Abstract
Severe acute respiratory syndrome coronavirus (SARS‐CoV)‐2 has spread worldwide, leading the World Health Organization (WHO) to declare a pandemic, on 11 March 2020. Variants of concern have appeared at regular intervals—Alpha, Beta, Gamma, Delta, and now Omicron. Omicron variant, first identified in Botswana in November 2021, is rapidly becoming the dominant circulating variant. In this review, we provide an overview regarding the molecular profile of the Omicron variant, epidemiology, transmissibility, the impact on vaccines, as well as vaccine escape, and finally, we report the pharmacological agents able to block the endocellular entry of SARS‐CoV‐2 or to inhibit its viral replication. The Omicron has more than 50 mutations, of which the spike protein has 26–35 amino acids different from the original SARS‐CoV‐2 virus or the Delta, some of which are associated with humoral immune escape potential and greater transmissibility. Omicron has a significant growth advantage over Delta, leading to rapid spread with higher incidence levels. The disease so far has been mild compared to the Delta. The two vaccination doses offer little or no protection against Omicron infection while the booster doses provide significant protection against mild illness and likely offer even greater levels of protection against serious illness. Recently, new oral antiviral agents such as molnupiravir and paxlovid have been approved and represent important therapeutic alternatives to antiviral remdesivir. In addition, monoclonal antibodies such as casirivimab/imdevimab bind different epitopes of the spike protein receptor; is this class of drugs effective against the Omicron variant? However, more research is needed to define whether Omicron is indeed more infectious and whether the vaccines, monoclonal antibodies, and antivirals currently available are effective.
Keywords: antiviral drugs, COVID‐19, epidemiology, monoclonal antibodies, mutations, pandemic, SARS‐CoV‐2, spike protein, vaccine, VOC

Introduction
On 11 March 2020, the World Health Organization (WHO) classified coronavirus disease 2019 (COVID‐19) as a global pandemic [1]. As of 18 December 2020, more than 1,600,000 deaths globally had been attributed to COVID‐19 [2]. The spread of severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) virus has been unprecedentedly fast, diffusing to more than 180 countries within 3 months with different severities. The COVID‐19 pandemic is responsible for great mortality and severe morbidity in both developed and developing nations. Development of sensitive, specific, and rapid COVID‐19 diagnostic tests aided to trace and control the infection [3, 4]. SARS‐CoV‐2 has the natural ability to undergo mutation and antigenic variation over a period of time. The SARS‐CoV‐2 virus was isolated and sequenced. The genome size varies from 29.8 kb to 29.9 kb and consists of four structural proteins: spike protein (S), envelope (E), membrane (M), and nucleocapsid (N) proteins [5]. S‐glycoprotein is the central transmembrane monomer of approximately 180 kDa with two subunits, S1 and S2. It mediates membrane fusion and ultimately facilitates virus entry. The receptor binding domain (RBD) (residues 319–541) of the S1 subunit interacts with the angiotensin‐converting enzyme 2 (ACE2), binding tightly to its peptidase domain [6]. The E protein, which is the smallest protein in the virus structure, plays a role in the maturation of this virus. The M protein is critical in determining the shape of the virus envelope and in stabilizing the nucleocapsids. The N protein is involved in processes related to the cellular response of host cells to viral infections, the viral replication cycle, and the viral genome. SARS‐CoV‐2 uses the ACE2 receptor for cellular entry in synergy with TMPRSS2, an important host factor that cleaves protein S, facilitating viral activation [7].
In the last 2 years, there have been a significant number of mutations that have led to the evolution of the virus and therefore to different variants [8]. WHO has classified them into three categories: variants of interest (VOIs), variants under monitoring (VUMs), and variants of concern (VOCs). For VOIs, there is still preliminary and uncertain evidence on genomic, epidemiological properties regarding a significant impact on transmissibility, severity, and/or immunity. The VUMs categories have been detected as signals through rule‐based genomic variant screening or preliminary scientific evidence. Some indications seem to assign them similar properties to those of a VOC, but the evidence is weak or has not yet been assessed by European Centre for Disease Prevention and Control (ECDC). For VOCs, clear evidence indicated a significant impact on transmissibility, severity, or virus virulence with consequences on the epidemiological situation by reducing the effectiveness of public health measures, diagnostics, vaccines, and therapies [9, 10]. So far, we have five VOCs: Alpha (B.1.1.7, U.K. variant), Beta (B.1.351, South Africa), Gamma (P.1, Brazil), Delta (B.1.617.2, India), and Omicron (B.1.1.529, Africa). The Alpha, Beta, and Gamma variants have the same mutation, N501Y, on RBD, which increases affinity for ACE2 by increasing its transmissibility; in addition, the Beta and Gamma variants also show K417N and E484K mutations, which by decreasing the binding of neutralizing antibodies lead to a partial immune escape favoring reinfections [11]. All these factors contributed to a new wave of pandemic and thousands of deaths around the world [9]. The highly contagious Delta variant quickly spread around the world and replaced Alpha as the more prevalent variant. The Omicron variant, first identified in Botswana in November 2021, is rapidly becoming the dominant circulating variant.
Molecular profile of Omicron variant
On 26 November 2021, the WHO declared the emerging SARS‐CoV‐2 Omicron variant (Pango Lineage B.1.1.529) as another VOC [12]. The Omicron variant, currently the dominant variant in circulation, has several mutations with 37 nonsynonymous changes in the spike protein, 11 in the amino terminal domain (N terminal domain [NTD]), and 15 in the RBD, some of which may be associated with humoral immune escape potential and higher transmissibility [13] (Fig. 1). The Omicron variant includes Pango lineages B.1.1.529, BA.1, BA.2, and BA.3. BA.1, which accounts for about 99% of sequences, and BA.3 has the 69–70 deletion in the spike protein while BA.2 does not. Knowledge of B.1.1.529 is still developing; it shares the 69–70 deletion present in about half of all available sequences [13].
Fig. 1.
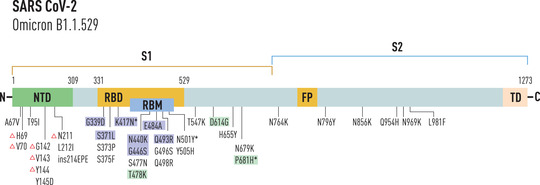
Mutations in spike protein of Omicron variant. Mutations responsible for escaping host immunity are indicated in blue, mutations shared with Delta variant of concern are indicated in green. *Mutations associated with hyper infectivity; Δ, deletion mutation; ins, insertion mutation; #mutation showed fusogenicity and pathogenicity.
Several mutations in the RBD of the Omicron variant are shared by other SARS‐CoV‐2 variants. These are K417N, E484K, N501Y, D614G, and T478K. The K417N mutation with asparagine substitution to lysine is shared by both Omicron and Beta variants; in E484K variant at residue 484, glutamic acid is replaced by lysine in both Beta and Gamma variants; the mutation at 484 residues in Omicron is E484A, where glutamic acid is mutated to alanine. This mutation, also shared by Beta and Gamma variants, can cause reinfection, while the Omicron variant might alter the interaction between RBD and human ACE2. N501Y mutation (asparagine to tyrosine), already detected in the Alpha, Beta, and Gamma variants, determines a higher binding affinity to human ACE2. The D614G mutation with substitution of aspartic acid to glycine in S1 subunit of the Omicron variant is also present in Alpha, Beta, Gamma, and Delta variants. The T478K mutation with the replacement of threonine to lysine is shared by Delta variants (Table 1) [14].
Table 1.
The schematic overview of spike protein mutations in Alpha, Beta, Gamma, Delta, Iota, Epsilon, Kappa, and Omicron variants
| SARS‐CoV‐2 variants | Spike mutations |
|---|---|
| Alpha (B.1.1.7) | ∆69/70, ∆144, N501Y, A570D, D614G, P681H, T716I, S982A, and D1118H |
| Beta (B.1.351) | L18F, D80A, D215G, ∆242–244, R246I, K417N, E484K, N501Y, D614G, and A701V |
| Gamma (P.1) | L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I, and V1176F |
| Delta (B.1.617.2) | T19R, FR1570158∆, L452R, T478K, D614G, P681R, and D950N |
| Iota (B.1.526) | L5F, T95I, D253G, E484K, D614G, and A701V |
| Epsilon (B.1.427/9) | S13I, W152C, L452R, and D614G |
| Kappa (B.1.617.1) | T95I, L452R, E484Q, D614G, P681R, and Q1071H |
| Omicron (B.1.1.529/BA.1) | A67V, HV69–70∆, T95I, G142D, VYY143–145∆, N211∆, L212I, R214EPEins, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, and L981F |
Note: Schematic representation of spike protein substitutions and deletions commonly detected in SARS‐CoV‐2 variants. The Omicron variant has several mutations with 37 nonsynonymous changes in the spike protein, some of which may be associated with humoral immune escape potential and higher transmissibility.
In addition, the Omicron variant SARS‐CoV‐2 shares the deletion of amino acids H69 and V70 in the S‐gene of its genome, which can cause an S gene target failure (SGTF) and for this reason, the PCR (polymerase chain reaction) tests most used to diagnose infection with SARS‐CoV‐2 do not detect the S gene on which these deletions are present. Although a small percentage of publicly shared sequences lack this deletion, SGTF can be used as a proxy marker for Omicron screening. In tests designed to detect multiple genetic targets, tests are expected to still detect the Omicron variant SARS‐CoV‐2, although one genetic target has reduced sensitivity due to mutation [15]. Therefore, the detection pattern, which shows dropout of the affected target, can be the indicative signal of the presence of the Omicron variant in a patient with a positive result. Molecular sequencing should be considered to characterize the variant since this deletion can also be found in other VOCs (e.g., Alpha and subsets of Gamma and Delta) that are circulating at low frequencies worldwide. However, it must be considered that the positive result with this test does not mean that the subject is infected with the Omicron variant; on the other hand, not all patient samples with the Omicron variant have this mutation, which leads to gene dropout since the Omicron variant could be present without a gene dropout detection pattern.
Epidemiology
The Omicron variant was confirmed in 110 countries on 23 December 2021. It became the dominant variant in South Africa, where it was first reported in mid‐November 2021 in Gauteng province, the economic hub of South Africa containing the cities of Tshwane and Johannesburg. Increasing positive cases in this area were accompanied by increased frequency of SGTF during PCR diagnostic tests, a phenomenon also observed previously for the Alpha variant due to the presence of the same deletion in amino acids 69 and 70 in the spike protein. Since the prevalence of Alpha was low in South Africa, the researchers immediately sequenced the entire genome of the SGTF samples and the results showed a genetically different lineage from the previous ones. Gauteng province continued to have the highest number of cases per week (27%). However, towards the end of December, the data began to show a decrease. In the neighboring countries of South Africa, with the exception of Zimbabwe, cases were recorded to be on the increase between 13 and 19 December, while minor increases were recorded in Lesotho, eSwatini, Namibia, and Mozambique [16].
Regarding the European region, Omicron became the dominant variant in the UK with 71.5% of cases for which the S gene status of COVID‐19 cases was known to have SGTF in the samples collected, in the second half of December 2021. Omicron is expected to become the dominant variant in other European countries as well in early 2022 and is expected to replace the Delta variant [17]. By 18 December, the Omicron variant became dominant in the United States with a case rate of approximately 73% [18]. However, starting from December 2021, the Omicron variant has spread to Denmark, Australia, Portugal, France, the Netherlands, Norway, Belgium, India, Israel, and Germany [19]. The greater prevalence of Omicron compared to Delta has now been confirmed by several pieces of evidence such as neutralization studies, the first estimates of the efficacy of vaccination against symptomatic disease, and studies on reinfection risk, which suggest that this is due to immune evasion against the infection. However, more data are needed to estimate the generation time of Omicron compared to the other variants and to understand whether Omicron has characteristics that make it more transmissible.
Impact on vaccines
Vaccines are the best and most reliable way to avoid and manage infectious diseases. The COVID‐19 pandemic and the resulting increase in deaths worldwide have made the development of an effective SARS‐CoV‐2 vaccine urgently important.
Sheikh et al. in their study evaluated the efficacy of the vaccine against the Omicron variant in Scotland. They studied the effect of second doses of Pfizer‐BioNTech (BNT162b2 COVID‐19), Oxford‐AstraZeneca (ChAdOx1 nCoV‐19), and Moderna (mRNA‐1273) vaccine and third doses of Pfizer‐BioNTech and Moderna vaccines in subjects exposed to these vaccines between 1 November and 19 December. This found that the Omicron variant is associated with a two‐thirds reduction in hospitalization risk compared to the Delta variant. The third vaccination dose shows maximum protection against Delta and a further 57% (95% confidence interval (CI): 55, 60) reduction in symptomatic infection for Omicron compared to ≥25 weeks after the second dose of the vaccines [20]. Andrews et al. conducted another study in the United Kingdom to evaluate the efficacy of different vaccination programs (BNT1622 and ChAdOx1‐S) against Omicron symptomatic disease versus Delta with two dose cycles of BNT1622 and ChAdOx1‐S, as well as booster doses of BNT162b2. The two doses of vaccination with BNT162b2 or ChAdOx1 are not sufficient to provide adequate levels of protection against mild infections and diseases with the Omicron variant. In particular, the effectiveness of BNT162b2 vaccine was 88.0% (95% CI: 65.9%–95.8%) 2–9 weeks after dose 2 for the Omicron variant, dropping to 34% (95% CI: 7.0%–59.0%) after 25 weeks, compared with vaccine effectiveness of 63.5% (95% CI: 61.4%–65.5%) against Delta post vaccination at all the time intervals investigated. In addition, vaccine effectiveness increased to 71.4% (95% CI: 41.8%–86.0%) after a BNT162b2 booster dose in subjects who received ChAdOx1 as the primary course and 75.5% (95% CI: 56.1%–86.3%) in subjects who received BNT162b2 as the primary course. Booster doses provide significant protection against mild disease and likely offer even greater levels of protection against severe disease. With the Delta variant, effectiveness increases over 90% 2 weeks following the booster after the primary course with both types of vaccines tested. These findings support maximizing coverage with third vaccine doses in a highly positive manner. However, little is known about the duration of booster vaccination protection [21].
Another analysis by Denmark using a nationwide cohort estimated COVID‐19 vaccine protection against Omicron or Delta infection up to 5 months after primary vaccination. Vaccine effectiveness against Omicron infections was 55.2% (95% CI: 23.5%–73.7%) and 36.7% (69.9%–76.4%) for the BNT162b2 and mRNA‐1273 vaccines, respectively, with evidence of rapid waning in 5 months, while vaccine effectiveness against Delta infections was substantially higher and better preserved during the same period. The vaccine effectiveness is restored to 54.6% (95% CI: 30.4%–70.4%) upon a booster dose with the BNT162b2 vaccine. Thus, the efficacy of the primary ongoing BNT162b2 or Mrna‐1273 vaccines against the Omicron variant rapidly decreases within a few months while the booster vaccination promotes increased protection [22].
A multinational research group, to understand the consequences of the numerous mutations found in the Omicron variant, studied the neutralization mediated by monoclonal and polyclonal antibodies using a pseudovirus test with Omicron RBD in sera of vaccinated individuals with six major COVID‐19 vaccines (Mrna‐1273, BNT162b2, AZD1222, Ad26.COV2.S, Sputnik V, and BBIBP‐CorV). In subjects vaccinated with Ad26.COV2.S or Sputnik V or BBIBO‐CorV against the Omicron spike, no neutralization activity was observed, while in individuals vaccinated with Mrna 1273, Pfizer BioNTech‐Comirnaty, and AstraZeneca‐Vaxzevria, a 33‐, 44‐, and 36‐fold reduction in neutralization was recorded, respectively [23].
Biotech company Moderna recently conducted a clinical trial to evaluate the immunogenicity, safety, and reactogenicity of multivalent vaccines against SARS‐CoV‐2 variants. In particular, the prototype vaccine (Mrna‐1273) and multivalent candidates were evaluated—Mrna‐1273.211 and Mrna‐1273.213 at the authorized dose (50 μg) and a higher dose (100 μg). The candidate (Mrna‐1273.211) includes several mutations present in the Omicron variant that were already present in the Beta variant. Preliminary results of the trial at 50‐μg booster dose against the Omicron variant showed an approximately 37‐fold increase in neutralizing antibody levels after 29 days of dosing, while the 100‐μg dose of Mrna‐1273 increased neutralizing antibody levels about 83 times. The multivalent candidate (Mrna‐1273.213) includes many mutations present in the Omicron variant and also in the Beta and Delta variants. Its trial for the 100‐μg dose (N = 584) and the 50‐μg dose in other 584 subjects is ongoing. In addition, the safety and tolerability data from the Phase 2/3 study of the 100‐μg full dose booster showed that this dose was generally safe and well tolerated. Systemic and local adverse events after 7 days were comparable to those observed after the 2‐dose primary series. Slightly more frequent adverse reactions were observed after the booster dose of 100 μg compared to the authorized dose of 50‐μg Mrna‐1273. However, Moderna will also continue to develop an Omicron variant–specific vaccine (Mrna‐1273.529) with human clinical trials evaluating the inclusion of Omicron in its multivalent booster program. Considering the strength of the current mRna‐1273 vaccine and the speed with which the Omicron variant is spreading, the first line of defense proposed by Moderna is represented by a booster dose of mRna‐1273 [24].
Vaccine escape
Immune evasion plays a significant role in the rapid growth in Omicron cases. The Omicron variant may be able to evade immunity from previous vaccines or infections more extensively than any other variant, making existing vaccines less effective against the variant [25].
In England, the risk of reinfection with Omicron was estimated to be 5.4 (95% CI: 4.87–6.00) times higher than with the Delta variant. The relative risks observed for unvaccinated and vaccinated cases were 6.36 (95% CI: 5.23–7.74) and 5.02 (95% CI: 4.47–5.67), respectively. Thus, the protection against reinfection can be as high as 19% [26].
The UK Health Security Agency determined that 5.9% of cases detected between 1 November and 13 December resulted from reinfection, evaluating the risk of reinfection with Omicron compared to other variants to be 3.3 (95% CI: 2.8–3.8) [27].
Pulliam et al. conducted a study analyzing routine surveillance data from South Africa, from which it emerged that the recent spread of the Omicron variant was associated with a decrease in the hazard coefficient for primary infection and an increase in the reinfection hazard coefficient [28].
A recent study with an Omicron spike pseudovirus showed that the Omicron variant significantly escaped from the neutralization of convalescent sera from early strain‐ or Delta‐infected individuals; it also still requires ACE2 as its receptor and shows significantly increased infectivity. Neutralizing antibody titer in convalescent sera from Delta‐infected patients decreased 39‐fold, while that in convalescent sera from early‐infected patients for Omicron decreased 36‐fold, significantly less than neutralizing antibody titers for other variants. This indicates a significant immune escape from existing protection created by virus infection or vaccination [29]. The analysis of the binding of the Omicron RBD to human ACE2 and the capacity of human sera from infected or vaccinated patients with respect to the RBDs of the variants Beta or Delta or the original strain highlighted a reduction in binding antibodies’ levels against the Omicron RBD. Serum titers against Beta and Delta RBDs were reduced, albeit to a lesser extent [30]. A further study showed that in convalescent and double vaccinated subjects, the neutralizing activity of sera against the Omicron variant was not detectable, while that of sera exposed to spikes three or four times, albeit at reduced levels, was maintained. In addition, binding to the Omicron RBD and NTD was reduced in convalescent not vaccinated but was mostly retained in vaccinated subjects. These studies add to the evidence that current vaccination protocols may be less effective against the Omicron variant [31].
Transmissibility
Currently available data indicate that the Omicron variant is highly transmissible and that it spreads much faster than the other variants. The Omicron variant is estimated to infect 3–6 times as many people as the Delta variant and also infects individuals immune to the other variants. As of 12 December, 77 countries with Omicron presence have been reported [25, 32]. In South Africa, Omicron cases spread rapidly in Gauteng province in November, particularly in schools and among young people. After 25 November, 249 samples were sequenced and 183 tested positive for the Omicron variant [32]. However, on 26 November 2021, South Africa recorded 3402 cases and 8561 cases on 1 December 2021; additionally, several hundred cases per day were registered in mid‐November [32]. The Omicron variant was already present in the Netherlands before the first cases were identified in South Africa. In fact, the Dutch health authority has reported cases dating back to 19 November 2021 and 23 November 2021 [33]. Japan reported the first Omicron variant case from a Namibian diplomat who tested positive at Narita airport near Tokyo upon his arrival. Belgium reported the first Omicron variant case in Europe on 26 November 2021. Germany reported, on 24 November 2021, two Omicron cases of travellers entering Munich airport. Norwegian health authorities have reported that Omicron infections will increase to 300,000 people per day compared to the previous variant's record of 1000 per day [34, 35, 36]. In San Francisco, California, the first case of Omicron in the United States was detected on 22 November 2021, by an individual returning from South Africa and was identified on 1 December 2021 [37]. At least 31 states in the United States have identified cases of Omicron variants as of 15 December 2021 [35, 36]. The United States accounted for 3% of coronavirus cases, with a weekly increase of 0.4%. On 20 December 2021, the death of the first patient for Omicron was reported [35]. On 27 November 2021, two cases of the Omicron variant were identified in the United Kingdom in individuals who had been to South Africa. Infections have increased rapidly and widely compared to the Delta strain and Omicron will likely become the dominant strain in Great Britain in a short period of time. Omicron infects 200,000 people per day and accounts for more than 1 in 5 cases [38, 39, 40]. As of 12 December 2021, the total number of cases in the UK became 3137 thanks to the addition of 1239 new cases of Omicron identified. Quickly, the total number of Omicron cases in the UK became 10,017. At least 10 people were hospitalized and Omicron's first death was reported on 13 December 2021, with an additional 20 deaths reported by 20 December 2021 [34]. The first case of Omicron in China was a person who arrived in Tianjin from a European country on 9 December 2021 and the second case was an individual who arrived from abroad identified in Guangzhou on 27 November 2021 [41].
Clinical severity
Although data are still limited, few studies prove that symptoms are less severe and the risk of hospitalizations is lower than with the Delta variant. In South Africa, hospitalizations have been declining since the start of the Omicron wave, with tendencies towards hospitalization during the fourth phase mainly in the province of Gauteng. Indeed, according to a study on serological surveys in this province, Omicron infection rates have increased rapidly compared to the Delta variant but hospitalizations and deaths rested relatively low [42, 43].
The analysis of the clinical and epidemiological data of both Delta and Omicron positive COVID‐19 cases within the California health system from 30 November 2021 to 1 January 2022 found 235 (0.5%) and 222 (1.3%) cases of hospital admissions for Omicron and Delta variants, respectively. Hospitalization rates and mortality rates were 0.26 (0.10–0.73) and 0.09 (0.01–0.75) times higher among Omicron‐infected cases compared to the Delta variant, respectively. No cases with Omicron variant infection received mechanical ventilation. The mean length of hospital stay was 3.4 (2.8–4.1) days shorter for hospitalizations of Omicron patients compared to those with the Delta variant, with a reduction of 69.6% (64.0%–74.5%) in the length of hospital stay [44].
In England, 195 hospitalizations and 18 deaths have been recorded as of 22 December [45]. A report from Imperial College London dated 22 December 2021 indicates a reduced risk of hospitalization for Omicron infection compared to the Delta variant. Omicron variant infection has been shown to have a 15%–20% reduced risk of hospitalization and a 41% reduced risk (95% CI: 37%–45%) of hospitalization with a stay of 1 or more nights [46]. The same trend was also observed in Scotland; in fact, the data on hospital admissions from 1 November to 19 December indicate a reduction of about two thirds of hospitalization risk for the Omicron variant compared to the Delta variant [20]. Furthermore, in Denmark, data collected up to 21 December indicate a lower percentage (0.6%) of hospitalizations for Omicron than that (1.6%) of other variants [47]. A Hong Kong study found that the Omicron variant replicates up to 70 times faster in human bronchi than Delta while replicating much slower in the lungs. This may be the reason for the less severe clinical presentation in patients with the Omicron variant [48].
Monoclonal antibodies and antiviral drugs against the Omicron variant
Since the beginning of the COVID‐19 pandemic, intense research activity has been carried out to identify, in the shortest time possible, pharmacological agents able to block the endocellular entry of SARS‐CoV‐2 or to inhibit its viral replication. An important contribution in this direction is represented by monoclonal antibodies against SARS‐CoV‐2 [49]. Monoclonal antibodies such as the combination casirivimab/imdevimab bind different epitopes of the spike protein receptor domain blocking interaction with ACE2 and causing inhibition of endocellular penetration of the virus [50]. The Omicron variant has at least 33 mutations in the S protein, and in particular 15 substitutions are in the RBD of the S protein, which is the primary molecular target of monoclonal antibodies. This may suggest that authorized monoclonal antibodies may be less effective against the Omicron variant. Recent in vitro studies have examined the neutralizing power of monoclonal antibodies against the Omicron variant [51].
Specifically, it was shown that almost all monoclonal antibodies have neutralizing efficacy against Alpha and Delta variants, and only bamlanivimab showed loss of efficacy against the Delta variant (Table 2). In addition, loss of neutralizing efficacy against the Omicron variant was shown for the monoclonal antibodies bamlanivimab, etesevimab, and imdevimab [52, 53]. However, clinical evidence is urgently needed to test the efficacy of monoclonal antibodies against the Omicron variant. The other group of drugs that have revolutionized therapy against SARS‐CoV‐2 infection are directoral antivirals such as molnupiravir and paxlovid. Recently, new oral antiviral agents such as molnupiravir and paxlovid have been approved and represent important therapeutic alternatives to remdesivir. Molnupiravir is a prodrug that is immediately metabolized in its active form by plasma esterases. The active form of the drug then undergoes intracellular phosphorylation by host cell kinases to form an alternative substrate for viral RNA polymerase [54]. In vitro and in vivo tests show that molnupiravir was found to be a potent inhibitor of SARS‐CoV‐2 replication. The new oral antiviral agent paxlovid is a combination of two active drugs—nirmatrelvir (PF‐07321332) acts by inhibiting SARS‐CoV‐2 viral replication by blocking the SARS‐CoV‐2‐3CL protease, and ritonavir, an antiretroviral indicated for the treatment of HIV, which is also an inhibitor of cytochrome P4503A and CYP2D6, was used to slow the metabolism of nirmatrelvir. In vitro studies confirm that nirmatrelvir is a potent 3CL protease inhibitor of the Omicron variant, suggesting that the drug demonstrates potential therapeutic efficacy against this novel variant as well [55]. The Omicron variant has mutations in both the RNA‐dependent RNA polymerase (RdRp) and the SARS‐CoV‐2 major protease, which are targets of antiviral drugs such as RdRp inhibitors (remdesivir and molnupiravir) and the major protease inhibitor nirmatrelvir. Current scientific evidence that analyzed in vitro 50% inhibitory concentration values (IC50) indicates that the susceptibility of Omicron to the three antiviral drugs is similar to that of previous variants [51]. A schematic overview of the efficacy of monoclonal antibodies and oral antivirals against SARS‐CoV‐2 variants is provided in Table 2. Data were collected from evidence in PubMed.
Table 2.
Efficacy of monoclonal antibodies and antiviral drugs against SARS‐CoV‐2 variants
| Monoclonal antibodies | Alpha/B.1.1.7 | Beta/B.1.351 | Gamma/P.1 | Delta/B.1.617.2 | Omicron/B.1.1.529 |
|---|---|---|---|---|---|
| Etesevimab | Effective | Marked reduction | Marked reduction | Effective | Marked reduction |
| Tixagevimab | Effective | Effective | Effective | Effective | Effective |
| Casirivimab | Effective | Effective | Effective | Effective | Effective |
| Bamlanivimab | Effective | Marked reduction | Marked reduction | Marked reduction | Marked reduction |
| Imdevimab | Effective | Effective | Effective | Effective | Marked reduction |
| Cilgavimab | Effective | Effective | Effective | Effective | Effective |
| Sotrovimab | Effective | Effective | Effective | Effective | Effective |
| Antiviral drugs | |||||
| Remdesivir | Effective | Effective | Effective | Effective | Effective |
| Molnupiravir | Effective | Effective | Effective | Effective | Effective |
| Nilmetravir | Effective | Effective | Effective | Effective | Effective |
Note: Schematic overview showing the efficacy and reduced neutralizing antiviral response of monoclonal antibodies and antiviral agents against SARS‐CoV‐2 variants. Data were collected from PubMed.
Early evidence suggests that all antivirals directed against SARS‐CoV‐2 may show clinical efficacy for the treatment of patients infected with the Omicron variant. However, further clinical studies are needed to fully demonstrate the efficacy of these antivirals against the Omicron variant.
Conclusions
Several aspects of the Omicron variant are yet to be fully defined, such as whether or not it is more infectious than previous SARS‐CoV‐2 variants; whether it can cause a higher incidence of severe COVID‐19 forms than other VOCs; and whether vaccines, monoclonal antibodies, and direct antivirals are effective.
To date, the available immunological and clinical data cannot provide definitive evidence. However, from genomic analysis, the deletions and mutations present in the Omicron variant are known to lead to increased transmissibility due to higher viral binding affinity.
Although early epidemiological data indicate rapid transmissibility and high contagiousness, to date no particularly alarming clinical concerns have been raised about an increased incidence of severe COVID‐19 cases. In addition, the potential impact of Omicron on the clinical efficacy of COVID‐19 infection vaccines is yet to be fully understood, even if the first epidemiological studies are very positive. Finally, early evidence indicates that for some monoclonal antibodies, there is a possible reduction in neutralizing power against the Omicron variant, while the new direct antivirals maintain their efficacy in inhibiting viral replication. Further clinical studies are needed to fully define these aspects. However, it is important to highlight that the preventive measures adopted with previous variants to protect public health, such as mask use, social distance, hand hygiene, and so on, and which have proven to be highly effective, still need to be adopted with the Omicron variant.
Conflict of interests
The authors declare no competing or financial interests.
Author contributions
Antonio Vitiello: conceptualization; methodology; supervision; validation; writing – original draft; writing – review and editing. Francesco Ferrara: conceptualization; formal analysis; methodology; validation; writing – original draft; writing – review and editing. Amogh M. Auti: methodology; writing – original draft; writing – review and editing. Marina Di Domenico: methodology; supervision; validation; writing – original draft; writing – review and editing. Mariarosaria Boccellino: methodology; validation; writing – original draft; writing – review and editing.
Acknowledgement
None.
Vitiello A, Ferrara F, Auti AM, Di Domenico M, Boccellino M. Advances in the Omicron variant development. J Intern Med. 2022;292:81–90.
References
- 1. World Health Organization . WHO Director‐General's opening remarks at the media briefing on COVID‐19, 11 March 2020. https://www.who.int/
- 2. Johns Hopkins University . New cases of COVID‐19 in world countries. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/
- 3. Di Domenico M, De Rosa A, Boccellino M. Detection of SARS‐COV‐2 proteins using an ELISA test. Diagnostics (Basel). 2021;11(4):698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Domenico M, De Rosa A, Di Gaudio F, Internicola P, Bettini C, Salzano N, et al. Diagnostic accuracy of a new antigen test for SARS‐CoV‐2 detection. Int J Environ Res Public Health. 2021;18(12):6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khailany RA, Safdar M, Ozaslan M. Genomic characterization of a novel SARS‐CoV‐2. Gene Rep. 2020;19:100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science 2020;367(6483):1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kupferschmidt K, Cohen J. Race to find COVID‐19 treatments accelerates. Science 2020;367(6485):1412–3. [DOI] [PubMed] [Google Scholar]
- 8. Winger A, Caspari T. The spike of concern—the novel variants of SARS‐CoV‐2. Viruses 2021;13(6):1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization . Tracking SARS‐CoV‐2 variants. https://www.who.int/en/activities/tracking‐SARS‐CoV‐2‐variants/ (2021). Accessed 25 Dec 2021.
- 10. Parums DV. Revised World Health Organization (WHO) terminology for variants of concern and variants of interest of SARS‐CoV‐2. Med Sci Monit. 2021;27:e933622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zahradník J, Marciano S, Shemesh M, Zoler E, Harari D, Chiaravalli J, et al. SARS‐CoV‐2 variant prediction and antiviral drug design are enabled by RBD in vitro evolution. Nature Microbiol. 2021;6:1188–98. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization . Update on Omicron. https://www.who.int/news/item/28‐11‐2021‐update‐on‐omicron (2021). Accessed 11 Jan 2021.
- 13. Ren SY, Wang WB, Gao RD, Zhou AM. Omicron variant (B.1.1.529) of SARS‐CoV‐2: mutation, infectivity, transmission, and vaccine resistance. World J Clin Cases. 2022;10(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kannan S, Shaik Syed Ali P, Sheeza A. Omicron (B.1.1.529)—variant of concern—molecular profile and epidemiology: a mini review. Eur Rev Med Pharmacol Sci. 2021;25:8019–22. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization HQ . Enhancing readiness for Omicron (B.1.1.529): technical brief and priority actions for Member States. 23 December 2021 (updated from the last version published on 17 December 2021). https://www.who.int/
- 16. World Health Organization WHO coronavirus (COVID‐19) dashboard. 2022. [Accessed January 2022]. https://covid19.who.int.
- 17. European Center for Disease Prevention and Control . Assessment of the further emergence of the SARS‐CoV‐2 Omicron VOC in the context of the ongoing Delta VOC transmission in the EU/EEA, 18th update. [Accessed January 2022]. https://www.ecdc.europa.eu/en/publications‐data/covid‐19‐assessment‐further‐emergence‐omicron‐18th‐risk‐assessmen (2021).
- 18. U.S. Centers for Disease Control and Prevention . COVID data tracker: monitoring variant proportions. [Accessed January 2022]. https://covid.cdc.gov/covid‐data‐tracker/#variant‐proportions.
- 19. Meo SA, Meo AS, Al‐Jassir FF, Klonoff DC. Omicron SARS‐CoV‐2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. 2021;25(24):8012–8. [DOI] [PubMed] [Google Scholar]
- 20. Sheikh A, Kerr S, Woolhouse M, McMenamin J & Robertson C. Severity of Omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. [Accessed January 2022]. https://www.pure.ed.ac.uk/ws/portalfiles/portal/245818096/Severity_of_Omicron_variant_of_concern_and_vaccine_effectiveness_against_symptomatic_disease (2021). [DOI] [PMC free article] [PubMed]
- 21. Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T & Gallagher E. Effectiveness of COVID‐19 vaccines against the Omicron (B.1.1.529) variant of concern. [Accessed January 2022]. https://khub.net/documents/135939561/430986542/Effectiveness+of+COVID‐19+vaccines+against+Omicron+variant+of+concern.pdf/f423c9f4‐91cb‐0274‐c8c5‐70e8fad50074 (2021).
- 22. Hansen CH, Blicher Schelde A, Moustsen‐Helm IR, Emborg HD, Krause TG, Mølbak K, et al. Vaccine effectiveness against SARS‐CoV‐2 infection with the Omicron or Delta variants following a two‐dose or booster BNT162b2 or mRNA‐1273 vaccination series: a Danish cohort study. 2021. 10.1101/2021.12.20.21267966. [DOI]
- 23. Cameroni E, Saliba C, Bowen JE. Broadly neutralizing antibodies overcome SARS‐CoV‐2 Omicron antigenic shift. 2021. 10.1101/2021.12.12.472269. [DOI] [PMC free article] [PubMed]
- 24. A study to evaluate the immunogenicity and safety of mRNA‐1273.211 vaccine for COVID‐19 variants. 2022. [Accessed January 2022]. (ClinicalTrials.gov Identifier: NCT04927065).
- 25. Callaway E. Omicron likely to weaken COVID vaccine protection. Nature. 2021;600:367–8. [DOI] [PubMed] [Google Scholar]
- 26. Ferguson N, Ghani A, Cori A & Imperial College London . Report: growth, population distribution and immune escape of Omicron in England. 2021. [Accessed January 2022]. https://www.imperial.ac.uk/mrc‐global‐infectious‐disease‐analysis/covid‐19/report‐49‐Omicron/
- 27. UK Health Security Agency . SARS‐CoV‐2 variants of concern and variants under investigation in England—technical briefing 32. 2021. [Accessed January 2022]. https://www.gov.uk/government/publications/investigation‐of‐sars‐cov‐2‐variants‐technical‐briefings
- 28. Pulliam J, van Schalkwyk C, Govender N. Increased risk of SARS‐CoV‐2 reinfection associated with emergence of the Omicron variant in South Africa. 2021. 10.1101/2021.11.11.21266068. [DOI] [PMC free article] [PubMed]
- 29. Zhang X, Wu S, Wu B, Yang Q, Chen A, Li Y, et al. SARS‐CoV‐2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Sig Transduct Target Ther. 2021;6(1):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schubert M, Bertoglio F, Steinke S. Human serum from SARS‐CoV‐2 vaccinated and COVID‐19 patients shows reduced binding to the RBD of SARS‐CoV‐2 Omicron variant. 2021. 10.1101/2021.12.10.21267523. [DOI] [PMC free article] [PubMed]
- 31. Carreno JM, Alshammary H, Tcheou J. Activity of convalescent and vaccine serum against a B.1.1.529 variant SARS‐CoV‐2 isolate. 2021. 10.1101/2021.12.20.21268134. [DOI]
- 32. Callaway E, Ledford H. How bad is Omicron? Nature 2021;600:197–9. [DOI] [PubMed] [Google Scholar]
- 33. Graham F. Daily briefing: Omicron was already spreading in Europe. Nature. 2021. 10.1038/d41586-021-03610-3. [DOI] [PubMed] [Google Scholar]
- 34. Xinhua . UK raises COVID‐19 alert level amid rising Omicron cases. [Accessed January 2022]. https://www.chinadaily.com.cn/a/202112/13/WS61b6a5d4a310cdd39bc7aea3.html (2021).
- 35. Zhang M. Omicron now accounts for 3% of US coronavirus cases. [Accessed January 2022]. https://global.chinadaily.com.cn/a/202112/15/WS61b95f63a310cdd39bc7b7f8.html (2021).
- 36. Xinhua . Omicron variant detected in California's wastewater before WHO raises alarms: US media. [Accessed January 2022]. https://global.chinadaily.com.cn/a/202112/11/WS61b40bd4a310cdd39bc7abbf.html (2021).
- 37. Xinhua . Omicron variant detected in California's wastewater before WHO raises alarms: US media. [Accessed January 2022]. https://global.chinadaily.com.cn/a/202112/11/WS61b40bd4a310cdd39bc7abbf.html (2021).
- 38. Brits E, Adepoju P. Omicron potential under close scrutiny. Nature. 2021. 10.1038/d44148-021-00119-9 [DOI] [Google Scholar]
- 39. Graham F. Daily briefing: Omicron coronavirus variant puts scientists on alert. Nature. 2021. 10.1038/d41586-021-03564-6 [DOI] [PubMed] [Google Scholar]
- 40. China Daily Global . Experts: Omicron likely no worse than other variants. [Accessed January 2022]. https://epaper.chinadaily.com.cn/a/202112/09/WS61b13202a31019b029ba2661.html (2021).
- 41. Veneti L, Salamanca BV, Seppälä E, Starrfelt J, Storm ML, Bragstad K, et al. No difference in risk of hospitalization between reported cases of the SARS‐CoV‐2 Delta variant and Alpha variant in Norway. Int J Infect Dis. 2022;115:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. NICD . COVID‐19 hospital surveillance update: South Africa—week 50, 2021. [Accessed January 2022]. https://www.nicd.ac.za/wp‐content/uploads/2021/12/NICD‐COVID‐19‐Weekly‐Sentinel‐Hospital‐Surveillnace‐update‐Week‐50‐2021.pdf (2021).
- 43. Madhi S, Kwatra G, Myers J, Jassat W, Dhar N, Mukendi CK, et al. South African population immunity and severe Covid‐19 with Omicron variant. 2021. 10.1101/2021.12.20.21268096. [DOI] [PMC free article] [PubMed]
- 44. Lewnard JA, Hong VX, Patel MM, Kahn R, Lipsitch M, Tartof SY. Clinical outcomes among patients infected with Omicron (B.1.1.529) SARS‐CoV‐2 variant in southern California. 2022. 10.1101/2022.01.11.22269045 [DOI] [PMC free article] [PubMed]
- 45. UK Health Security Agency . Omicron daily overview: 22 December 2021. [Accessed January 2022]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1043466/20211222_OS_Daily_Omicron_Overview.pdf (2021).
- 46. Ferguson N, Ghani A, Volz E & Imperial College London . Report 50: Hospitalisation risk for Omicron cases in England. [Accessed January 2022]. https://www.imperial.ac.uk/media/imperial‐college/medicine/mrc‐gida/2021‐12‐22‐COVID19‐Report‐50.pdf (2021).
- 47. Statens Serum Institut . Status of the SARS‐CoV‐2 variant Omicron in Denmark. [Accessed January 2022]. https://files.ssi.dk/covid19/omikron/statusrapport/rapport‐omikronvarianten‐21122021‐14tk (2021).
- 48. HKU Med . HKUMed finds Omicron SARS‐CoV‐2 can infect faster and better than Delta in human bronchus but with less severe infection in lung. [Accessed January 2022]. https://www.med.hku.hk/en/news/press/20211215‐omicron‐sars‐cov‐2‐infection (2021).
- 49. Vitiello A, Porta R, Pianesi L, Ferrara F. COVID‐19 pandemic: vaccine and new monoclonal antibodies, point of view. Ir J Med Sci. 2022;191(1):487–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hwang YC, Lu RM, Su SC, Chiang PY, Ko SH, Ke FY, et al. Monoclonal antibodies for COVID‐19 therapy and SARS‐CoV‐2 detection. J Biomed Sci. 2022;29(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Takashita E, Kinoshita N, Yamayoshi S, Fujisaki S, Ito M, Chiba S, et al. Efficacy of antibodies and antiviral drugs against Covid‐19 Omicron variant. N Engl J Med. 2022;386:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Corti D, Purcell LA, Snell G, Veesler D. Tackling COVID‐19 with neutralizing monoclonal antibodies. Cell 2021;184:3086–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel‐Benhassine F, Rajah MM, et al. Reduced sensitivity of SARS‐CoV‐2 variant Delta to antibody neutralization. Nature 2021;596:276–80. [DOI] [PubMed] [Google Scholar]
- 54. Zhao J, Guo S, Yi D, Li Q, Ma L, Zhang Y, et al. A cell‐based assay to discover inhibitors of SARS‐CoV‐2 RNA dependent RNA polymerase. Antiviral Res. 2021;190:105078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pfizer Featured Article. 2022. [Accessed January 2022]. https://www.pfizer.com/news/


