FUNDING INFORMATION
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL STATEMENT
Ethical approval was waived by the local Ethics Committee of University of Bologna in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
AUTHOR CONTRIBUTION
Dr Andrea Sechi and Dr. Federico Bardazzi had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Starace is involved in study concept and design and critical revision of the manuscript for important intellectual content. Guglielmo is involved in acquisition, analysis, and interpretation of data. Sechi and Guglielmo are involved in drafting of the manuscript. Sacchelli is involved in administrative, technical, or material support. Sechi is involved in study supervision.
To the editor,
Alopecia areata (AA) is an autoimmune disease characterized by non‐scarring hair loss due to inflammatory responses that target the hair follicles. Infectious agents, including viruses, have been associated with the onset or progression of AA. The link with vaccines is more controversial, despite some cases have been reported. 1 We present six cases of AA, 3 after SARS‐CoV‐2 infection, and 3 beginning after COVID‐19 vaccination (Table S1).
We herein report the case of three patients (2 female patients and 1 male patient) aged 28–56 years who presented with patchy AA on the scalp after a moderate SARS‐CoV‐2 infection, beginning after a time gap of 2–3 weeks (Figure 1). In all the cases, COVID‐19 was confirmed by PCR test. None of them required hospitalization, and eventually healed within 3 weeks after symptoms onset. Only one of them had a previous episode of a single alopecic patch of the scalp 4 years before. All patients applied local corticosteroids and underwent spontaneous resolution within 4 months.
FIGURE 1.
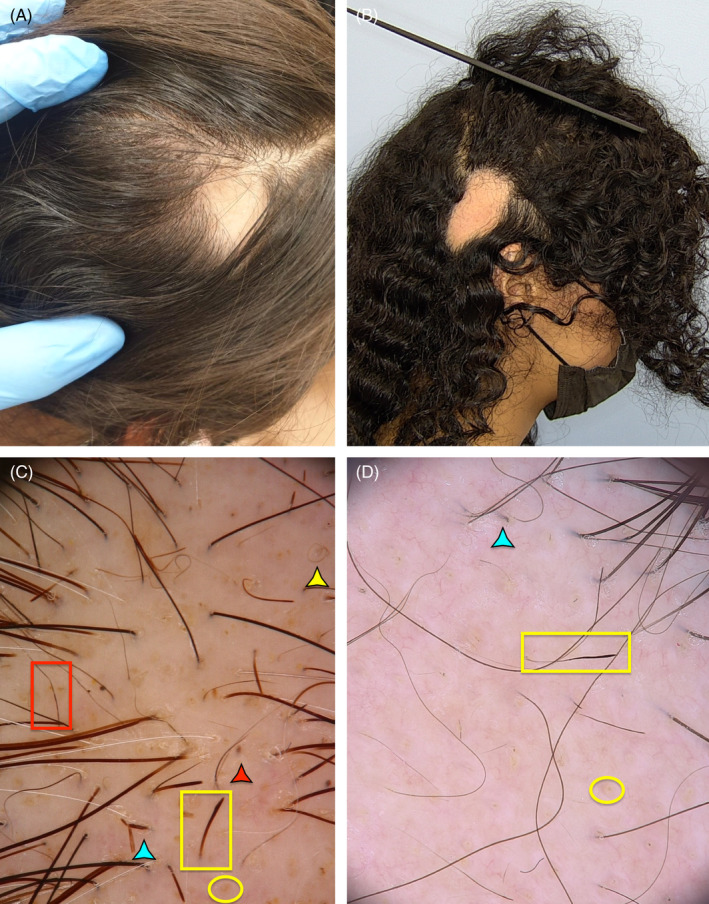
Clinical and trichoscopic features of cases 1 and 3, presenting with single enlarging patch of non‐scarring alopecia (A,B). Trychoscopy displays yellow dots and pigtail hairs along with features of disease activity, including black dots, broken hairs, Pohl–Pinkus restrictions
Three other patients developed widespread AA following 1–2 weeks after the first dose of the vaccine. In all the cases, a mRNA‐based vaccine was administered: BioNTech‐/Pfizer mRNABNT162b2 (Comirnaty®) in patients 4 and 5, and Moderna mRNA −1273 (Spikevax®) in patient 6. Patient 4 had a history of chronic recurrent AA, and witnessed a recrudescence after being vaccinated, whereas patient 5 had a history of single patch AA in pediatric age, and was then only one who progressed to AA totalis in the following 6 weeks, being unresponsive to topical and systemic steroids (Figure 2).
FIGURE 2.
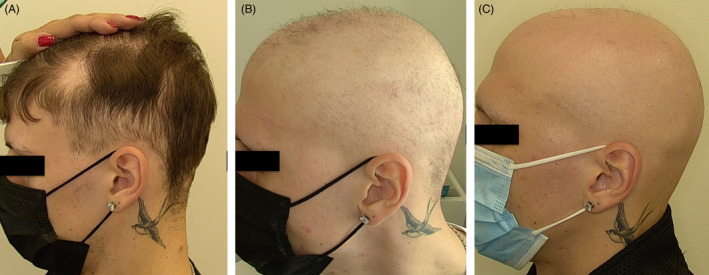
Rapidly progressing alopecia areata in a 24‐year‐old patient (Patient 5), occurring a week after the first dose of Comirnaty®. Note the concomitant partial loss of eyebrows. Timeframe: (A) 2 weeks after vaccination; (B) 5 weeks; (C) 8 weeks
Trichoscopy of all cases showed features of disease activity, including black dots, broken hairs, and exclamation mark hairs. Median SALT scores for disease activity were higher in the post‐vaccination group than in the post‐infectious one (56 ± 38.9 vs. 10.6 ± 7).
Written informed consent was obtained by all patients.
During the COVID‐19 pandemic, an increased incidence of AA has been observed, but only a few cases were associated with a confirmed SARS‐CoV‐2 infection. 2 COVID‐19 set off a interferon‐driven cytokines storm by triggering T‐ and B‐lymphocytes and plasmacytoid dendritic cells (pDC) immune response, with consequent raised levels of IL‐6, IL‐4 and tumor necrosis factor‐α. Activated pDC Th1‐generates a cellular immunity/CD8 + T‐ and NK cell‐dependent cytotoxic response, which is crucial for the pathogenesis of AA. 3 At the same time, raised INF‐I levels upregulated MHC class I proximal outer root sheath, favoring the loss of hair follicle immune privilege. 4
In our case series, AA was mild, limited to the scalp, and showed a good response to prescribed treatment. Although, rapidly progressive forms of AA, including alopecia universalis, have been observed in association with COVID‐19. In patients with preexisting AA, no increase in disease severity was observed when patients contracted mild‐to‐moderate SARS‐CoV‐2 infection. 5
Recent advances point outs genetic similarities between human endogenous cross‐reactive antigens and spike protein, claiming the reason for common adverse events to both COVID‐19 and COVID‐vaccines, since both mRNA (Comirnaty® and Spikevax®) and inactivated COVID‐vaccine uses the technology, respectively, of incapsulated mRNA lipid nanoparticles and chimpanzee adenovirus‐vectored vaccine to encode the spike protein. Likely, individuals with genetic susceptibility for alopecia areata or autoimmune disorders are at greater risk. 6 Interestingly, Patient 5 had a sister affected by androgenetic alopecia and alopecia areata incognita. AA has been reported after vaccinations against several pathogens, including hepatitis B virus, Influenza, Human Papillomavirus, Varicella‐Zoster, and Japanese encephalitis vaccines. 4 , 6
Previous reports of post‐vaccine alopecia areata have been published in limited case series. Rossi et al. published three cases of recrudescing AA after the first dose of COVID‐vaccine, 4 similar to the case reported by Essam and coworkers regarding AA onset in young woman with a previous episode of AA years before. 6 Differently, our Case 6 had no previous history of AA.
Overall, several mechanism including molecular mimicry ad antibody‐dependent response toward cross‐reacting human antigens and IFN‐I‐mediated immune response to viral antigens may accounts for SARS‐CoV‐2 infection and COVID‐vaccine‐related AA onset or recurrence. Finally, it is important to mention that the major limitation of this study is the small sample size. Further investigation with a larger cohort of patients is needed to clarify the causal link between COVID‐19 infection or vaccination and AA onset or recurrence.
Supporting information
Table S1
Bardazzi F, Guglielmo A, Abbenante D, Sacchelli L, Sechi A, Starace MVR. New insights into alopecia areata during COVID‐19 pandemic: When infection or vaccination could play a role. J Cosmet Dermatol. 2022;21:1796–1798. doi: 10.1111/jocd.14864
Andrea Sechi and Michela Valeria Rita Starace equally contributed to this study.
REFERENCES
- 1. Richardson CT, Hayden MS, Gilmore ES, Poligone B. Evaluation of the Relationship between Alopecia Areata and Viral Antigen Exposure. Am J Clin Dermatol. 2018;19(1):119‐126. [DOI] [PubMed] [Google Scholar]
- 2. Kutlu Ö, Aktaş H, İmren IG, Metin A. Short‐term stress‐related increasing cases of alopecia areata during the COVID‐19 pandemic. J Dermatolog Treat. 2020;19:1. [DOI] [PubMed] [Google Scholar]
- 3. Abou‐Rahal J, Abdullah L, Kurban M. Letter to the editor regarding the article ‘‘Rossi A, Magri F, Michelini S, et al. New onset of alopecia areata in a patient with SARS‐COV‐2 infection: Possible pathogenetic correlations? J Cosmet Dermatol. 2021;20(7):2004‐2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rossi A, Magri F, Michelini S, et al. Recurrence of alopecia areata after COVID‐19 vaccination: A report of three cases in Italy. J Cosmet Dermatol. 2021;20(12):3753‐3757. [DOI] [PubMed] [Google Scholar]
- 5. Rudnicka L, Rakowska A, Waskiel‐Burnat A, Kurzeja M, Olszewska M. Mild‐to‐moderate COVID‐19 is not associated with worsening of alopecia areata: A retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85(3):723‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Essam R, Ehab R, Al‐Razzaz R, Khater MW, Moustafa EA. Alopecia areata after ChAdOx1 nCoV‐19 vaccine (Oxford/AstraZeneca): a potential triggering factor? J Cosmet Dermatol. 2021;20:3727‐3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


