment outcomes and survival.10 While the prevalence of CRF is high during active treatment, many cancer survivors also continue to report moderate to severe fatigue at 12 months post diagnosis and for several years after treatment completion.13 Additionally, caregivers of cancer survivors can also face significant emotional, physical, psychosocial and spiritual fatigue burden that affects their productivity, particularly while those they are caring for receive active treatment.11 14–17
Many studies have assessed pharmacological and non-pharmacological strategies to reduce CRF.13 Despite their prior use, pharmacological treatments (eg, modafinil, erythropoietin, methylphenidate) are largely ineffective for CRF, and may be potentially harmful to its users.18–20 Several guidelines, including the ‘National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for CRF’, now recommend non-pharmacological treatments including physical activity (ie, aerobic exercise, resistance exercise, yoga), psychological interventions (ie, cognitive–behavioural therapy, psychoeducational therapy), physical therapies (ie, massage, acupuncture) and energy conservation techniques.1 10 A meta-analysis including 113 randomised clinical trials that involved 11 525 cancer survivors identified that exercise (weighted effect size (WES): 0.30; 95% CI 0.25 to 0.36; p<0.001), psychological (WES: 0.27; 95% CI 0.21 to 0.33; p<0.001) or combined (WES: 0.26; 95% CI 0.13 to 0.38; p<0.001) interventions as the most effective strategies for reducing CRF during and after cancer treatment when compared with pharmacologic interventions and other therapies.21 In addition, there is also recent evidence suggesting that the management of concurrent symptoms also improves CRF.22
Despite high-quality evidence of effective management strategies for CRF, it remains an unmet need for most cancer survivors, suggesting that current management strategies are not well implemented in clinical practice.1 2 A recent scoping review on the implementation of CRF management strategies into clinical practice identified a lack of high-quality studies in the literature which also highlights the disconnect between effective CRF interventions and routine clinical care.23 As a key implementation strategy, the concept of a ‘CRF clinic’ is one successful method to facilitate the systematic assessment and management of CRF in cancer survivors.24 25 These clinics are often physician-led, provided in well-resourced centres, and require cancer survivors to attend face-to-face appointments at the cancer centre.24 25 With CRF being one of the most common unmet needs reported by cancer survivors, it is key to develop and test more accessible and sustainable methods for delivering such CRF clinics. First, with the increasing use of telehealth, especially in the post-COVID era, it is extremely important to determine if a CRF clinic can be sufficiently delivered using telehealth.26 27 Second, trained cancer nurses28 are already managing a myriad of cancer symptoms and delivering psychological and physical activity interventions in their practice,29 which are key evidence-based strategies for managing CRF. Nurses in partnership with allied health practitioners, key members of multidisciplinary cancer care, are the ideal workforce to lead CRF clinics and enhance service accessibility, ultimately facilitating implementation of evidence-based care and improving CRF outcomes in cancer survivors.
Our pilot randomised controlled trial (RCT) seeks to determine the feasibility of a community-based and cancer nurse-led, telehealth CRF (T-CRF) intervention and assess the preliminary efficacy of the intervention on CRF and other clinical factors in comparison to usual care for cancer survivors and carers. Specifically, this trial will evaluate the feasibility of implementing the T-CRF intervention within the community setting into routine care by assessing recruitment, attrition, functionality, acceptability, satisfaction with care, adherence among participants and intervention fidelity among programme administrators. This trial will also evaluate the preliminary efficacy of the T-CRF intervention according to clinical and resource outcomes including cancer survivor fatigue, symptom burden, physical activity, productivity loss, hospital resource utilisation and carer fatigue and productivity loss. Preliminary efficacy data will be used to determine appropriate effect sizes and other statistical data that can be used in future statistical models to estimate sample sizes required to run the definitive clinical effectiveness trials.
Methods and analysis
Study design
A parallel-group, pilot RCT (1:1, intervention vs usual care) study design will be used to determine the feasibility and evaluate the preliminary efficacy of the T-CRF intervention. More specifically, this pilot RCT will provide feasibility and process data to inform the design of a fully powered RCT that will compare the effects of a novel clinic model of care verses usual care on the severity of CRF and related symptom outcomes. The pilot study design will incorporate individualised treatment flexibility in a real-world setting to provide realistic estimates of effects when implemented in the definitive RCT.30 The study protocol (v1.1) has been prepared in accordance with the Standard Protocol Items: Recommendations for Interventional Trials statement.31
Study setting
Cancer survivors and their carers will be recruited through outpatient clinics of the Division of Cancer Services at the Princess Alexandra Hospital (PAH), a tertiary hospital located in Brisbane, Queensland, Australia.
Participants
Eligibility criteria
Cancer survivors experiencing moderate to severe fatigue (ie, Brief Fatigue Inventory (BFI) score of 4 or greater32), and who are receiving cancer treatment at the PAH will be approached for recruitment. Eligible participants will be over 18 years of age and be at least 6 weeks post completion of primary cancer treatment (ie, surgery, radiotherapy, chemotherapy) or have completed at least 3 months of maintenance treatment (ie, hormone therapy, immunotherapy, chemotherapy). One informal carer of recruited cancer survivors will also be invited to participate if they are over 18 years of age. Further details of eligibility criteria for carers and cancer survivors are provided in table 1.
Table 1.
Study eligibility criteria
| Cancer Survivor (Inclusion Criteria) |
Cancer Survivor (Exclusion Criteria) |
Carer (Inclusion Criteria) |
Withdrawal Criteria (If Applicable) |
| ≥18 years of age | Presence of severe mental, cognitive or physical conditions that would limit the person’s ability to participate. This ensures patients have the capacity to provide informed consent, and participation in the study will not pose unethical burden on the person. | ≥18 years of age | Altered mental capacity resulting in inability to provide continuing informed consent. |
| Have a definitive diagnosis of solid tumour or haematological cancer Receive care at the Princess Alexandra Hospital outpatient clinics |
Self-endorsing or identified by cancer survivors as ‘a relative, friend, or partner who you have a close relationship with and who assists you with medical care on a regular basis and who may or may not live in the same residence as you and who is not paid for their help’. | ||
| Be 6-week post completion of primary cancer treatment (ie, surgery, radiotherapy, chemotherapy) or have completed at least 3 months of maintenance treatment (ie, hormone therapy, immunotherapy chemotherapy) | Known prognosis of <6 months at the discretion of the treating clinician. This ensures participation in this study will not pose unethical burden on cancer survivors nearing end of life. | The caregiver’s care recipient must be participating in the study. | Unforeseeable circumstances where participation in this study may pose unethical burden on the cancer survivor and/or carer or hinder their ability to provide informed consent. |
| ≥4 on the global fatigue score of the Brief Fatigue Inventory | Medical conditions or circumstances (eg, active infections) where participation in this study may pose unethical burden on the cancer survivor or hinder their ability to provide informed consent or participate. | Death | |
| Eastern Cooperative Oncology Group performance status of ≤2 | |||
| Not currently receiving specialist palliative care | |||
| Have access to a telephone/mobile device or a computer and internet connection. Agrees and has the capacity to upload wearable device data |
Recruitment and consent
Potentially eligible cancer survivors will be identified and approached by their treating clinicians who will gauge their interest in the study, provide a study brochure and gain verbal consent to being approached by the research team. Cancer survivors will be contacted by a research team member, screened for eligibility and provided with study information. After a time of reflection (at least 24 hours), cancer survivors will be invited to sign a consent form (online supplemental material 1) to indicate their willingness to participate. At the time of consent, cancer survivor participants will be asked for their consent to contact their primary informal carer. Informal carers (individuals self-endorsed or identified by cancer survivors as a relative, friend or partner they have a close relationship with and who assists them with care) will be contacted by the research team, provided with study information and after a time of reflection (at least 24 hours), they will be invited to sign a consent form (online supplemental material 2) to indicate willingness to participate in the trial.
bmjopen-2021-059952supp001.pdf (534.7KB, pdf)
Trial procedures
Sample size
Sixty cancer survivors experiencing moderate or severe fatigue (n=30 per arm) will be recruited. This study is not hypothesis testing; thus, power level is not the consideration underpinning sample size. Our chosen sample size for this study falls within the range of recommendations for preliminary studies of this nature.33 34 PAH service data indicate a throughput of more than 30 cancer survivors per week. Of these, approximately 30%–50% will report moderate-to-severe CRF.2 As our research team is embedded within the clinical care team at the PAH, we anticipate a high referral rate (~10 per week) and a recruitment rate of ~5 per week following full eligibility screening. All consented cancer survivors will be invited to refer their informal carer to participate in the trial. The sample size for informal carers is expected to be approximately 30, as we anticipate 50% of the carers referred by recruited cancer survivors will agree to participate in the study.
Randomisation and allocation
Randomisation occurs at the level of the cancer survivor participant. Carer participants are assigned to the same group as their cancer survivor. Computer-generated random numbers will be used to allocate cancer survivor participants in a 1:1 ratio by a researcher not involved in recruitment, intervention implementation or data collection. Allocation numbers will be sealed in opaque envelopes prepared by an independent researcher. Randomisation will be blocked using random permuted blocks of four and six to ensure that the groups are balanced periodically within stratification groups. To ensure equal distribution of participants with different levels of fatigue, participants will be stratified by their fatigue severity (moderate: 4–6 or severe 7–10 on the BFI scale) at baseline.
Blinding
Outcome assessors and data analysts will be blinded to group allocation. Participants will be advised not to reveal their group allocation to the outcome assessor. Due to the nature of intervention, trial participants and intervention administrators will not be blinded to group allocation.
Intervention
All participants will be provided with a written 3-page booklet on ‘Fatigue and Cancer’ published by Cancer Council Australia,35 regardless of arm assignment.
Arm 1: the T-CRF clinic (intervention)
The overarching aim of the intervention is to systematically implement evidence-based strategies including, but not limited to the promotion of physical activities/exercise intervention; delivery of psychological interventions; management of concurrent symptoms; and general coping. The design of the T-CRF clinic is informed by the NCCN CRF guidelines1 and incorporates CRF assessment, the development of management strategies, and the provision of referral pathways. Specific components of the T-CRF clinic intervention are listed in online supplemental material 3.
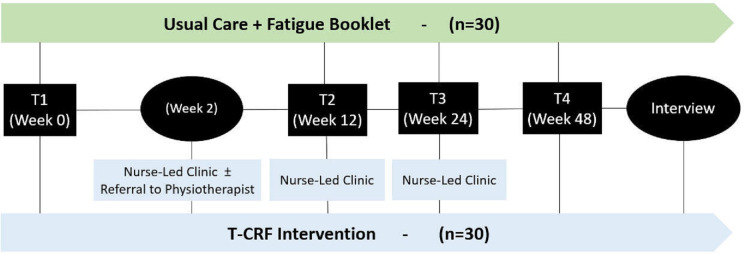
Briefly, after cancer survivor participant enrolment, nurses working at the non-government organisation Cancer Council Queensland (CCQ) will receive a referral from the research team indicating cancer survivor medical and treatment histories; fatigue severity; physical activity behaviours; nutritional status; any contraindications to unsupervised exercise recommendations; and a recommended clinic schedule at weeks 0–2, week 12–14 and week 24–26 post baseline (see figure 1). The CCQ nurse will contact cancer survivor participants directly to arrange three telehealth clinic appointments and four booster phone calls, two between each clinic appointment. During clinic consultations, nurses will: (1) conduct a CRF assessment; (2) provide verbal education on fatigue management addressing: physical activity, current symptoms and/or general coping; (3) codevelop a CRF management plan including up to three Specific, Measurable, Achievable, Relevant, and Time bound (SMART) goals that address physical activity, current symptoms and/or general coping; and (4) facilitate referrals. During consultations, CCQ nurses will be guided by a nurse clinic checklist that details the required components of each clinic session. Where referral pathways at PAH are not available or appropriate, CCQ nurses will refer cancer survivors to community organisations or to their primary care provider to coordinate community referrals. Cancer survivor participants will be emailed or posted a copy of their CRF management plan developed by the research team (see online supplemental material 4).
Figure 1.
The telehealth cancer-related fatigue clinic model for cancer survivors follow-up. Schematic of the trial design. T1: baseline; T2: 12–14 weeks post baseline; T2: 24–26 weeks post baseline; T4: 48 weeks post baseline. T-CRF, telehealth cancer-related fatigue.
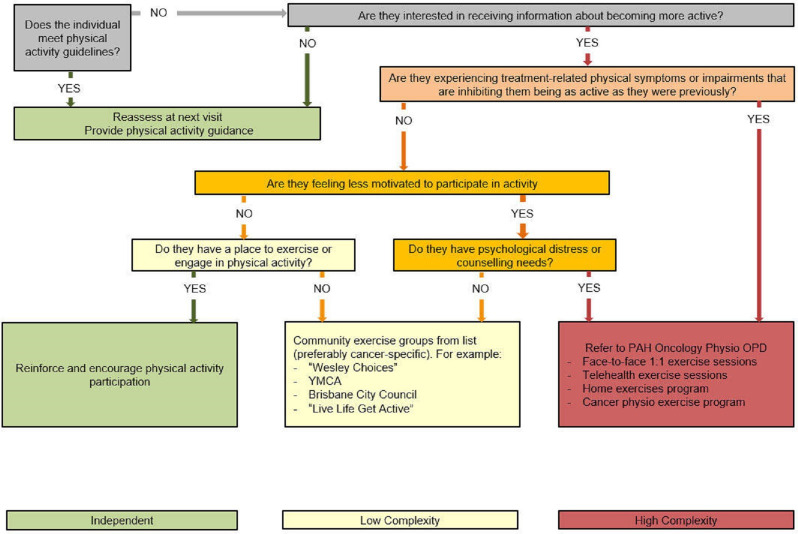
CCQ nurses will make general recommendations for exercise intensity levels and supervision based on an adapted clinical pathway triage algorithm developed by Stout et al36 (figure 2). This decision-making support tool enables personalised condition assessment, risk stratification and referral to optimal settings for exercise promotion in cancer survivors—in this regard, to address CRF. Participants who require exercise supervision will be referred to the cancer physiotherapist of the PAH, who will offer face-to-face or telehealth group exercise sessions over 12 weeks (once weekly) or 6 weeks (twice weekly) or one-on-one exercise sessions including aerobic, resistance, flexibility and balance activities depending on individual need and available equipment. Face-to-face group exercise allows for eight participants supervised by two physiotherapists, and telehealth group exercise allows for five participants supervised by one physiotherapist. Attendance at supervised exercise sessions or referrals to community exercise programmes will be recorded as a measure of adherence to the intervention. Between the first and second T-CRF clinics, CCQ nurses will provide two 10–20 min follow-up booster phone calls to participants to monitor progress towards meeting SMART goals and offer support. Adherence to the intervention will be monitored using clinic and phone review checklists.
Figure 2.
Algorithm for determining exercise intensity and levels of supervision. The pathway is intended to stratify individuals to higher (red), intermediate (yellow) or lower (green) condition complexity, which provides insight into the level of supervision and guidance that individuals may need to successfully engage in exercise and informs referrals. OPD, outpatient department; PAH, Princess Alexandra Hospital; YMCA, Young Men's Christian Association.
Intervention training and adverse events (AEs)
CCQ nurses have extensive experience in caring for cancer survivors. Intervention physiotherapists are nationally accredited by the Australian Physiotherapy Association and have extensive experience caring for cancer survivors. CCQ nurses will receive additional training with regards to all components of the T-CRF intervention. Briefly, training will comprise a written manual with information on how to deliver the intervention, and material on communication, motivational interviewing and cognitive behavioural techniques; and a 1-day workshop incorporating a mix of written mock intervention case studies and motivational interviewing role play activities.
Participants requiring supervised exercise require medical clearance from their treating oncology team and will undergo a comprehensive initial assessment with vital signs monitored pre and post exercise by the intervention physiotherapist to ensure safety. Procedural guidelines are in place to deal with unexpected exercise-related AEs as clinically indicated. Existing incident reporting structures at the PAH will be followed and the participant’s treating clinician, cancer nurse coordinator and CCQ intervention nurses will be informed. A detailed review of cancer survivor participant assessment forms and exercise history will be undertaken by an independent oncologist. For participants who experience any emotional distress during CCQ intervention nurse consults will be referred to the CCQ counselling service consisting of nurse counsellors and psychologists for evaluation and clinical management.
Intervention fidelity
In addition to the use of clinic and phone booster checklists, participants and CCQ nurses will be asked to consent to the audio recording of all nurse-led clinics for quality assurance and to recheck any data or information. Fidelity of the intervention will be assessed using the framework for behavioural interventions recommended by the National Institutes of Health37 38 as outlined in table 2. It is expected that some of these strategies will be refined through the conduct of the pilot trial.
Table 2.
Intervention fidelity strategies
| Study design |
Study design procedures have been designed to ensure tha the study can adequately test its hypotheses in relation to underlying theory and clinical practices. |
| Training providers | Standardised provider training includes procedures to ensure that interventionists have been satisfactorily trained to deliver the intervention to cancer survivor participants. This training will involve:
|
| Delivery of treatment | Intervention procedures will be monitored to improve delivery of intervention and comparison of conditions, and ensure that the intervention is delivered as intended, through:
|
| Receipt of treatment | Treatment receipt focuses on the cancer survivor participant and includes procedures to assure that the treatment was both received and understood. This goal will be achieved by:
|
| Enactment of treatment skills | Enactment of treatment skills includes processes to monitor and improve cancer survivor participant ability to perform treatment-related behavioural skills and cognitive strategies in relevant real-life settings as intended. This goal will be achieved by:
|
CRF, cancer-related fatigue; NCCN, National Comprehensive Cancer Network.
Arm 2: control (usual care)
The control arm consists of usual follow-up care plus a written 3-page booklet on Fatigue and Cancer published by Cancer Council Australia.35 Follow-up arrangements at the PAH will vary primarily according to cancer type, and is determined by the treating surgeon, medical oncologist or radiation oncologist through a specialist-led model.
Baseline and follow-up procedures
Study schedules for data collection and a schematic of the trial design are shown in table 3 and figure 1, respectively. Clinical characteristics and demographics (ie, age, gender, ethnicity, highest level of education, living arrangements, marital status, employment) will be collected directly from participants and medical records by outcome assessors at baseline (T1). All participant-reported outcomes will be collected at baseline (T1), 12–14 weeks (T2), 24–26 weeks (T3) and 48 weeks (T4) post baseline. Instruments will be self-administered via online surveys using Research Electronic Data Capture (REDCap) or interviewer-administered by blinded outcome assessors via telephone. Participants and healthcare providers will be invited to opt into a semistructured interview at T4 either face to face, by telephone or through videoconferencing as per interviewee preference. Semistructured interviews will be used to collect data on intervention functionality, acceptability and satisfaction that will be guided by the Consolidated Framework for Implementation Research (CFIR) (see online supplemental material 5 for the interview guide).
Table 3.
Study schedule for data collection
| Process | EST time (min) | Consent | Baseline (T1) |
Week 2* | Week 13±1* (T2) |
Week 25±1* (T3) |
Week 49±1 (T4) |
| Self-report Data Collection — Cancer Survivor Participant | |||||||
| Garmin Education | 5 | X | |||||
| PG-SGA SF (10 items) | 5 | X | |||||
| BFI (10 items) | 3 | X | X | X | X | ||
| MSAS (32 items) | 5 | X | X | X | X | ||
| AAS and Fruit and Vegetable intake (8 items) | 5 | X | X | X | X | ||
| AES-S (18 items) | 5 | X | X | X | X | ||
| Productivity (3 items) | 2 | X | X | X | X | ||
| Interview | 15 | X | |||||
| Medical Record Data Collection — Cancer Survivor Participant | |||||||
| Participant Characteristics | X | ||||||
| Garmin Data | X | X | X | X | |||
| Health Resource Data | X | X | X | ||||
| Process Outcomes Data | X | X | X | ||||
| Referral to Services† | O | O | O | ||||
| Self-Report Data Collection — Carer | |||||||
| BFI (10 items) | 5 | X | X | X | X | ||
| iVICQ (14 items) | 10 | X | X | X | X | ||
| Interview† | 15 | X | |||||
*Data collection will occur as close as practically possible to the timepoint.
†Only completed for participants in the telehealth cancer-related fatigue intervention group.
AAS, The Active Australia Survey; AES-S, self-reported apathy; BFI, Brief Fatigue Inventory; iVICQ, institute for Medical Technology Assessment Valuation of Informal Care Questionnaire; MSAS, Memorial Symptom Assessment Scale; O, conducted by intervention nurse; PG-SGA SF, Patient-Generated Subjective Global Assessment Short Form; T, time point; X, conducted by research assistant.
Outcomes
Primary outcomes include measurements relevant to the feasibility of conducting large-scale RCT. Secondary outcomes involve measurements of preliminary clinical efficacy intended for use in the full-scale trial.
Trial feasibility
Feasibility of the T-CRF trial is the primary outcome of this pilot RCT and will be assessed using the following process outcomes: recruitment and uptake, attrition, adherence, fidelity, apathy, functionality, acceptability and satisfaction with the intervention (see table 4).
Table 4.
Study outcome measures
| Outcome domain | Specific measurement | Metric and method of aggregation | Time-point of interest |
| Process measures | |||
| Apathy | Self-Reported Apathy Evaluation Scale39 | Effect of time on mean change score between groups | Baseline, 12 weeks, 24 weeks and 48 weeks |
| Adherence and fidelity among programme administrators: completion of clinic records | Completion of items on the nurse clinic checklist and booster phone checklist. | 12 weeks, 24 weeks and 48 weeks | |
| Adherence and fidelity: referrals to allied health and community services | Number and type of allied health and community service referrals raised and number actioned and attended as reported by research assistant, intervention nurse or cancer survivor participant and verified with electronic hospital medical records | 12 weeks, 24 weeks and 48 weeks | |
| Treatment fidelity among programme administrators: intervention delivery | Audio and video recording of telehealth sessions. | 12 weeks, 24 weeks and 48 weeks | |
| Intervention functionality, acceptability and satisfaction. | Semistructured interviews with stakeholders (ie, cancer survivor participants, carer participants, CCQ nurses, other healthcare providers) to discuss acceptability, and barriers and facilitators to implementing of T-CRF intervention. | 48 weeks | |
| Recruitment and attrition | Information from research assistant records and hospital records. | Baseline, 48 weeks. | |
| Clinical outcome measures | |||
| Fatigue | Brief Fatigue Inventory (BFI)32 | Effect of time on mean change score between groups | Baseline, 12 weeks, 24 weeks and 48 weeks |
| Symptom distress | Memorial Symptom Assessment Scale44 | Effect of time on mean change score between groups | Baseline, 12 weeks, 24 weeks and 48 weeks |
| Physical activity (subjective) | Active Australia Survey45 International Physical Activity Questionnaire short-form46 |
Effect of time on mean change score between groups | Baseline, 12 weeks, 24 weeks and 48 weeks |
| Physical activity (objective) | Number of steps per day, number of stairs climbed per day, total hours doing moderate intensity exercise per day, and total hours slept per day as measured by Garmin wrist-worn activity tracker (VívoSmart 4, Garmin Australasia Pty Ltd, NSW, Australia) | Effect of time on mean change score between groups | Baseline, 12 weeks, 24 weeks and 48 weeks |
| Productivity loss | Incidence and severity of financial distress and employment interference as measured by a 3-item survey developed by the research team.47 48 | Effect of time on mean change score between groups | Baseline, 12 weeks, 24 weeks and 48 weeks |
| Hospital resource utilisation | Electronic hospital medical records | Number of hospital admissions and emergency presentations | Baseline, 12 weeks, 24 weeks and 48 weeks |
| Carer’s fatigue | BFI32 | Effect of time on mean change score between groups | Baseline, 12 weeks, 24 weeks and 48 weeks |
| Carer’s productivity loss | Modified version of the Institute for Medical Technology Assessment Valuation of Informal Care Questionnaire.49 | Effect of time on mean change score between groups | Baseline, 12 weeks, 24 weeks and 48 weeks |
| Cancer survivor participant characteristcs | |||
| Demographics | Participant interview | Collection of age, gender, ethnicity, education, living arrangements, marital status and employment | Baseline |
| Clinical characteristics | Participant interview Malnutrition Screening Tool50 |
Past and current medical conditions and syndromes, current medications and supplements, cancer diagnosis, previous cancer treatment, current cancer treatment and fatigue history using participant interview, and nutrition risk | Baseline |
CCQ, Cancer Council Queensland; T-CRF, telehealth cancer-related fatigue.
Recruitment, intervention uptake and attrition will be assessed using screening logs and online REDCap survey data. Intervention adherence and fidelity of intervention nurses will be evaluated through assessing the number of items completed on the nurse-clinic checklist during consultations and booster phone calls. Apathy will be measured using the Self-Reported Apathy Evaluation Scale.39 Intervention functionality, acceptability and satisfaction will be evaluated using a cancer survivor satisfaction survey as well as stakeholder semistructured interviews. Semistructured interviews will be conducted with intervention nurses and other healthcare providers involved in providing care for cancer survivor participants. Cancer survivors and carers allocated to the T-CRF trial arm will be invited to participate in an interview at the 12-month time point. Guiding questions (see online supplemental file 5) and analysis of the interviews will be guided by the CFIR.
Clinical outcomes
A secondary goal is to assess the preliminary efficacy of the T-CRF intervention on cancer survivor’s fatigue, symptom burden, productivity loss, hospital resource utilisation, level of physical activity, as well as carer’s fatigue, and carer’s productivity loss. These outcomes will be assessed using validated self-report measures and medical record data as described in table 3. Additionally, participants will be required to wear a Garmin wrist-worn activity monitoring device at no additional cost. This will measure physical activity (pedometer: number of steps per day, altimeter: number of stairs climbed, total hours doing moderate intensity exercise based on heart rate per day and total hours slept per day).
Withdrawal and study termination
Any participant can withdraw from the study at any time and for any reason without prejudice. If a cancer survivor or carer participant is withdrawn because of an AE, the investigator will arrange for appropriate follow-up care until the AE is resolved or has stabilised. Unresolved AEs will be followed until the last scheduled follow-up visit or until no longer indicated, per investigator discretion. In addition to AEs, other reasons for removal of participants from the study might include, but are not limited to, withdrawal of consent, administrative decision by the principal investigator or responsible organisation, or protocol deviation. If a participant asks or decides to withdraw, all efforts will be made to complete and report the observations, especially primary and secondary objectives, as thoroughly as possible up to the date of withdrawal. The primary reason for withdrawal (where known) will be identified and recorded on a case report form, along with the date of withdrawal. Withdrawal criteria are listed in table 1.
Statistical analysis
Descriptive statistics will be used to report on feasibility and process-related outcomes (eg, recruitment rate, retention and attrition rates, adherence) as well as clinical and resource-use outcomes (eg, fatigue, physical activity, hospital resource utilisation). Preliminary effect size estimates for cancer survivor and resource use outcomes will be calculated following intention-to-treat principles using linear mixed models to account for repeated measures and missing data. Effect sizes will be reported as estimates with a 95% CI, and without p values due to the underpowered nature of the study. Models will include group, time and their interaction and be adjusted by fatigue severity and current cancer treatment. Balance of demographic variables between the usual care and intervention group will be examined and adjusted for potential confounders. Assumptions of all models (normality, linearity, homoscedasticity) will be examined using the residuals of the model and will be described using mean, median, skewness, kurtosis and plots such as histograms and QQ-plots. If assumptions are violated, models will be either bootstrapped or log transformed, as appropriate. Missing data will be explored using descriptive statistics. Generalised linear models will be used to provide estimates for categorical outcomes such as adherence, with appropriate link models used based on the outcome distribution. All statistical analysis will be undertaken by an independent statistician blinded to treatment allocation. Semistructured interviews will be audio or video recorded and will be transcribed verbatim for thematic analysis; a method for systematically identifying, organising and offering insight into, patterns of meaning (themes) across a dataset.40
Data management
Data management and confidentiality
All data will be recorded in electronic case report forms. Participants will only be identified by a unique participant study number on the case report forms and other documents. A secure system for online and offline data capture will be used for direct data entry by both participants and research staff. Case report forms will be accessed by the project manager for data checking. Data queries will be generated and sent to the relevant research team member for response before the database is locked and released for statistical analysis. Other study-related documents (eg, signed informed Participant Information and Consent Form (PICF)) will be kept in strict confidence by the Chief Investigator.
Data checking
Data will be directly entered into REDCap by members of the research team using a tablet or desktop computer. All research team members will receive training regarding data collection from the Project Manager. To maximise data integrity and completeness, the project manager will undertake routine audits with data validation performed via REDCap. Any discrepancies and missing data will be alerted and resolved with the relevant research team member(s) as soon as practical. All electronic case report forms will be maintained on the system with details of any changes logged accordingly.
Data protection
Participants will be informed that data will be archived at PAH and that these data may be viewed by staff including the project manager and by external auditors on behalf of PAH and appropriate regulatory authorities including Metro South Health Human Research Ethics Committee (MSH HREC) and PAH Research Governance. Participants will be informed that a study report will be submitted to regulatory authorities and for publication and conference presentation. However, participants will be deidentified in such reports with only their study identification number, gender and age used for recording or linkage purposes.
Data retention
Audio and video recordings of the telehealth intervention will be stored electronically at Queensland University of Technology in a secure repository and will be securely destroyed after analysis is conducted. All other source data, clinical records and laboratory data relating to the study will be archived at PAH for at least 15 years after study completion and remain available for retrospective review or audit. The investigator and study staff will be responsible for maintaining a comprehensive filing system of all essential study-related documentation. All essential documentation will be retained by PAH as per the requirements of the responsible organisation for the same period required for medical records retention. No study document will be destroyed without written agreement between the PAH and the principal investigator. If the principal investigator wishes to assign the study records to another party or move them to another location, they will notify the responsible organisation in writing of the new responsible person or the new location.
Patient and public involvement
To ensure cancer survivor perspectives were represented and accommodated in the intervention design and implementation, the patient support and advocacy group at CCQ were invited, have provided input into the study. Consumers were invited to provide comments and critical appraisal of the study protocol. They will also assist with raising the profile of the study through their consumer and clinical networks. CCQ will also be providing the intervention cancer nurses who will deliver the T-CRF clinics.
Ethics and dissemination
Prior to the commencement of the study, written approval was acquired by MSH HREC (ID: HREC/2020/QMS/63495), with Research Governance approval provided by the PAH Research Governance Office. This study will be conducted in accordance with the principles of the Declaration of Helsinki,41 Good Clinical Practice (CPMP/ICH/135/95)42 and the National Health and Medical Research Council National Statement on Ethical Conduct in Research Involving Humans.43
Archiving and regulatory inspection
In accordance with the guidance on Good Clinical Practice (GCP), this study may be selected for audit. Inspection of site facilities and review of study-related records may occur by a representative of the responsible organisation or regulatory authority to evaluate the study conduct and compliance with the protocol, GCP and applicable regulatory requirements. All study-related documents and records will be retained for a minimum of 15 years after trial completion. Written agreement from the responsible organisation will precede destruction of the same.
Dissemination
Publication and reporting of results and outcomes of this trial will be accurate and honest, undertaken with integrity and transparency. Trial results will be disseminated to all participants with a summary sheet that will outline the trial findings in lay language. It is intended that the findings from this trial will be disseminated at academic, clinical and professional conferences, and published in high-quality, international peer-reviewed journals.
Protocol amendments and deviation
Neither the principal investigator nor the PAH will modify or alter this protocol without the agreement of the other. All agreed protocol amendments will be clearly recorded on a protocol amendment form and will be signed and dated by the original protocol approving signatories. All protocol amendments will be submitted to the MSH HREC for approval before implementation. The only exception will be when the amendment is necessary to eliminate an immediate hazard to the trial participants. In this case, the necessary action will be taken first, with the relevant protocol amendment following shortly thereafter. Should any protocol deviation occur, it will be reported to the study project manager as soon as is practical. The deviation and the reason for its occurrence will be included in the study report.
Trial status
This protocol (V.1.1) was approved and registered on the Australian New Zealand Clinical Trial Registry (ANZCTR) on the 10 December 2020 (ID: ACTRN12620001334998). The study started recruitment on 17 February 2021, and as of 1 November 2021, 40 cancer survivor participants and 16 carers have been enrolled. Data collection is anticipated to conclude in January 2023 (48 weeks following the final participant enrolled). Data analysis and manuscript preparation are anticipated to occur over 6 months, concluding in July 2023.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Cancer Council Queensland who have provided feedback on the study and contributed their telehealth facilities and the cancer nurses who will be delivering the intervention.
Footnotes
Twitter: @Fiona_CW1, @DrNicolasHart, @rayychan
Contributors: RJC and RL conceptualised the study. RJC, RL, PY, SMM, CPE, JFA MBP, BH, and GL acquired and received the funding. JT, BH, JFA provided input on motivational interviewing, counselling techniques, and resources for the nurse-led intervention. OAA, EPP, JN, LT, FCW and NHH led the design, development, and writing of the pilot RCT protocol. LJ provided data and statistical analysis methods. All authors contributed important intellectual content to the trial design and written protocol and reviewed and approved the final version for publication.
Funding: This work is financially supported by the Princess Alexandra Research Foundation (Award Number: RSS_2020_095). RJC (#1194051), PY (#2009529), and SMM (#1161138) receive salary support from National Health and Medical Research Council administered fellowships. The funding bodies have no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Berger AM, Mooney K, Alvarez-Perez A, et al. Cancer-Related fatigue, version 2.2015. J Natl Compr Canc Netw 2015;13:1012–39. 10.6004/jnccn.2015.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molassiotis A, Yates P, Li Q, et al. Mapping unmet supportive care needs, quality-of-life perceptions and current symptoms in cancer survivors across the Asia-Pacific region: results from the International step study. Ann Oncol 2017;28:2552–8. 10.1093/annonc/mdx350 [DOI] [PubMed] [Google Scholar]
- 3.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol 2000;18:743–53. 10.1200/JCO.2000.18.4.743 [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez Antolín A, Martínez-Piñeiro L, Jiménez Romero ME, et al. Prevalence of fatigue and impact on quality of life in castration-resistant prostate cancer patients: the vital study. BMC Urol 2019;19:92. 10.1186/s12894-019-0527-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the fatigue coalition. Oncologist 2000;5:353–60. 10.1634/theoncologist.5-5-353 [DOI] [PubMed] [Google Scholar]
- 6.Lis CG, Rodeghier M, Grutsch JF, et al. Distribution and determinants of patient satisfaction in oncology with a focus on health related quality of life. BMC Health Serv Res 2009;9:190. 10.1186/1472-6963-9-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luthy C, Cedraschi C, Pugliesi A, et al. Patients' views about causes and preferences for the management of cancer-related fatigue-a case for non-congruence with the physicians? Support Care Cancer 2011;19:363–70. 10.1007/s00520-010-0826-9 [DOI] [PubMed] [Google Scholar]
- 8.Banipal RPS, Singh H, Singh B. Assessment of cancer-related fatigue among cancer patients receiving various therapies: a cross-sectional observational study. Indian J Palliat Care 2017;23:207–11. 10.4103/IJPC.IJPC_135_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charalambous A, Kouta C. Cancer related fatigue and quality of life in patients with advanced prostate cancer undergoing chemotherapy. Biomed Res Int 2016;2016:3989286 10.1155/2016/3989286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bower JE. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat Rev Clin Oncol 2014;11:597–609. 10.1038/nrclinonc.2014.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofman M, Ryan JL, Figueroa-Moseley CD, et al. Cancer-related fatigue: the scale of the problem. Oncologist 2007;12 Suppl 1:4–10. 10.1634/theoncologist.12-S1-4 [DOI] [PubMed] [Google Scholar]
- 12.Wolvers MDJ, Leensen MCJ, Groeneveld IF, et al. Predictors for earlier return to work of cancer patients. J Cancer Surviv 2018;12:169–77. 10.1007/s11764-017-0655-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thong MSY, van Noorden CJF, Steindorf K, et al. Cancer-Related fatigue: causes and current treatment options. Curr Treat Options Oncol 2020;21:17. 10.1007/s11864-020-0707-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark MM, Atherton PJ, Lapid MI, et al. Caregivers of patients with cancer fatigue: a high level of symptom burden. Am J Hosp Palliat Care 2014;31:121–5. 10.1177/1049909113479153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher BS, Paul SM, Dodd MJ, et al. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol 2008;26:599–605. 10.1200/JCO.2007.12.2838 [DOI] [PubMed] [Google Scholar]
- 16.Jean-Pierre P, Figueroa-Moseley CD, Kohli S, et al. Assessment of cancer-related fatigue: implications for clinical diagnosis and treatment. Oncologist 2007;12 Suppl 1:11–21. 10.1634/theoncologist.12-S1-11 [DOI] [PubMed] [Google Scholar]
- 17.Jensen S, Given B. Fatigue affecting family caregivers of cancer patients. Support Care Cancer 1993;1:321–5. 10.1007/BF00364970 [DOI] [PubMed] [Google Scholar]
- 18.Finnegan-John J, Molassiotis A, Richardson A, et al. A systematic review of complementary and alternative medicine interventions for the management of cancer-related fatigue. Integr Cancer Ther 2013;12:276–90. 10.1177/1534735413485816 [DOI] [PubMed] [Google Scholar]
- 19.Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer 2014;111:33–45. 10.1038/bjc.2014.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson D, Robinson PD, Oberoi S, et al. Pharmacologic interventions for fatigue in cancer and transplantation: a meta-analysis. Curr Oncol 2018;25:152–67. 10.3747/co.25.3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol 2017;3:961–8. 10.1001/jamaoncol.2016.6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Raaf PJ, de Klerk C, Timman R, et al. Systematic monitoring and treatment of physical symptoms to alleviate fatigue in patients with advanced cancer: a randomized controlled trial. J Clin Oncol 2013;31:716–23. 10.1200/JCO.2012.44.4216 [DOI] [PubMed] [Google Scholar]
- 23.Agbejule OA, Hart NH, Ekberg S, et al. Bridging the research to practice gap: a systematic scoping review of implementation of interventions for cancer-related fatigue management. BMC Cancer 2021;21:809. 10.1186/s12885-021-08394-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escalante CP, Kallen MA, Valdres RU, et al. Outcomes of a cancer-related fatigue clinic in a comprehensive cancer center. J Pain Symptom Manage 2010;39:691–701. 10.1016/j.jpainsymman.2009.09.010 [DOI] [PubMed] [Google Scholar]
- 25.Escalante CP, Manzullo E, Valdres R. A cancer-related fatigue clinic: opportunities and challenges. J Natl Compr Canc Netw 2003;1:333–43. 10.6004/jnccn.2003.0030 [DOI] [PubMed] [Google Scholar]
- 26.Chan RJ, Crichton M, Crawford-Williams F, et al. The efficacy, challenges, and facilitators of telemedicine in post-treatment cancer survivorship care: an overview of systematic reviews. Ann Oncol 2021;32:1552–70. 10.1016/j.annonc.2021.09.001 [DOI] [PubMed] [Google Scholar]
- 27.Chan A, Ashbury F, Fitch MI, et al. Cancer survivorship care during COVID-19-perspectives and recommendations from the MASCC survivorship Study Group. Support Care Cancer 2020;28:3485–8. 10.1007/s00520-020-05544-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monterosso L, Platt V, Bulsara M, et al. Systematic review and meta-analysis of patient reported outcomes for nurse-led models of survivorship care for adult cancer patients. Cancer Treat Rev 2019;73:62–72. 10.1016/j.ctrv.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 29.Tuominen L, Stolt M, Meretoja R, et al. Effectiveness of nursing interventions among patients with cancer: an overview of systematic reviews. J Clin Nurs 2019;28:2401–19. 10.1111/jocn.14762 [DOI] [PubMed] [Google Scholar]
- 30.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new medical Research Council guidance. BMJ 2008;337:a1655. 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the brief fatigue inventory. Cancer 1999;85:1186–96. [DOI] [PubMed] [Google Scholar]
- 33.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health 2008;31:180–91. 10.1002/nur.20247 [DOI] [PubMed] [Google Scholar]
- 34.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287–91. 10.1002/pst.185 [DOI] [Google Scholar]
- 35.Cancer Council Australia . Fatigue and cancer, 2019. [Google Scholar]
- 36.Stout NL, Brown JC, Schwartz AL, et al. An exercise oncology clinical pathway: screening and referral for personalized interventions. Cancer 2020;126:2750–8. 10.1002/cncr.32860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellg AJ, Borrelli B, Resnick B, et al. Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH behavior change Consortium. Health Psychol 2004;23:443–51. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- 38.Robb SL, Burns DS, Docherty SL, et al. Ensuring treatment fidelity in a multi-site behavioral intervention study: implementing NIH behavior change Consortium recommendations in the smart trial. Psychooncology 2011;20:1193–201. 10.1002/pon.1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res 1991;38:143–62. 10.1016/0165-1781(91)90040-V [DOI] [PubMed] [Google Scholar]
- 40.Braun V, Clarke V. Thematic analysis. In: Cooper H, Camic PM, Long DL, et al., eds. APA Handbook of research methods in psychology, vol. 2: research methods: quantitative, qualitative, neuropsychological, and biological. Washington, DC: American Psychological Association, 2012: 57–71. [Google Scholar]
- 41.World Medical Association declaration of Helsinki . Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–6. [PubMed] [Google Scholar]
- 42.Therapeutic Goods Administration . Note for guidance on good clinical practice (CPMP/ICH/135/95), 2000. [Google Scholar]
- 43.National H, Medical Research C, Australian Research C, Australian Vice-Chancellors C . National statement on ethical conduct in human research 2007 (updated 2018). Canberra: National Health and Medical Research Council (NHMRC), 2007. [Google Scholar]
- 44.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial symptom assessment scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 1994;30A:1326–36. 10.1016/0959-8049(94)90182-1 [DOI] [PubMed] [Google Scholar]
- 45.Australian Institute of Health and Welfare . The active Australia survey: a guide and manual for implementation, analysis and reporting. Canberra: AIHW, 2003. [Google Scholar]
- 46.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 47.Chan R, Cooper B, Paul S, et al. Distinct financial distress profiles in patients with breast cancer prior to and for 12 months following surgery. BMJ Support Palliat Care 2020:bmjspcare-2020-002461. 10.1136/bmjspcare-2020-002461 [DOI] [PubMed] [Google Scholar]
- 48.Chan RJ, Cooper B, Koczwara B, et al. A longitudinal analysis of phenotypic and symptom characteristics associated with inter-individual variability in employment interference in patients with breast cancer. Support Care Cancer 2020;28:4677–86. 10.1007/s00520-020-05312-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoefman R, Van-Excel NJA, Brouwer WBF. iMTA valuation of informal care questionnaire, 2013. [Google Scholar]
- 50.Ferguson M, Capra S, Bauer J, et al. Development of a valid and reliable malnutrition screening tool for adult acute hospital patients. Nutrition 1999;15:458–64. 10.1016/S0899-9007(99)00084-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059952supp001.pdf (534.7KB, pdf)