Dear Editor,
We describe two patients with diffuse large B‐cell lymphoma (DLBCL), which developed as axillary lymphadenopathy after BNT162b2 COVID‐19 vaccination.
Case 1 was a 67‐year‐old Japanese man who visited Tokyo‐kita Medical Center complaining of a 6.0‐cm subcutaneous mass in the left axilla 2 weeks after the second BNT162b2 vaccination. Tenderness and a palpable lymph node (LN) in the left axilla were noted 1 day after the first BNT162b2 vaccination. Computed tomography revealed an enlarged LN in the left axilla (Fig. 1a), and it was suspected as a reactive lymphadenopathy. However, the nodule became bigger and was accompanied with redness of the surrounding skin. Hence, biopsy specimens were taken from the swollen LN and erythematous skin (Fig. 1b). Histopathological examination revealed a diffuse infiltration of large, atypical lymphocytes with centroblast and immunoblast in the LN (Fig. 1c) and the skin. The large, atypical lymphocytes were stained strongly with CD20, BCL2 and MUM‐1/IRF4 (Fig. 1d–f) and were negative for CD3. The Ki‐67 positivity was over 80%. He was diagnosed with DLBCL, and R‐CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone) regimen was initiated, resulting in the shrinkage of the LN.
Figure 1.
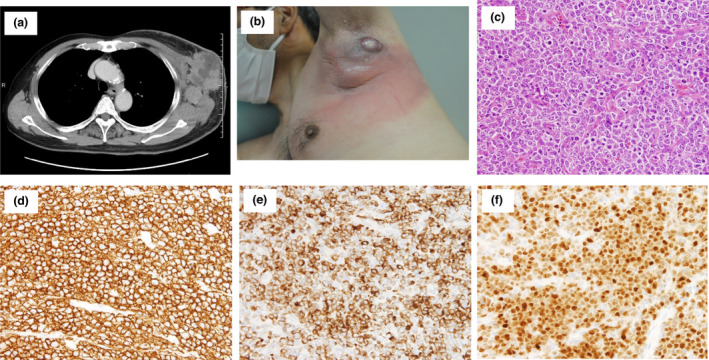
Clinical and histopathological characteristics of case 1. (a) CT image revealed enlarged mass in the left axilla. (b) A clinical image of the case 1 at the biopsy was presented. (c) An image of Haematoxylin and Eosin staining was shown (×200). (d–f) Images of immunohistochemical staining for CD20 (d × 100), BCL‐2 (e × 100) and MUM1/IRF4 (f × 100) were shown. [Colour figure can be viewed at wileyonlinelibrary.com]
Case 2 was an 80‐year‐old Japanese woman who visited the University of Yamanashi Hospital due to an enlarging nodule in her left axilla 1 day after the second BNT162b2 vaccination. The nodule appeared 2 days after the first vaccination. Ultrasonography detected a 4.1‐cm round mass with blood flow (Fig. 2a), which was suggestive of lymphadenopathy. Two months after the first consultation, the nodule gradually enlarged, and computed tomography revealed a 6.0‐cm mass in the left axilla (Fig. 2b) and another 2.8‐cm mass in the left mesentery. A biopsy of the nodule in the left axilla (Fig. 2c) demonstrated a sheet‐like diffuse infiltration of atypical lymphocytes (Fig. 2d). The atypical cells were positive for CD20 (Fig. 2e), BCL6 and BCL2 and negative for CD3 and MUM‐1/IRF4. The Ki‐67 positivity was over 90%. A diagnosis of germinal centre B‐cell DLBCL was made. The patient complained of diplopia and left eyelid ptosis 8 days after the biopsy. Magnetic resonance imaging detected a small tumour, a suspicious DLBCL lesion, in the left cavernous sinus (Fig. 2f). The dose‐attenuated CHOP regimen with standard dose of rituximab was initiated. Besides, radiotherapy (40 Gy) targeting the brain nodule was performed. Through combined modality therapy, the nodules in the left axilla and left cavernous sinus disappeared.
Figure 2.
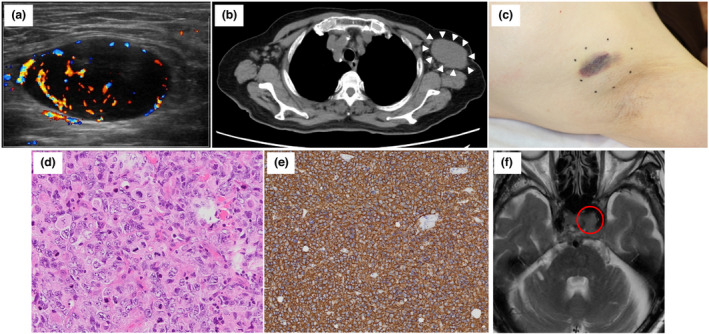
Clinical and histopathological characteristics of case 2. (a) An image of ultrasound sonography at the initial consultation was presented. (b) CT image revealed enlarged mass in the left axilla. (c) A clinical image of the case 2 at the biopsy was presented. (d and e) Images of Haematoxylin and Eosin staining (d × 200) and immunohistochemical staining for CD20 (e × 100) were shown. (f) An MRI image of a tumour in the left cavernous sinus was shown. [Colour figure can be viewed at wileyonlinelibrary.com]
This is the first case report of DLBCL developed shortly after BNT162b2 vaccination, although the recurrence of remitted T‐cell lymphoma cases has been reported. 1 , 2 Reactive lymphadenopathy after COVID‐19 vaccination has been repeatedly reported; hence, both cases were initially suspected as temporal LN swelling. The influence of vaccination on the development of DLBCL is uncertain. BNT162b2 vaccines have been reported to induce a cytokine signature featuring IL‐15, IFN‐γ, CXCL10 and IL‐6. 3 On the contrary, the elevation of these cytokines was observed in the sera of patients with pretreated DLBCL, 4 suggesting some roles of these cytokines in the growth or survival of DLBCL. Thus, it might be conceivable that pre‐existing or subclinical DLBCL may rapidly grow in a specific condition induced by BNT162b2 vaccination. Nevertheless, the precise mechanism regulating the induction of DLBCL by this vaccination must await further investigations, including interaction between lymphoma cells and tumour microenvironment, genetic instability and so on. 5 , 6
In conclusion, DLBCL may rapidly grow after BNT162b2 vaccination. Dermatologists should pay attention to enlarging LNs or mass near the injection site of BNT162b2 vaccine. This case report might become an emergent alert for the candidates receiving anti‐COVID‐19 vaccination.
Conflicts of interest
None declared.
Funding sources
None.
Acknowledgement
The patients in this manuscript have given written informed consent to the publication of their case details.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Panou E, Nikolaou V, Marinos L et al. Recurrence of cutaneous T‐cell lymphoma (CTCL) post viral vector COVID‐19 vaccination. J Eur Acad Dermatol Venereol 2022; 36: e91–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldman S, Bron D, Tousseyn T et al. Rapid progression of Angioimmunoblastic T cell lymphoma following BNT162b2 mRNA vaccine booster shot: a case report. Front Med (Lausanne) 2021; 8: 798095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergamaschi C, Terpos E, Rosati M et al. Systemic IL‐15, IFN‐g, and IP‐10/CXCL10 signature associated with effective immune response to SARS‐CoV‐2 in BNT162b2 mRNA vaccine recipients. Cell Rep 2021; 36: 109504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Charbonneau B, Maurer MJ, Ansell SM et al. Pretreatment circulating serum cytokines associated with follicular and diffuse large B‐cell lymphoma: a clinic‐based case‐control study. Cytokine 2012; 60: 882–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phelan JD, Young RM, Webster DE et al. A multiprotein supercomplex controlling oncogenic signalling in lymphoma. Nature 2018; 560: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasqualucci L, Dalla‐Favera R. Genetics of diffuse large B‐cell lymphoma. Blood 2018; 131: 2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


