Abstract
SARS‐CoV‐2 infection can damage the nervous system with multiple neurological manifestations described. However, there is limited understanding of the mechanisms underlying COVID‐19 neurological injury. This is a cross‐sectional exploratory prospective biomarker cohort study of 21 patients with COVID‐19 neurological syndromes (Guillain–Barre Syndrome [GBS], encephalitis, encephalopathy, acute disseminated encephalomyelitis [ADEM], intracranial hypertension, and central pain syndrome) and 23 healthy COVID‐19 negative controls. We measured cerebrospinal fluid (CSF) and serum biomarkers of amyloid processing, neuronal injury (neurofilament light), astrocyte activation (GFAp), and neuroinflammation (tissue necrosis factor [TNF] ɑ, interleukin [IL]‐6, IL‐1β, IL‐8). Patients with COVID‐19 neurological syndromes had significantly reduced CSF soluble amyloid precursor protein (sAPP)‐ɑ (p = 0.004) and sAPPβ (p = 0.03) as well as amyloid β (Aβ) 40 (p = 5.2 × 10−8), Aβ42 (p = 3.5 × 10−7), and Aβ42/Aβ40 ratio (p = 0.005) compared to controls. Patients with COVID‐19 neurological syndromes showed significantly increased neurofilament light (NfL, p = 0.001) and this negatively correlated with sAPPɑ and sAPPβ. Conversely, GFAp was significantly reduced in COVID‐19 neurological syndromes (p = 0.0001) and this positively correlated with sAPPɑ and sAPPβ. COVID‐19 neurological patients also displayed significantly increased CSF proinflammatory cytokines and these negatively correlated with sAPPɑ and sAPPβ. A sensitivity analysis of COVID‐19‐associated GBS revealed a non‐significant trend toward greater impairment of amyloid processing in COVID‐19 central than peripheral neurological syndromes. This pilot study raises the possibility that patients with COVID‐19‐associated neurological syndromes exhibit impaired amyloid processing. Altered amyloid processing was linked to neuronal injury and neuroinflammation but reduced astrocyte activation.
Keywords: Alzheimer's disease, amyloid processing, APP, beta amyloid, COVID‐19
SARS‐CoV‐2 can result in neurological conditions, but the mechanisms of COVID‐19‐associated nervous system injury remain unclear. Using cerebrospinal fluid (CSF) and blood biomarkers of amyloid processing collected from patients with COVID‐19‐associated neurological injury and healthy controls, we find COVID‐19 neurological patients display impaired amyloid processing characterized by decreased soluble amyloid precursor proteins and Amyloid β. Reductions in amyloid processing biomarkers correlated with increases in neuronal injury (NfL) and neuroinflammatory biomarkers but with decreases in astrocyte reactivity (GFAp). This supports the possibility that patients with COVID‐19‐associated neurological syndromes exhibit impaired amyloid processing during their acute illness.
Abbreviations
- Aβ
amyloid β
- AD
Alzheimer's disease
- ADEM
acute disseminated encephalomyelitis
- APP
amyloid precursor protein
- CNS
central nervous system
- CSF
cerebrospinal fluid
- CTF
C‐terminal fragment
- GBS
Guillain–Barre syndrome
- GFAp
glial fibrillary acidic protein
- IL
interleukin
- IQR
interquartile range
- NfL
neurofilament light
- PCR
polymerase chain reaction
- sAPP
soluble amyloid precursor protein
- TNF
tissue necrosis factor
- WC
white cells β
1. INTRODUCTION
Patients with COVID‐19 can suffer from loss of smell, cognitive impairment, encephalitis, stroke, and Guillain–Barre Syndrome (GBS) as well as other neurological conditions (Ellul et al., 2020; Helms et al., 2020; Mao et al., 2020; Matschke et al., 2020; Paterson et al., 2020; Varatharaj et al., 2020). COVID‐19 has also been linked to the development of cognitive impairment, particularly among hospitalized patients (Al‐Aly, Xie, & Bowe, 2021; Beaud et al., 2021; Becker et al., 2021; Meppiel et al., 2021; Miners, Kehoe, & Love, 2020; Romero‐Sánchez et al., 2020; Taquet, Geddes, Husain, Luciano, & Harrison, 2021; Zhou et al., 2020). It remains unclear if COVID‐19 neuropathology arises because of direct viral CNS infection or indirectly from the accompanying immune response and resulting hypercoagulability and critical illness (Iadecola, Anrather, & Kamel, 2020; Varatharaj et al., 2020). SARS‐CoV‐2 can cross the blood–brain barrier and infect vascular and immune cells, where it may damage the CNS (Cantuti‐Castelvetri et al., 2020; Jacob et al., 2020; Meinhardt et al., 2021; Neman & Chen, 2015; Solomon, 2021; Song et al., 2021; Yang et al., 2021).
Mechanistic links have been reported between COVID‐19 and Alzheimer's Disease (AD), centered around neuroinflammation and microvascular injury (Zhou et al., 2021). Accumulation of amyloid plaques is associated with neurodegeneration, particularly in AD (van der Kant & Goldstein, 2015). Neurotoxic β‐amyloid (Aβ) is formed from cleavage of the trans‐membrane amyloid precursor protein (APP), by β and 𝛾 secretases. ɑ‐secretase releases soluble APP (sAPP)‐ɑ (large N‐terminal soluble fragment) and ɑ‐CTF (short C‐terminal fragment) via the non‐amyloidogenic pathway while β‐secretase generates sAPPβ and β‐CTF via the amyloidogenic pathway. CTFs can be further processed by 𝛾‐secretase, generating Aβ monomers that can self‐associate forming toxic Aβ oligomers. Although Aβ aggregation increases in the brain in AD and during normal aging, Aβ accumulation also accelerates in other contexts, including some infections, for example, HIV (Chai et al., 2017). As yet, it remains unclear whether COVID‐19 impairs APP processing, and if so whether this could directly contribute to future neurodegeneration (Abbott, 2020; Miners et al., 2020). Here, we aimed to investigate biomarkers of APP processing and how these relate to amyloidosis, neuronal injury, astrocyte activation, and neuroinflammation in patients with COVID‐19 who manifest clinically apparent neurological syndromes.
2. MATERIALS AND METHODS
2.1. Subjects
We prospectively recruited patients presenting to University College London Hospital between March and June 2020. Participants were included if they met both (i) the European Centre for Disease Prevention and Control definition of COVID‐19 and (ii) had new neurological symptoms or signs according to agreed definitions (Ellul et al., 2020) within 40 days of symptomatic COVID‐19 infection. Neurological diagnoses were made according to clinical, biochemical, and imaging findings and based on internationally accepted criteria. Patients not meeting both of these criteria were excluded. These COVID‐19 neurological cases were compared to neurologically healthy controls, which were derived from the healthy aging study from individuals who are amyloid and tau PET‐negative (non‐COVID controls) as previously reported (ethics approval IUSMD 16–60) (Gobom et al., 2021; Paterson et al., 2021). All cerebrospinal fluid (CSF) samples were collected in polypropylene tubes and spun, aliquoted, and frozen within 24 hours, alongside samples collected as part of standard clinical care with written informed consent. All CSF samples were SARS‐CoV‐2 PCR‐negative. Subjects' consent was obtained according to the Declaration of Helsinki. This exploratory study was approved by the Queen Square ethics committee (12‐LO‐1540) and was not pre‐registered.
2.2. Biomarker collection, analysis, and interpretation
CSF sAPPα, sAPPβ (cat. no. K15120E), NfL, GFAp (in‐house assays as previously described in detail (Gaetani et al., 2018; Rosengren et al., 1992)), TNFα, IL6, IL8, and IL1β (cat. no. K15052D) were measured by ELISA. For TNFα and IL1β, when samples were below 0.165 and 0.285 pg/ml, respectively (the lower limits of their assay detection) the values were truncated. T‐tau (cat. no. 230312), P‐tau (cat. no. 230350), amyloid β 1–40 (Aβ40; cat. no. 231524), and 1–42 (Aβ42; cat. no. 230336) were analyzed by Lumipulse assays (Fujirebio). The Aβ42/Aβ40 ratio was calculated and used as a marker of amyloid accumulation (Hansson, Lehmann, Otto, Zetterberg, & Lewczuk, 2019). Serum NfL (cat. no. 103186) and GFAp (cat. no. 102336) were measured by single‐molecule array (Simoa). All assays except NfL were performed using a single batch of reagents (intra‐assay coefficient of variation was <10%), on randomized samples and blinded to the clinical data. In patients with COVID‐19, assays were performed at a single time point during the acute phase of the illness.
2.3. Statistical analysis
Continuous variables were summarized using medians and interquartile range (IQR, reported as median ± IQR), and compared using the two‐sided Wilcoxon test. Assessment of the normality of the data was performed with the Shapiro–Wilk test, which revealed that although Aβ42, Aβ40, sAPPα, and sAPPβ were normally distributed all other assays were non‐normally distributed. Outliers were tested for using the boxplot method using thresholds of < Q1–1.5 * IQR and > Q3 + 1.5 * IQR. Effect sizes were calculated with Cohen's d measure and post hoc power analysis was performed with the pwr.t2n.test function from the pwr R package. Correlation coefficients were calculated using Spearman's rank correlation and relationships were measured using linear regression, with an examination of the distribution of residual errors. To improve the visualization of outlier values, scales are log10 transformed. All statistical analyses and graphs were generated using R v4.0.3; p < 0.05 was considered significant. Boxplots represent the median, hinges correspond to the first and third quartiles and whiskers to 1.5 * IQR and outliers are plotted individually.
3. RESULTS
A total of 44 individuals were included. Twenty‐one COVID‐19 neurological cases and 23 non‐COVID controls were included. The COVID‐19 neurological group consisted of Guillain–Barre Syndrome (GBS) n = 8; encephalopathy n = 6; encephalitis n = 3; acute disseminated encephalomyelitis (ADEM) n = 2; intracranial hypertension n = 1; and central pain syndrome n = 1. All patients were hospitalized, of which six patients required oxygen therapy and four required intensive care for ventilatory support. The COVID‐19 neurological groups were younger (57 ± 15 years, median ± IQR) than the non‐COVID control group (68 ± 4 years, p = 0.01) and there were relatively more males in the COVID‐19 neurological group compared to the non‐COVID controls (COVID neuro 13 [62%] and non‐COVID 8 [35%], p = 0.1; Table 1). In the COVID‐19 neurological groups, CSF was acellular (<5 white cells [WC] /μl) except for a case of encephalitis (95 WC/μl) and a case of GBS (12 WC/μl). CSF protein was normal (<0.5 g/dl) except in two cases of GBS (0.6 g/dl and 1.2 g/dl) and in one individual with encephalopathy (1.0 g/dl).
TABLE 1.
Demographic information for the COVID neurological CNS and PNS syndrome and non‐COVID control group
| COVID neurological syndrome | Non‐COVID controls | p‐value | |
|---|---|---|---|
| N | 21 | 23 | |
| Diagnosis |
Guillain–Barre syndrome 9 Encephalopathy 6 Encephalitis 3 Acute disseminated encephalomyelitis 2 Idiopathic intracranial hypertension 1 Central pain syndrome 1 |
NA | |
| median Age (IQR), years | 57 (15) | 68 (4) | 0.01 |
| Male sex (%) | 62 | 35 | 0.13 |
| Ethnicity | |||
| Non‐white, % | 58 | NA | |
| White, % | 42 | NA |
3.1. Diminished amyloid processing in COVID‐19 neurology syndromes
To examine how amyloid processing differs between patients with COVID‐19‐associated neurological syndromes and controls, we measured CSF sAPPɑ and sAPPβ. We first confirmed that, as reported previously (Gabelle et al., 2010), sAPPɑ with sAPPβ were strongly positively correlated (Spearman's R + 0.89, p = 9.5 × 10−16; Figure 1a), irrespective of COVID‐19 status. Comparing amyloid processing biomarkers between groups revealed that both sAPPɑ and sAPPβ were significantly lower in COVID‐19 neurological patients compared to non‐COVID controls (sAPPɑ 194 ± 154 vs. 304 ± 177 pg/ml [median ± IQR], Wilcoxon test p = 0.004, Cohen's d = 0.98; sAPPβ 497 ± 366 vs. 635 ± 346 pg/ml, p = 0.03, d = 0.80; Figure 1b,c). This suggests that amyloid processing is altered in the central nervous system in acute COVID‐19 infection.
FIGURE 1.
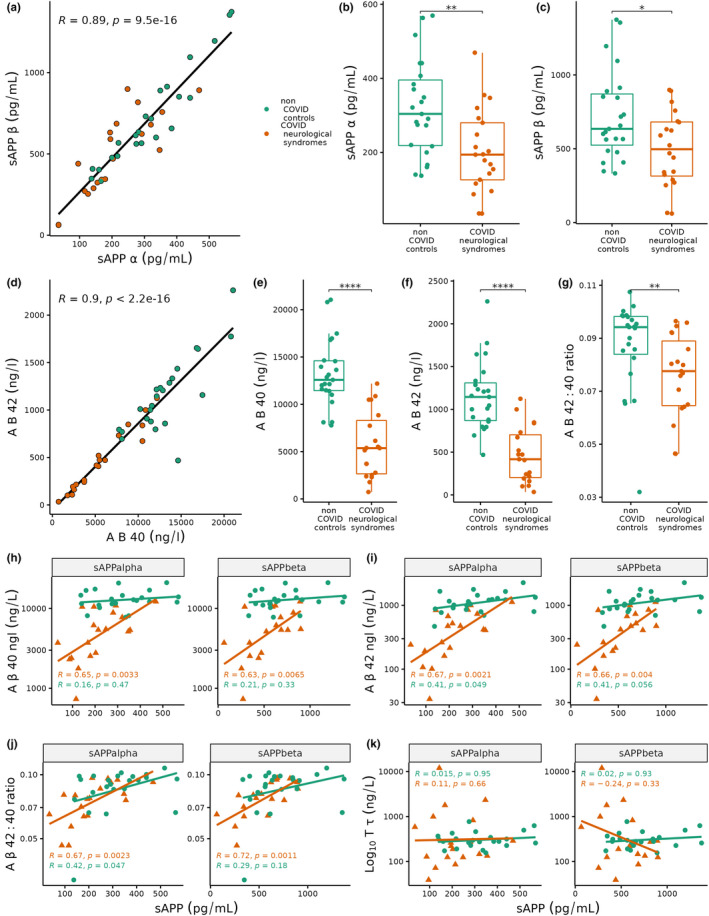
(a) Scatter plot of soluble amyloid precursor protein (sAPP)‐ɑ (x‐axis) against sAPPβ (y‐axis). Linear regression correlation spearman coefficient R = +0.89. Red dots represent COVID‐19 neurological patients (n = 21), while green dots represent non‐COVID controls (n = 23). (b–c) Boxplots of (b) sAPPɑ and (c) sAPPβ in COVID neurological syndromes (red) and non‐COVID controls (green). (d) Scatter plot of amyloid‐β (Aβ) 40 (x‐axis) against Aβ42 (y‐axis). Linear regression correlation spearman coefficient R = +0.9. (e–g) Boxplots of (e) Aβ40, (f) Aβ42 and (g) Aβ42/Aβ40 ratio in COVID neurological syndromes and non‐COVID controls. ****p < 0.0001 ***p < 0.001, **p < 0.01, *p < 0.05 from Wilcoxon test; ns, non‐significant. (h–k) Scatterplots for sAPPɑ (left facet) and sAPPβ (right facet) on x‐axis against Aβ40, Aβ42, Aβ42/Aβ40 ratio, total tau (y‐axis). Wilcoxon test *p < 0.05, **<0.01, ***<0.001, ****<0.0001
To understand the relationship between Alzheimer's Disease (AD) pathology and amyloid processing in acute COVID‐19, CSF Aβ42, and Aβ40 were measured and compared against local clinical cut‐points. COVID‐19 neurological patients exhibited significantly lower Aβ40 and Aβ42 than controls (Aβ40: 5367 ± 5638 vs. 12 582 ± 3154 ng/L, p = 5.2 × 10−8, d = 2.1; Aβ42: 417 ± 499 vs. 1145 ± 441 ng/L, p = 3.5 × 10−7, d = 1.9; Figure 1d–f). Given that a reduced Aβ42/Aβ40 ratio is a more predictive indicator of amyloid pathology, we next examined the Aβ42/Aβ40 ratio and found that it was significantly reduced in COVID‐19 neurological patients compared to controls (0.077 ± 0.02 vs. 0.094 ± 0.014 ratio, p = 0.005, d = 0.71; Figure 1g). Despite this difference, only one individual had an Aβ42/Aβ40 below the clinical threshold to suggest AD pathology. We correlated both sAPPɑ and sAPPβ with Aβ40, Aβ42, and the Aβ42/Aβ40 ratio and found that patients with COVID‐19 neurological syndromes displayed stronger positive correlations (R ~ +0.6 to +0.7) than controls (R ~ +0.1 to +0.4; Figure 1h–j). In contrast, neither T‐tau nor P‐tau was correlated with sAPPɑ or sAPPβ in either COVID‐19 neurological patients or controls (Figure 1k).
3.2. Biomarkers of neuronal damage and blunted astrocyte activation correlate with impaired amyloid processing
We next explored the relationship between amyloid processing and neuronal injury and astrocyte activation biomarkers. We first confirmed that serum and CSF NfL were significantly raised in COVID‐19 neurological patients compared to controls (serum 91.5 ± 188 vs. 16.7 ± 12.9 pg/ml, p = 0.001, d = 0.80; CSF 881 ± 1975 vs. 862 ± 496 pg/ml; p = 0.047, d = 0.61, Figure 2a,b). In COVID‐19 neurological patients, we found weak negative correlations between serum NfL with both sAPPɑ (R = −0.1) and sAPPβ (R = −0.08; Figure 2a). However, CSF NfL showed weak positive correlations with sAPPɑ (R = 0.3) and sAPPβ (R = 0.05; Figure 2b).
FIGURE 2.
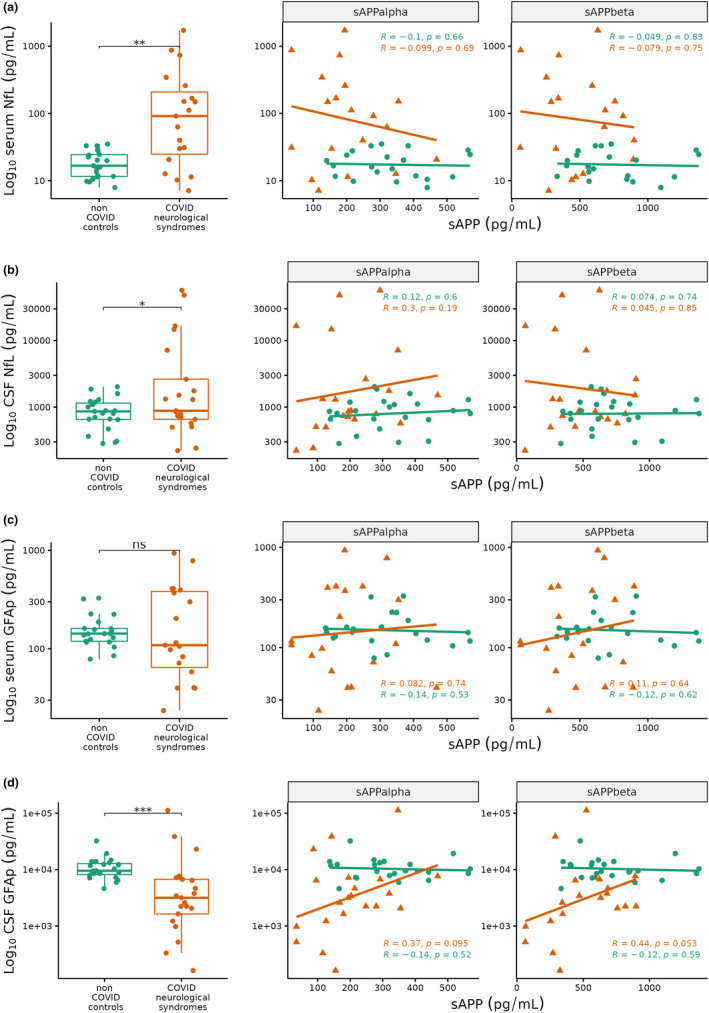
Boxplots (left) and scatterplots (right) of (a) serum neurofilament light (NfL), (b) cerebrospinal fluid (CSF) NfL, (c) serum glial fibrillary acidic protein (GFAp), (d) CSF GFAp in COVID neurological syndromes (red, n = 21) and non‐COVID controls (green, n = 23). Scatterplots show soluble amyloid precursor protein (sAPP) ɑ (left facet) and sAPPβ (right facet) on x‐axis against Log10 NfL and GFAp (y‐axis). Correlation coefficients and p‐values are shown in each scatterplot for non‐COVID controls (green), COVID neurological patients (red). Wilcoxon test *p < 0.05, **<0.01, ***<0.001, ns, non‐significant
Conversely, GFAp was reduced in COVID‐19 neurological patients compared to control, although this was only significant in CSF (serum 109 ± 318 vs. 143 ± 41.9 pg/ml, p = 0.5, d = 0.44; CSF 3160 ± 5091 vs. 9533 ± 4650 pg/mL, p = 0.0001, d = 0.02; Figure 2c,d). COVID‐19 neurological syndromes displayed a positive correlation between GFAp and both sAPPɑ and sAPPβ in serum (R = +0.08, + 0.11 respectively; Figure 2c), and CSF (R = +0.37, +0.44 respectively; Figure 2d), while controls showed weak negative correlations (serum R = −0.14,‐0.12; CSF R = −0.14, −0.12).
3.3. Amyloid processing and CSF biomarkers of inflammation
COVID‐19 neurological syndromes have been linked to aberrant neuroinflammation (Amruta et al., 2021; Yang et al., 2021; Zhou et al., 2021). Consistent with this, we found that CSF proinflammatory cytokines (TNFɑ, IL6, IL1β, IL8) were significantly increased in COVID‐19 neurological syndromes compared to controls (Figure 3a–d). To investigate this further, we inspected correlations between amyloid processing and these pro‐inflammatory biomarkers. Individuals with COVID‐19 neurological syndromes exhibited weak negative correlations between sAPPɑ and sAPPβ with TNFɑ (R = −0.01 and −0.16, respectively), while controls showed weak positive correlations (R = +0.28, +0.15; Figure 3a). Similarly, correlations between sAPPɑ and sAPPβ with the other inflammatory cytokines (IL6, IL1β, IL8) generally displayed stronger negative correlations in patients with COVID‐19 neurological syndromes relative to controls (Figure 3b–d). Thus, the neuroinflammatory sAPP correlation profiles of COVID‐19 neurological patients are divergent to controls and this may in part explain the impaired amyloid processing in COVID‐19 neurological syndromes.
FIGURE 3.
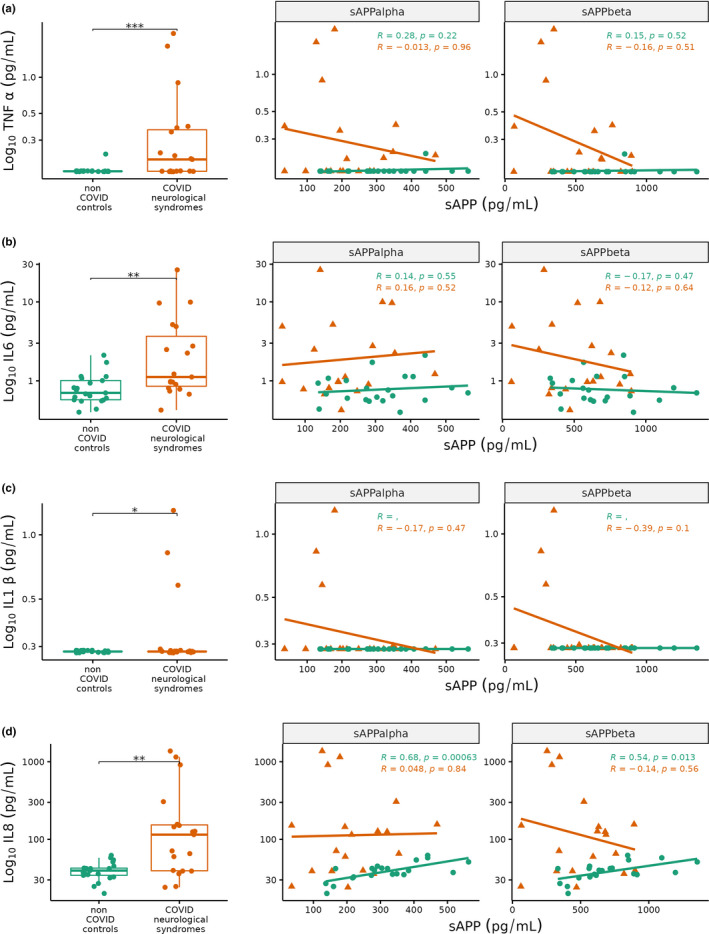
Boxplots (left) and scatterplots (right) of cerebrospinal fluid (CSF) (a) tissue necrosis factor (TNF) ɑ, (b) interleukin (IL) 6, (c) IL1β, (d) IL8 in COVID neurological syndromes (red, n = 21) and non‐COVID controls (green, n = 23). Scatterplots show soluble amyloid precursor protein (sAPP) ɑ (left facet) and sAPPβ (right facet) on x‐axis against Log10 TNFɑ, IL6, IL1β, IL8 (y‐axis). Correlation coefficients and p‐values are shown in each scatterplot for non‐COVID controls (green), COVID neurological patients (red). Wilcoxon test *p < 0.05, **<0.01, ***<0.001
3.4. Heterogeneity in amyloid processing among COVID‐19 neurological syndromes
The amyloid processing correlations in this study are generally weak and substantial heterogeneity can be observed between patients in the COVID‐19 neurological group. Although most of the neurological syndromes in this study represent CNS pathology, GBS predominantly affects the peripheral nervous system (PNS). To identify whether amyloid processing differs between COVID‐19‐associated CNS conditions and COVID‐19‐associated GBS, we performed a sensitivity analysis splitting the COVID‐19 neurological syndrome group into patients with GBS and those with CNS conditions. Comparing amyloid processing biomarkers between GBS and CNS syndromes revealed a non‐significant decrease in both sAPPɑ and sAPPβ in CNS syndromes compared to GBS (sAPPɑ 194 ± 71 vs. 214 ± 165 pg/ml, p = 0.8; sAPPβ 470 ± 343 vs. 623 ± 396 pg/ml, p = 0.2; Figure 4a,b). Although these subgroups are small (GBS n = 8 and CNS n = 13), these non‐significant trends suggest that amyloid processing may be more impaired in central than peripheral COVID‐19 neurological syndromes. Consistent with this we found that CSF Aβ40 and Aβ42 were also non‐significantly increased in COVID‐19‐associated CNS syndromes compared to GBS (Aβ40 5389 ± 5402 vs. 3950 ± 3972 ng/L, p = 0.3; Aβ42 472 ± 451 vs. 300 ± 419 ng/L, p = 0.4; Figure 4c,d). Furthermore, we found that T‐tau and P‐tau were increased in CNS syndromes compared to GBS, however, P‐tau did not achieve statistical significance (T‐tau 289 ± 1186 vs. 126 ± 57.8 pg/ml, p = 0.02; P‐tau 26.3 ± 10.7 vs. 17.5 ± 5.7 pg/ml, p = 0.09; Figure 4e).
FIGURE 4.
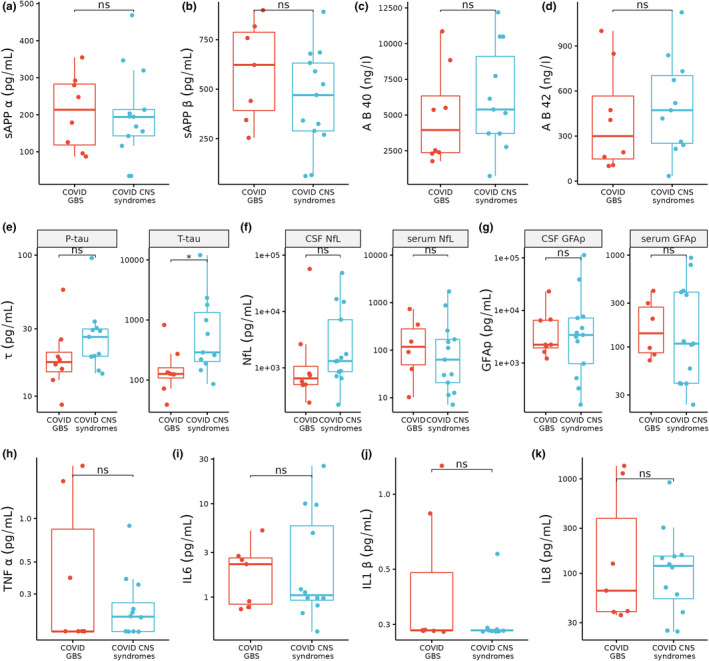
Boxplots comparing COVID‐19‐associated Guillain–Barre syndrome (GBS, red, n = 8) versus central nervous system (CNS) syndromes (blue, n = 13). Statistics shown are from the Wilcoxon test *p < 0.05; ns, non‐significant
Comparing serum NfL between COVID‐19 CNS syndromes and GBS showed a partial decrease in NfL in CNS syndromes (63 ± 148 vs. 121 ± 243 pg/ml, p = 0.6), whereas CSF NfL was increased in CNS syndromes (1320 ± 6304 vs. 656 ± 744 pg/ml, p = 0.1; Figure 4f). This disparity may be explained by CSF NfL being more representative of central neuronal injury, while serum NfL better represents peripheral nervous damage (Körtvelyessy et al., 2020). Similarly, we found that serum GFAp was non‐significantly decreased in CNS syndromes compared to GBS (109 ± 355 vs. 150 ± 189 pg/ml, p = 0.8), whereas CSF GFAp trended toward an increase in CNS syndromes (3420 ± 6209 vs. 2231 ± 4557 pg/ml, p = 0.8; Figure 4g). Examining the CSF proinflammatory cytokines TNFɑ, IL6, IL1β, and IL8 revealed no significant differences between CNS syndromes and GBS (Figure 4h–k).
4. DISCUSSION
We report the first exploratory biomarker study to examine CNS amyloid processing in patients with COVID‐19‐associated neurological syndromes. Using assays for soluble forms of APPs and neurodegenerative markers from the serum and CSF, we provide insights into the pathobiology of COVID‐19. As well as relating these biomarkers to clinical syndromes, we have correlated them with biomarkers of neurodegeneration, neuronal injury, astrocyte activation, and neuroinflammation. The main finding is that sAPP and Aβ biomarkers are lower in patients with COVID‐19 neurological diseases, and this change correlated with raised NfL and neuroinflammatory markers but decreased GFAp.
Although this is an exploratory study and the correlations are generally weak, the association between higher NfL levels and lower sAPPs raises the possibility that patients with more significant neuronal damage have reduced amyloid processing capacity. One possibility could be a blunted protective astrocyte response in severe infection. Consistent with this, we found lower sAPPs were linked to lower GFAp levels, suggesting less astrocyte reactivity which may contribute to a diminished regenerative response in COVID‐19 neurological syndromes. Indeed, astrocyte reactivity and sAPP have previously been reported to correlate in autism as well as AD, where astrocyte dysfunction contributes to impaired amyloid processing possibly because of a direct influence on secretase activity (Cai, Wan, & Liu, 2017; Ishiki et al., 2016; Lananna et al., 2020; Viejo et al., 2022).
An important question is whether the substantial reduction in both sAPPs and Aβ biomarkers in COVID‐19 neurological syndromes is because of (i) reduced amyloid production or (ii) enhanced clearance. We observed stronger correlations between sAPPs and Aβ42 and Aβ40 in COVID‐19 neurological disease than controls, raising the possibility that amyloid processing is reduced and consequently Aβ40 and Aβ42 production is attenuated in parallel via both the normal non‐amyloidogenic and abnormal amyloidogenic APP processing routes. The reduction in the Aβ42:40 ratio suggests the reduction in Aβ42 is greater than the reduction in Aβ40, however, this is not fully in keeping with a pathological amyloid setting like AD, which is characterized by a selective reduction in Aβ42. These findings are consistent with previous reports in neuroinflammation and CNS infection where there are decreases in both Aβ42 and Aβ40 (Zetterberg & Blennow, 2020).
It is possible that altered sAPPs levels in COVID‐19 neurological syndromes is an appropriate or even protective response against infection. A report in HIV infection found that sAPPs function as an innate antiviral defense factor in macrophages and microglia, restricting the release of HIV (Chai et al., 2017). However, this viral evasion mechanism was linked to increased production of neurotoxic Aβ42 and raises the possibility that this contributes to neuronal damage in CNS infection. Indeed, the greatest sAPPs reductions were found in HIV patients with CNS opportunistic infections and AIDS dementia complex, implicating a role of CNS immune activation on neuronal amyloid processing (Gisslén et al., 2009). Although it remains unclear whether SARS‐CoV‐2 infects neurons with studies reporting conflicting results (Matschke et al., 2020; Ramani et al., 2020)—expression of sAPPs in infected CNS microglia may serve as a restriction to protect against COVID‐19 spread across the BBB (Solomon, 2021).
We hypothesized that CSF amyloid processing biomarkers would be deranged to a greater degree in CNS than peripheral neurological syndromes. However, it is noteworthy that although we found divergences in amyloid processing between CNS syndromes and GBS in COVID‐19, these generally did not reach statistical significance. While the subgroup sizes are underpowered to detect these changes, it is plausible that patients with COVID‐19‐associated GBS exhibit subclinical CNS injury. However, the absence of a control group that is COVID‐19 positive but without neurological disease means that we cannot rule out that this is a direct effect of COVID‐19 itself. We were unable to obtain CSF for biomarker analysis from COVID‐19 patients without neurological disease because of the ethical issue of sampling from patients when not clinically indicated.
A strength of this study is that participants were prospectively recruited and clinically classified according to agreed case definitions, across a heterogeneous group of neurological conditions. However, numbers are relatively small and samples were collected during acute infection and because of the absence of serial longitudinal measurements, we are unable to assess the chronic effects on amyloid processing. The correlation results between biomarkers do not equate to causation and further work is required to validate these relationships and examine the underlying mechanisms. Furthermore, these results should be interpreted with caution given possible known and masked confounders, particularly with respect to differences in baseline characteristics between groups. For instance, the COVID‐19 cohort was younger than controls, which may act to underestimate the effect of COVID‐19 on amyloid processing impairment.
In summary, our study uses sAPPs and neurodegenerative biomarkers in COVID‐19 neurological disease to explore altered amyloid processing. sAPPs and Aβ are reduced in patients with COVID‐19 neurological diseases, and this change is linked to increased NfL and neuroinflammatory markers but decreased GFAp. These findings shed light on the mechanisms of neuronal damage associated with COVID‐19. To clarify whether the impairment in amyloid processing is specific to COVID‐19 patients with neurological syndromes, further study is required in COVID‐19 patients who do not exhibit overt neurological manifestations.
CONFLICT OF INTEREST
All authors have completed the ICMJE conflict of interest statement. OJZ, NJA, PRM, RB, DA, JH, AJH, ALB, AMC, and CFH report no conflicts of interest. SG has served as a consultant on the scientific advisory boards of Alzheon, Boeringher, Cerveau, and TAURX and has given lectures sponsored by Lundbeck. KB has served as a consultant or at advisory boards for Axon, Biogen, COGRX, Lilly, MAGQU, Novartis, and Roche Diagnostics, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. PRN has served as a consultant for Cerveau, Enigma, and is a clinical trials site investigator with TAURX, Jannssn, Eli Lilly, Eisai, and Biogen. JMS declares personal fees from Axon Neuroscience, Roche, Eli Lilly, General Electric Healthcare, Merck Sharp & Dohme, Oxford University Press, Biogen, and EU Horizon 2020 outside the submitted work. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and COGRX, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). LB reports a research grant from GLAXOSMITHKLINE, outside the submitted work. RWP is co‐principal investigator of the Neurofilament Light consortium, an industry‐funded consortium, and has received honoraria from GE Healthcare, outside the scope of this study.
AUTHOR CONTRIBUTIONS
OJZ, LAB, and RWP were involved in conceptualization, methodology, software, formal analysis, data curation, writing—original draft, and visualization. OJZ, NJA, PRM, RB, DA, JH, AJH, ALB, KB, AMC, CFH, SG, PRN, NCF, JMC, HZ, LAB, and RWP carried out investigation and writing—review and editing. LAB and RWP were involved in resources, supervision, project administration, and funding acquisition of the manuscript.
ACKNOWLEDGMENTS
We are grateful to Coraline Daeninck, Jane Douglas, and Anne Parnell for administrative support in running this study. This work is supported by UCLH NIHR Biomedical Research Centre (BRC), UCL Queen Square BRC and Moorfields BRC. LB is supported by Global Challenges Research Fund and a Wellcome Trust Fellowship (222102/Z/20/Z). RWP is supported by an Alzheimer’s Association Clinician Scientist Fellowship and the UK Dementia Research Institute. KB is supported by the Swedish Research Council (#2017‐00915), the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB‐201809‐2016615), the Swedish Alzheimer Foundation (#AF‐742881), Hjärnfonden, Sweden (#FO2017‐0243), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF‐agreement (#ALFGBG‐715986), and European Union Joint Program for Neurodegenerative Disorders (JPND2019‐466‐236). HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018‐02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG‐720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809‐2016862), and the UK Dementia Research Institute at UCL. Weston Brain Institute, Canadian Institutes of Health Research (CIHR) [MOP‐11‐51‐31; RFN 152985, 159815, 162303], Canadian Consortium of Neurodegeneration and Aging (CCNA; MOP‐11‐51‐31 ‐team 1), Brain Canada Foundation (CFI Project 34874; 33397), the Fonds de Recherche du Québec – Santé (FRQS; Chercheur Boursier, 2020‐VICO‐279314). P.R‐N and SG are members of the CIHR‐CCNA Canadian Consortium of Neurodegeneration in Aging.
Ziff, OJ, Ashton, NJ, Mehta, PR, Brown, R, Athauda, D, Heaney, J et al. (2022) Amyloid processing in COVID‐19‐associated neurological syndromes. Journal of Neurochemistry. 161:146–157. 10.1111/jnc.15585
Laura A. Benjamin and Ross W. Paterson contributed equally to this work.
DATA AVAILABILITY STATEMENT
Raw data were generated at University College London Hospitals NHS Foundation Trust. Derived data supporting the findings of this study are available from the corresponding author [RWP] on request.
REFERENCES
- Abbott, A. (2020). Are infections seeding some cases of Alzheimer's disease? Nature, 587, 22–25. [DOI] [PubMed] [Google Scholar]
- Al‐Aly, Z. , Xie, Y. , & Bowe, B. (2021). High‐dimensional characterization of post‐acute sequelae of COVID‐19. Nature, 594, 259–264. [DOI] [PubMed] [Google Scholar]
- Amruta, N. , Chastain, W. H. , Paz, M. , Solch, R. J. , Murray‐Brown, I. C. , Befeler, J. B. , Gressett, T. E. , Longo, M. T. , Engler‐Chiurazzi, E. B. , & Bix, G. (2021). SARS‐CoV‐2 mediated neuroinflammation and the impact of COVID‐19 in neurological disorders. Cytokine & Growth Factor Reviews, 58, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaud, V. , Crottaz‐Herbette, S. , Dunet, V. , Vaucher, J. , Bernard‐Valnet, R. , Du Pasquier, R. , Bart, P. A. , & Clarke, S. (2021). Pattern of cognitive deficits in severe COVID‐19 [Internet]. Journal of Neurology, Neurosurgery & Psychiatry, 92, 567–568. 10.1136/jnnp-2020-325173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker, J. H. , Lin, J. J. , Doernberg, M. , Stone, K. , Navis, A. , Festa, J. R. , & Wisnivesky, J. P. (2021). Assessment of cognitive function in patients after COVID‐19 infection. JAMA Network Open, 4, e2130645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, Z. , Wan, C.‐Q. , & Liu, Z. (2017). Astrocyte and Alzheimer's disease. Journal of Neurology, 264, 2068–2074. [DOI] [PubMed] [Google Scholar]
- Cantuti‐Castelvetri, L. , Ojha, R. , Pedro, L. D. , Djannatian, M. , Franz, J. , Kuivanen, S. , van der Meer, F. , Kallio, K. , Kaya, T. , Anastasina, M. , Smura, T. , Levanov, L. , Szirovicza, L. , Tobi, A. , Kallio‐Kokko, H. , Österlund, P. , Meunier, F. A. , Butcher, S. J. , & Simons, M. (2020). Neuropilin‐1 facilitates SARS‐CoV‐2 cell entry and infectivity. Science, 370, 856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, Q. , Jovasevic, V. , Malikov, V. , Sabo, Y. , Morham, S. , Walsh, D. , & Naghavi, M. H. (2017). HIV‐1 counteracts an innate restriction by amyloid precursor protein resulting in neurodegeneration. Nature Communications, 8, 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul, M. A. , Benjamin, L. , Singh, B. , Lant, S. , Michael, B. D. , Easton, A. , Kneen, R. , Defres, S. , Sejvar, J. , & Solomon, T. (2020). Neurological associations of COVID‐19. Lancet Neurol. Elsevier, 19, 767–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabelle, A. , Roche, S. , Gény, C. , Bennys, K. , Labauge, P. , Tholance, Y. , Quadrio, I. , Tiers, L. , Gor, B. , Chaulet, C. , Vighetto, A. , Croisile, B. , Krolak‐Salmon, P. , Touchon, J. , Perret‐Liaudet, A. , & Lehmann, S. (2010). Correlations between soluble α/β forms of amyloid precursor protein and Aβ38, 40, and 42 in human cerebrospinal fluid. Brain Research, 1357, 175–183. [DOI] [PubMed] [Google Scholar]
- Gaetani, L. , Höglund, K. , Parnetti, L. , Pujol‐Calderon, F. , Becker, B. , Eusebi, P. , Sarchielli, P. , Calabresi, P. , Di Filippo, M. , Zetterberg, H. , & Blennow, K. (2018). A new enzyme‐linked immunosorbent assay for neurofilament light in cerebrospinal fluid: Analytical validation and clinical evaluation. Alzheimer's Research & Therapy, 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisslén, M. , Krut, J. , Andreasson, U. , Blennow, K. , Cinque, P. , Brew, B. J. , Spudich, S. , Hagberg, L. , Rosengren, L. , Price, R. W. , & Zetterberg, H. (2009). Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurology, 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobom J., Parnetti L., Rosa‐Neto P., Vyhnalek M., Gauthier S., Cataldi S., Lerch, O. , Laczo, J. , Cechova, K. , Clarin, M. , Benet, A. L. , Pascoal, T. A. , Rahmouni, N. , Vandijck, M. , Huyck, E. , Le Bastard, N. , Stevenson, J , Chamoun, M. , Alcolea, D. , … Blennow, K. (2021). Validation of the LUMIPULSE automated immunoassay for the measurement of core AD biomarkers in cerebrospinal fluid. Clinical Chemistry and Laboratory Medicine[Internet], 60, 207–219. 10.1515/cclm-2021-0651 [DOI] [PubMed] [Google Scholar]
- Hansson, O. , Lehmann, S. , Otto, M. , Zetterberg, H. , & Lewczuk, P. (2019). Advantages and disadvantages of the use of the CSF amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer's disease. Alzheimers Res Ther. BioMed Central, 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms, J. , Kremer, S. , Merdji, H. , Clere‐Jehl, R. , Schenck, M. , Kummerlen, C. , Collange, O. , Boulay, C. , Fafi‐Kremer, S. , Ohana, M. , Anheim, M. , & Meziani, F. (2020). Neurologic features in severe SARS‐CoV‐2 infection. The New England Journal of Medicine, 382, 2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola, C. , Anrather, J. , & Kamel, H. (2020). Effects of COVID‐19 on the nervous system. Cell, 183, 16–27.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiki, A. , Kamada, M. , Kawamura, Y. , Terao, C. , Shimoda, F. , Tomita, N. , Arai, H. , & Furukawa, K. (2016). Glial fibrillar acidic protein in the cerebrospinal fluid of Alzheimer's disease, dementia with Lewy bodies, and frontotemporal lobar degeneration. Journal of Neurochemistry, 136, 258–261. [DOI] [PubMed] [Google Scholar]
- Jacob, F. , Pather, S. R. , Huang, W.‐K. , Zhang, F. , Wong, S. Z. H. , Zhou, H. , Cubitt, B. , Fan, W. , Chen, C. Z. , Xu, M. , Pradhan, M. , Zhang, D. Y. , Zheng, W. , Bang, A. G. , Song, H. , Carlos de la Torre, J. , & Ming, G. L. (2020). Human pluripotent stem cell‐derived neural cells and brain organoids reveal SARS‐CoV‐2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell, 27, 937–50.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körtvelyessy, P. , Kuhle, J. , Düzel, E. , Vielhaber, S. , Schmidt, C. , Heinius, A. , Leypoldt, F. , Schraven, B. , Reinhold, D. , Leppert, D. , & Goihl, A. (2020). Ratio and index of neurofilament light chain indicate its origin in Guillain‐Barré syndrome. Annals of Clinical Translational Neurology, 7, 2213–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lananna, B. V. , McKee, C. A. , King, M. W. , Del‐Aguila, J. L. , Dimitry, J. M. , Farias, F. H. G. , Nadarajah, C.J. , Xiong, D.D. , Guo, C. , Cammack, A. J. , Elias, J. A. , Zhang, J. , Cruchaga, C. , & Musiek, E. S. (2020). Chi3l1/YKL‐40 is controlled by the astrocyte circadian clock and regulates neuroinflammation and Alzheimer's disease pathogenesis. Science Translational Medicine, 12, eaax3519. 10.1126/scitranslmed.aax3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, L. , Jin, H. , Wang, M. , Hu, Y. , Chen, S. , He, Q. , Chang, J. , Hong, C. , Zhou, Y. , Wang, D. , Miao, X. , Li, Y. , & Hu, B. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology, 77, 683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke J., Lütgehetmann M., Hagel C., Sperhake J. P., Schröder A. S., Edler C., Mushumba, H. , Fitzek, A. , Allweiss, L. , Allweiss, M. , Dottermusch, M. , Heinemann, A. , Pfefferle, S. , Schwabenland, M. , Sumner Magruder, D. , Bonn, S. , Prinz, M. , Gerloff, C. , Püschel, K. , … Glatzel, M. (2020). Neuropathology of patients with COVID‐19 in Germany: A post‐mortem case series. Lancet Neurology; Elsevier, 19, 919–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt, J. , Radke, J. , Dittmayer, C. , Franz, J. , Thomas, C. , Mothes, R. , Laue, M. , Schneider, J. , Brünink, S. , Greuel, S. , Lehmann, M. , Hassan, O. , Aschman, T. , Schumann, E. , Chua, R.L. , Conrad, C. , Eils, R. , Stenzel, W. , Windgassen, M. , … Heppner, F.L. (2021). Olfactory transmucosal SARS‐CoV‐2 invasion as a port of central nervous system entry in individuals with COVID‐19. Nature Neuroscience, 24, 168–175. [DOI] [PubMed] [Google Scholar]
- Meppiel, E. , Peiffer‐Smadja, N. , Maury, A. , Bekri, I. , Delorme, C. , Desestret, V. , Gorza, L. , Hautecloque‐Raysz, G. , Landre, S. , Lannuzel, A. , Moulin, S. , Perrin, P. , Petitgas, P. , Sellal, F. , Wang, A. , Tattevin, P. , & de Broucker, T. (2021). Neurologic manifestations associated with COVID‐19: A multicentre registry. Clinical Microbiology and Infection, 27, 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miners, S. , Kehoe, P. G. , & Love, S. (2020). Cognitive impact of COVID‐19: Looking beyond the short term. Alzheimer's Research & Therapy, 12, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neman, J. , & Chen, T. C. (2015). The choroid plexus and cerebrospinal fluid: Emerging roles in CNS development, maintenance, and disease progression. Academic Press. [Google Scholar]
- Paterson R. W., Benjamin L. A., Mehta P. R., Brown R. L., Athauda D., Ashton N. J., Leckey, C. A. , Ziff, O. J. , Heaney, J. , Heslegrave, A. J. , Benedet, A. L. , Blennow, K. , Checkley, A. M. , Houlihan, C. F. , Mummery, C. J. , Lunn, M. P. , Manji, H. , Zandi, M. S. , Keddie, S. , … Schott, J. M. (2021). Serum and cerebrospinal fluid biomarker profiles in acute SARS‐CoV‐2‐associated neurological syndromes. Brain Communications, 3, fcab099. 10.1093/braincomms/fcab099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson, R. W. , Brown, R. L. , Benjamin, L. , Nortley, R. , Wiethoff, S. , Bharucha, T. , Jayaseelan, D. L. , Kumar, G. , Raftopoulos, R. E. , Zambreanu, L. , Vivekanandam, V. , Khoo, A. , Geraldes, R. , Chinthapalli, K. , Boyd, E. , Tuzlali, H. , Price, G. , Christofi, G. , Morrow, J. , … Zandi, M. S. (2020). The emerging spectrum of COVID‐19 neurology: Clinical, radiological and laboratory findings. Brain, 143, 3104–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani, A. , Müller, L. , Ostermann, P. N. , Gabriel, E. , Abida‐Islam, P. , Müller‐Schiffmann, A. , Mariappan, A. , Goureau, O. , Gruell, H. , Walker, A. , Andrée, M. , Hauka, S. , Houwaart, T. , Dilthey, A. , Wohlgemuth, K. , Omran, H. , Klein, F. , Wieczorek, D. , Adams, O. , … Gopalakrishnan, J. (2020). SARS‐CoV‐2 targets neurons of 3D human brain organoids. The EMBO Journal, 39, e106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero‐Sánchez, C. M. , Díaz‐Maroto, I. , Fernández‐Díaz, E. , Sánchez‐Larsen, Á. , Layos‐Romero, A. , García‐García, J. , González, E. , Redondo‐Peñas, I. , Perona‐Moratalla, A. B. , Del Valle‐Pérez, J. A. , Gracia‐Gil, J. , Rojas‐Bartolomé, L. , Feria‐Vilar, I. , Monteagudo, M. , Palao, M. , Palazón‐García, E. , Alcahut‐Rodríguez, C. , Sopelana‐Garay, D. , Moreno, Y. , … Segura, T. (2020). Neurologic manifestations in hospitalized patients with COVID‐19: The ALBACOVID registry. Neurology, 95, e1060–e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren, L. E. , Ahlsén, G. , Belfrage, M. , Gillberg, C. , Haglid, K. G. , & Hamberger, A. (1992). A sensitive ELISA for glial fibrillary acidic protein: Application in CSF of children. Journal of Neuroscience Methods, 44, 113–119. [DOI] [PubMed] [Google Scholar]
- Solomon, T. (2021). Neurological infection with SARS‐CoV‐2 ‐ the story so far. Nature Reviews. Neurology, 17, 65–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, E. , Zhang, C. , Israelow, B. , Lu‐Culligan, A. , Prado, A. V. , Skriabine, S. , Lu, P. , Weizman, O. E. , Liu, F. , Dai, Y. , Szigeti‐Buck, K. , Yasumoto, Y. , Wang, G. , Castaldi, C. , Heltke, J , Ng, E , Wheeler, J. , Alfajaro, M. M. , & Iwasaki, A. (2021). Neuroinvasion of SARS‐CoV‐2 in human and mouse brain. Journal of Experimental Medicine, 218, e20202135. 10.1084/jem.20202135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet, M. , Geddes, J. R. , Husain, M. , Luciano, S. , & Harrison, P. J. (2021). 6‐month neurological and psychiatric outcomes in 236 379 survivors of COVID‐19: A retrospective cohort study using electronic health records. Lancet Psychiatry, 8, 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kant, R. , & Goldstein, L. S. B. (2015). Cellular functions of the amyloid precursor protein from development to dementia. Developmental Cell, 32, 502–515. [DOI] [PubMed] [Google Scholar]
- Varatharaj, A. , Thomas, N. , Ellul, M. A. , Davies, N. W. S. , Pollak, T. A. , Tenorio, E. L. , Sultan, M. , Easton, A. , Breen, G. , Zandi, M. , Coles, J. R. , Manji, H. , Al‐Shahi Salman, R. , Menon, D. K. , Nicholson, T. R. , Benjamin, L. A. , Carson, A. , Smith, C. , Turner, M. , … Michael, B. D. (2020). Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: A UK‐wide surveillance study. Lancet Psychiatry, 7, 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viejo, L. , Noori, A. , Merrill, E. , Das, S. , Hyman, B. T. , & Serrano‐Pozo, A. (2022). Systematic review of human post‐mortem immunohistochemical studies and bioinformatics analyses unveil the complexity of astrocyte reaction in Alzheimer's disease. Neuropathology and Applied Neurobiology, 48, e12753. 10.1111/nan.12753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, A. C. , Kern, F. , Losada, P. M. , Agam, M. R. , Maat, C. A. , Schmartz, G. P. , Fehlmann, T. , Stein, J. A. , Schaum, N. , Lee, D. P. , Calcuttawala, K. , Vest, R. T. , Berdnik, D. , Lu, N. , Hahn, O. , Gate, D. , McNerney, M. W. , Channappa, D. , Cobos, I. , … Wyss‐Coray, T. (2021). Dysregulation of brain and choroid plexus cell types in severe COVID‐19. Nature, 595, 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg, H. , & Blennow, K. (2020). Blood biomarkers: Democratizing Alzheimer's diagnostics. Neuron, 106, 881–883. [DOI] [PubMed] [Google Scholar]
- Zhou, H. , Lu, S. , Chen, J. , Wei, N. , Wang, D. , Lyu, H. , Shi, C. , & Hu, S. (2020). The landscape of cognitive function in recovered COVID‐19 patients. Journal of Psychiatric Research, 129, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Xu, J. , Hou, Y. , Leverenz, J. B. , Kallianpur, A. , Mehra, R. , Liu, Y. , Yu, H. , Pieper, A. A. , Jehi, L. , & Cheng, F. (2021). Network medicine links SARS‐CoV‐2/COVID‐19 infection to brain microvascular injury and neuroinflammation in dementia‐like cognitive impairment. Alzheimer's Research & Therapy, 13, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated at University College London Hospitals NHS Foundation Trust. Derived data supporting the findings of this study are available from the corresponding author [RWP] on request.


