Abstract
Background and Aim
Gut dysbiosis is associated with immune dysfunction and severity of COVID‐19. Whether targeting dysbiosis will improve outcomes of COVID‐19 is unknown. This study aimed to assess the effects of a novel gut microbiota‐derived synbiotic formula (SIM01) as an adjuvant therapy on immunological responses and changes in gut microbiota of hospitalized COVID‐19 patients.
Methods
This was an open‐label, proof‐of‐concept study. Consecutive COVID‐19 patients admitted to an infectious disease referral center in Hong Kong were given a novel formula of Bifidobacteria strains, galactooligosaccharides, xylooligosaccharide, and resistant dextrin (SIM01). The latter was derived from metagenomic databases of COVID‐19 patients and healthy population. COVID‐19 patients who were admitted under another independent infectious disease team during the same period without receiving SIM01 acted as controls. All patients received standard treatments for COVID‐19 according to the hospital protocol. We assessed antibody response, plasma proinflammatory markers, nasopharyngeal SARS‐CoV‐2 viral load, and fecal microbiota profile from admission up to week 5.
Results
Twenty‐five consecutive COVID‐19 patients received SIM01 for 28 days; 30 patients who did not receive the formula acted as controls. Significantly more patients receiving SIM01 than controls developed SARS‐CoV‐2 IgG antibody (88% vs 63.3%; P = 0.037) by Day 16. One (4%) and 8 patients (26.7%) in the SIM01 and control group, respectively, failed to develop positive IgG antibody upon discharge. At week 5, plasma levels of interleukin (IL)‐6, monocyte chemoattractant protein‐1 (MCP‐1), macrophage colony‐stimulating factor (M‐CSF), tumor necrosis factor (TNF‐α), and IL‐1RA reduced significantly in the SIM01 but not in the control group. There was a significant negative correlation of nasopharyngeal SARS‐CoV‐2 viral load and SIM01 intervention. Metagenomic analysis showed that bacterial species in SIM01 formula were found in greater abundance leading to enrichment of commensal bacteria and suppression of opportunistic pathogens in COVID‐19 patients by week 4 and week 5.
Conclusions
This proof‐of‐concept study suggested that the use of a novel gut microbiota‐derived synbiotic formula, SIM01, hastened antibody formation against SARS‐CoV‐2, reduced nasopharyngeal viral load, reduced pro‐inflammatory immune markers, and restored gut dysbiosis in hospitalised COVID‐19 patients.
Keywords: immunity, microbiota, probiotics, SARS‐CoV‐2
Introduction
Growing evidence indicates that gut microbiota plays an important role in COVID‐19. 1 , 2 , 3 , 4 , 5 We previously reported that patients with COVID‐19 had altered fecal microbiomes characterised by enrichment of opportunistic pathogens and depletion of beneficial commensals. 1 , 2 Our results showed that gut dysbiosis correlated with the severity of COVID‐19 and persisted even after clearance of SARS‐CoV‐2 in respiratory samples and resolution of respiratory symptoms. 1 , 2
Emerging evidence suggests that probiotics modulate host immune functions 6 and reduce the severity of upper respiratory tract infections in children and adults. 7 , 8 Accordingly, a national health authority recommended the use of probiotics in the management of COVID‐19. 9 However, whether modulation of gut microbiota can improve the outcomes of COVID‐19 is largely unknown.
SIM01 is an oral encapsulated formulation of 3 lyophilised Bifidobacteria that was derived from our previous study 1 , 2 and 3 prebiotics that are beneficial for growth of these bacteria. 10 , 11 , 12 , 13 , 14 , 15 This formula aims to replenish the missing bacteria associated with immune defense in COVID‐19 patients. In this open‐label pilot study, we aimed to evaluate the efficacy of SIM01 as an adjuvant therapy on immunologic response and gut microbiota amongst patients hospitalised for COVID‐19. We hypothesised that restoring gut dysbiosis with SIM01 would improve antibody response and suppress proinflammatory response in COVID‐19.
Methods
Study design and participants
This was an open‐label, proof‐of‐concept study of consecutive hospitalised patients with a confirmed diagnosis of SARS‐CoV‐2 infection based on positive reverse‐transcriptase polymerase chain reaction (PCR) in a tertiary referral center in Hong Kong. Patients were excluded if they were: below age of 18; on mechanical ventilation, admitted to intensive care unit, on peritoneal dialysis or hemodialysis, immunocompromised (e.g., organ or hematopoietic stem cell transplant, neutropenia < 500 cells/μL, HIV positive with CD4 < 200 cells/μL), having active or known history of infective endocarditis, pregnant, or having a history of suspected intolerance to the product (SIM01) or its components. The latter was defined as any condition such as allergic reaction or any discomfort that rendered the subject not suitable to participate in this study. Using big data analysis and metagenomics derived from COVID‐19 patients and healthy subjects, we have developed a unique oral microbiome formula, SIM01, to target gut dysbiosis. The three probiotic species included in the formula were selected based on positive correlations with bacterial species found to be depleted in COVID‐19 patients from our published work. 1 , 2 In addition, the abundance of these bacteria also demonstrated strong positive correlations with abundance of beneficial bacteria from two independent healthy cohorts (N = 1400; data in files). The ratio of three bacteria was derived from the average natural accruing ratio in over 3000 healthy population. We also included three specific prebiotics (galactooligosaccharides, xylooligosaccharide, and resistant dextrin) in SIM01 to stimulate the favorable growth and/or enhance activities of the indigenous probiotic bacteria. 10 , 11 , 12 , 13 , 14 , 15 The SIM01 formula used in this study was a combination of Bifidobacterium strains, galactooligosaccharides, xylooligosaccharide, and resistant dextrin. 1 , 2 The three Bifidobacterium strains are commercially available from Chambio Co., Ltd and WECARE‐PROBIOTICS. A blend of Bifidobacterium strains (25 billion CFU per capsule) and prebiotics (galactooligosaccharides, xylooligosaccharide, and resistant dextrin) were prepared by the Centre for Gut Microbiota Research, The Chinese University of Hong Kong according to the International Good Manufacturing Practice Guidelines for Pharmaceuticals. Consecutively sealed bottles of the study capsules were stored in a double‐locked cabinet with temperature and humidity control in the Clinical Research Pharmacy. A designated pharmacist was responsible for dispensing the study capsules. Eligible patients received two doses of SIM01 (100 billion CFU) per day. All the subjects were recruited consecutively, and the baseline stool samples were collected before SIM01 administration. All patients took study capsules together with standard meals for 28 days. The control group consisted of 30 COVID‐19 patients who were admitted under another independent infectious disease team during the same period without receiving SIM01. Both study group and control group received the same treatment protocol for COVID‐19 endorsed by the local health authority. This study was approved by the Clinical Research Ethics Committees (2020.407) and was registered in the Clinical Trials Registry (NCT04581018). Written informed consents were obtained from all patients.
Clinical data collection
Demographic data including age, gender, smoking and drinking status were recorded. COVID‐19 specific clinical data including symptoms on admission, chest X‐ray findings on admission, treatments and disease severity were collected using an electronic medical record system of the Hong Kong Hospital Authority. Severity of COVID‐19 was categorized as (i) mild, if there was no radiographic evidence of pneumonia; (ii) moderate, if pneumonia was associated with fever and respiratory symptoms; (iii) severe, if respiratory rate ≥ 30/min, oxygen saturation ≤ 93% when breathing ambient air, or PaO2/FiO2 ≤ 300 mm Hg (1 mm Hg = 0.133 kPa); or (iv) critical, if there was respiratory failure requiring mechanical ventilation, shock, or organ failure requiring intensive care. 16 Safety was assessed based on adverse events reported from baseline till week 5.
Blood tests
Immunoglobulin G (IgG) antibody tests against SARS‐CoV‐2 were performed in all patients before discharge as per local hospital policy. A chemiluminescent microparticle immunoassay for qualitative detection of IgG against SARS‐CoV‐2 nucleoprotein (SARS‐CoV‐2 IgG for use with ARCHITECT; Abbott Laboratories, Abbott Park, IL, USA; reference 06R8620). The amount of IgG antibodies to SARS‐CoV‐2 in each sample was determined by comparing its chemiluminescent relative light unit (RLU) to the calibrator RLU (index S/C). Using an index S/C threshold of 1·4, the manufacturer reported a sensitivity of 86·4% after 7 days from symptom onset and 100% after 14 days, and a specificity of 99·6%, using RT‐PCR as the gold standard. 17 Plasma samples were collected after centrifugation of whole blood at 1500× g for 10 min at room temperature (RT) without brake. Concentrations of cytokines and chemokines in plasma were measured using the Custom Premix Human Cyto Panel A 47 Plex (Millipore, #HCYTA‐60 K‐47C). All samples were measured upon the first thaw.
Detection of nasopharyngeal SARS‐CoV‐2 viral load
Serial conventional nasopharyngeal swabs were collected by healthcare workers under strict infection control precautions, using flocked swabs (FLOQSwabs, Copan, Italy) contained in a sterile bottle with viral transport medium. RNA was extracted using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany). SARS‐CoV2 RNA was detected and quantified by real‐time reverse‐transcriptase polymerase chain reaction (RT‐PCR), with primers and probes targeting the N gene of SARS‐CoV‐2. The primer‐probe set N1 (2019‐nCoV_N1‐F: 5’‐GAC CCC AAA ATC AGC GAA AT‐3′, 2019‐nCoV_N1‐R: 5′‐TCT GGT TAC TGC CAG TTG AAT CTG‐3′ and 2019‐nCoV_N1‐P: 5′‐FAMACC CCG CAT TAC GTT TGG TGG ACC‐BHQ1‐3′) was purchased from Integrated DNA Technologies, USA. The one‐step real‐time RT‐PCR reaction contained 5 μL of the extracted preparation, 4 μL of TaqMan™ Fast Virus 1‐Step Master Mix (Applied Biosystems, USA) in a final reaction volume of 20 μL. The primer and probe concentrations were 0.5 and 0.125 μM, respectively. The cycling conditions were set at 25°C for 2 min, 50°C for 15 min, and 95°C for 2 min; followed by 45 cycles of 95°C for 15 s, and 55°C for 30 s, and performed using the StepOnePlus Real‐Time PCR System (Applied Biosystems, USA). Samples were considered as negative if the Ct values exceeded 39.9 cycles. 18
Stool metagenomic sequencing and profiling
Fecal samples were collected at baseline, and week 2, 4 and 5 for metagenomic profiling as previously described. 1 Briefly, fecal DNA was extracted using Maxwell® RSC PureFood GMO and Authentication Kit (Promega, Madison, Wisconsin) following the manufacturer's instructions. Sequencing libraries were prepared from extracted DNA using the Nextera DNA Flex Library Prep Kit (Illumina, California USA), and sequenced with paired‐end 150 bp sequencing strategy by Illumina NovaSeq 6000 System, generating 93.5 ± 15.2 million (mean ± SD) raw reads per sample. Raw sequence reads were filtered and quality‐trimmed using Trimmomatic v0.36 19 and decontaminated against human genome (Reference: hg38) by Kneaddata (V.0.7.2, https://bitbucket.org/biobakery/kneaddata/wiki/Home). MetaPhlAn2 (v2.6.0) was used to generate a species‐level abundance table. The definition of bacteria species enriched in COVID‐19 and non‐COVID‐19 subjects were based on fecal metagenomic analysis as previously described. 2
Statistical analysis
Continuous variables were expressed as median (interquartile range [IQR]) and categorical variables as number (percentage). Qualitative and quantitative differences between subgroups were analyzed using chi‐square or Fisher's exact tests for categorical parameters and Wilcoxon rank‐sum test for continuous parameters where appropriate. Friedman test, which is a non‐parametric alternative to one‐way ANOVA with repeated measures was used and can account for multiple measures from the same subject. Post‐hoc analysis was performed using Wilcoxon signed‐rank test with Bonferroni corrections. DESeq2 was used in microbiome analysis for paired samples from the same subject before and after treatment. Pearson's correlation was used to assess the correlation between fecal microbiome functional pathways with inflammatory markers. Kendall rank correlation analyses were conducted to associate longitudinal nasopharyngeal SARS‐CoV‐2 viral loads across 34 patients with COVID‐19 in both arms. All statistical tests were two‐sided. Statistical significance was taken at P < 0.05. Statistical analysis was performed using Statistical Product and Service Solutions (SPSS) version 25.0 (SPSS, Chicago, IL) or R v4.0.4.
Results
Antibody formation against SARS‐CoV‐2 virus
From August 8, 2020 to October 9, 2020, 25 consecutive eligible patients received SIM01 as an adjuvant therapy for COVID‐19. Thirty patients with comparable demographics who did not receive SIM01 acted as controls. Demographics are shown in Table 1. There were no significant differences in age, co‐morbidities, presenting symptoms, treatment and disease severity at baseline. One patient in the control arm required mechanical ventilation at week 1, while none required high flow oxygen/mechanical ventilation during hospitalization in the SIM01 arm. A significantly higher proportion of patients in the SIM01 group developed antibody against SARS‐CoV‐2 compared with the control by Day 16 (88% vs 63.3%; P = 0.037) (Fig. 1).
Table 1.
Baseline characteristics of patients with COVID‐19 in SIM01 and control arms
| SIM01 (n = 25) | Controls (n = 30) | P value | |
|---|---|---|---|
| Male, n (%) | 14 (56) | 9 (30) | 0.052 |
| Median age, years (IQR) | 50 (39–59) | 46.5 (29.5–56) | 0.151 |
| Smokers/ex‐smokers, n (%) | 7 (28) | 6 (20) | 0.298 |
| Drinkers/ex‐drinkers, n (%) | 2 (8) | 1 (3) | 0.585 |
| Comorbidities, n (%) | 11 (44) | 16 (53) | 0.729 |
| Recent exposure history, n (%) | |||
| Travel history | 0 (0) | 2 (6.7) | 1.000 |
| Contact with person with COVID‐19 | 18 (72) | 19 (63) | 0.448 |
| Symptoms at admission, n (%) | |||
| Fever | 20 (80) | 15 (50) | 0.030 |
| Ageusia/anosmia | 4 (16) | 4 (13) | |
| Myalgia | 5 (20) | 1 (3) | 0.085 |
| Malaise | 3 (12) | 7 (23) | 0.309 |
| Gastrointestinal symptoms | |||
| Diarrhea | 6 (24) | 5 (17) | 0.736 |
| Respiratory symptoms | |||
| Cough | 15 (60) | 16 (53) | 0.721 |
| Sputum | 3 (12) | 8 (27) | 0.156 |
| Sore throat | 7 (28) | 11 (37) | 0.440 |
| Rhinorrhea | 4 (16) | 5 (17) | 1.000 |
| Shortness of breath | 4 (16) | 3 (10) | 0.692 |
| Medications for COVID‐19, n (%) | |||
| Antibiotic | 4 (16) | 6 (20) | 0.741 |
| Antiviral drug | 12 (48) | 19 (63) | 0.254 |
| Dexamethasone | 4 (16) | 5 (17) | 1.000 |
| Pneumonia changes on chest X‐ray at admission, n (%) | 10 (40) | 13 (43) | 1.000 |
| Disease severity on admission, n (%) | |||
| Mild | 16 (64) | 21 (70) | 0.637 |
| Moderate | 8 (32) | 8 (27) | 0.665 |
| Severe | 1 (4) | 1 (3) | 1.000 |
| Critical | 0 (0) | 0 (0) | N/A |
IQR, interquartile range.
Figure 1.
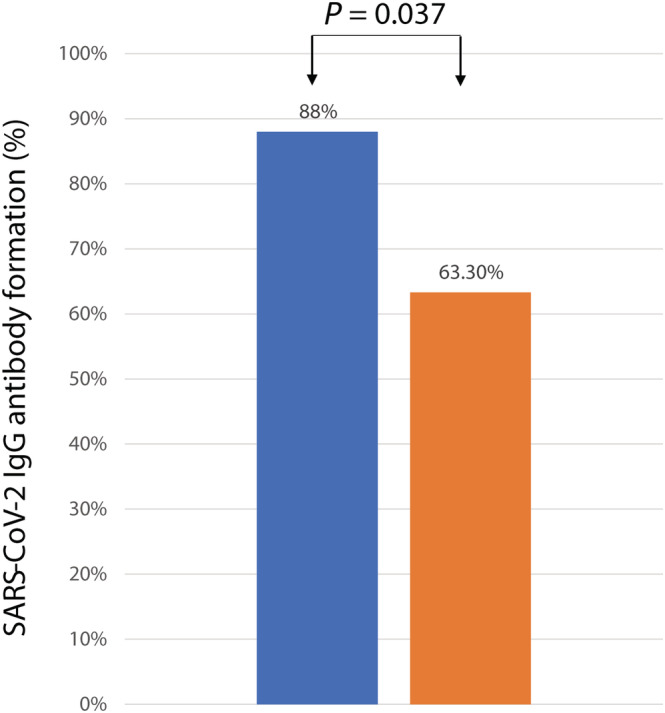
Antibody formation against SARS‐CoV‐2 in COVID‐19 patients in SIM01 and control groups.  , SIM01;
, SIM01;  , Controls. [Color figure can be viewed at wileyonlinelibrary.com]
, Controls. [Color figure can be viewed at wileyonlinelibrary.com]
Adverse events
There were no serious adverse events reported in both groups during the study period. Mild adverse effects were reported in 3 patients in the SIM01 group (one dizziness, one tinea infection, and one case of hypertension) and 4 patients in the control group (one hand inflammation and 3 cases of constipation).
Quantification of immune markers in plasma
Blood samples for analysis of immune markers were available from all 25 patients in the SIM01 arm and 10 patients in the control arm. At baseline, inflammatory markers were not significantly different between the SIM01 group and the control group. At week 5, the levels of plasma interleukin (IL)‐6, monocyte chemoattractant protein‐1 (MCP‐1), macrophage colony‐stimulating factor (M‐CSF), tumor necrosis factor (TNF‐α), and IL‐1RA dropped significantly in the SIM01 group but not in the control group. IL‐18, CXC motif chemokine ligand 10 (CXCL10), and monokine induced by interferon‐γ (MIG) were significantly reduced in both control and SIM01 group (Fig. 2).
Figure 2.
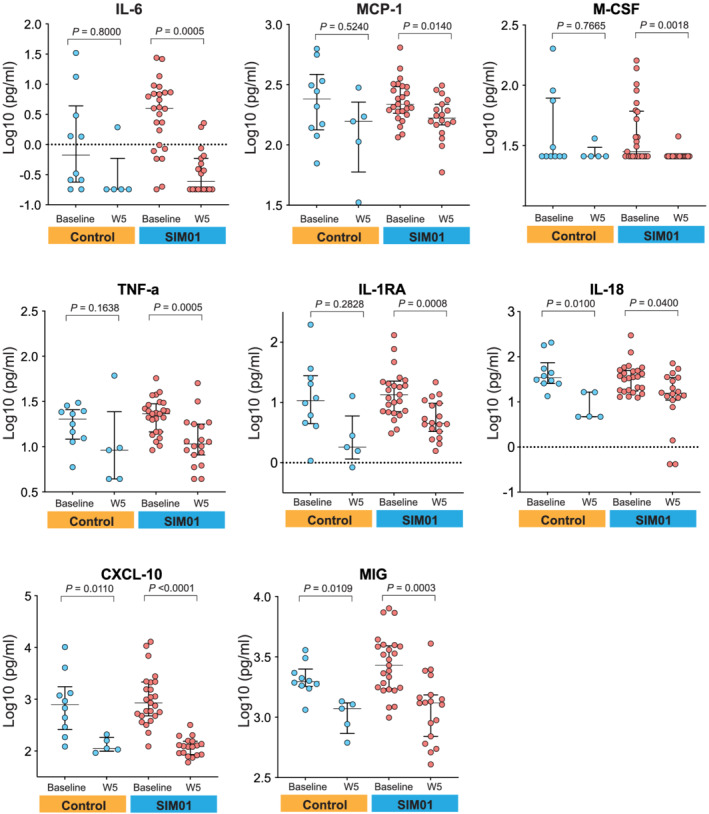
Quantification of plasma immune markers plotted as log10 transformation at baseline and at week 5 in SIM01 and control groups. For all boxplots, the center was the median of the measurement while the lower and upper bounds of the box corresponded to the first and third percentiles, respectively. P values were determined by two‐sides, Wilcoxon rank‐sum test for each arm.  , Controls;
, Controls;  , SIM01. [Color figure can be viewed at wileyonlinelibrary.com]
, SIM01. [Color figure can be viewed at wileyonlinelibrary.com]
Alterations in fecal microbiota composition
Stool samples were collected from the 25 patients in the SIM01 arm and 10 patients in the control arm. Using shotgun metagenomic sequencing, we compared the fecal microbiota profile at baseline, weeks 2, 4, and 5. The relative abundance of probiotic species in SIM01 arm was higher than the control arm at baseline (P = 0.044, Wilcoxon rank sum test) (Fig. 3). In subjects who received SIM01, their fecal samples showed an increased in relative abundance of probiotic species contained in SIM01 (P = 0.01419, Friedman test) as an aggregate and individual probiotics species (Fig. S1) at week 2 (P = 0.0533 vs baseline, Wilcoxon signed‐rank test with Bonferroni correction) and at week 4 compared with baseline (P = 0.0211 vs baseline, Wilcoxon signed‐rank test with Bonferroni correction) (Fig. 3a). The SIM01 group also showed a significant increase in the abundance of the phyla Actinobacteria and Firmicutes represented by beneficial commensals such as Bifidobacterium spp., Eubacterium spp. and Faecalibacterium prausnitzii at week 5 (adjusted P < 0.05 vs baseline, Table S1, Fig. S2). There was a significant reduction in opportunistic pathogens such as Escherichia coli in the Proteobacteria phylum and Bacteroides spp. in the Bacteroidetes phylum at week 5 (adjusted P < 0.05 vs baseline, Table S1, Fig. S2).
Figure 3.
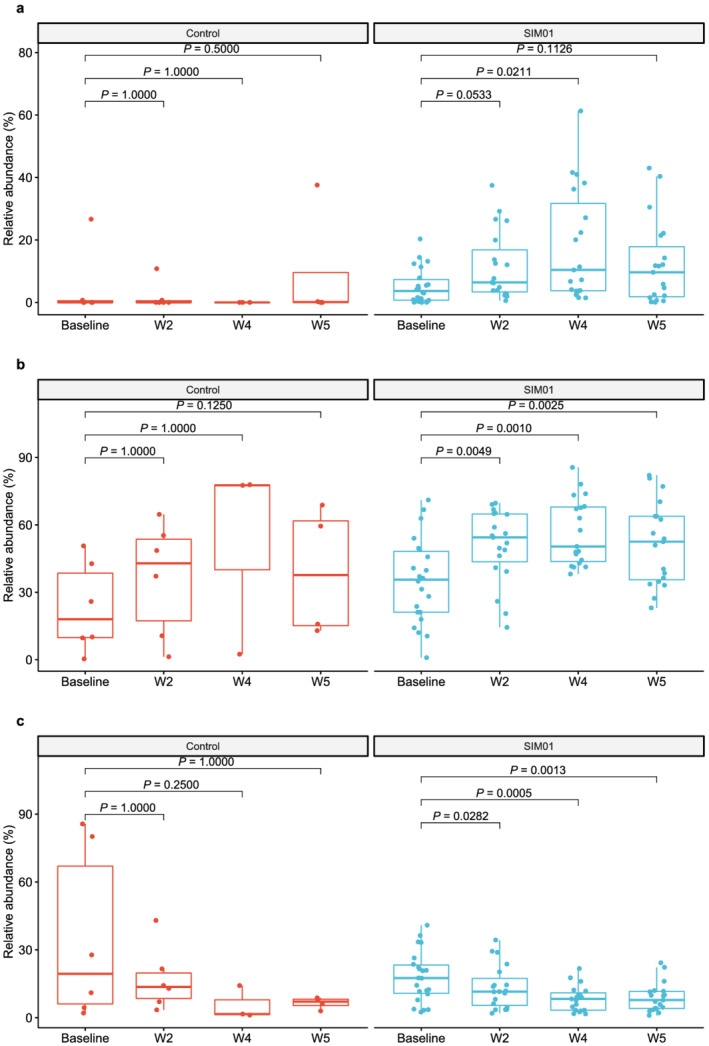
(a) Relative abundance of three probiotic species contained in SIM01 as an aggregate at baseline, week 2, week 4 and week 5 in the SIM01 and control groups. (b) Relative abundance of bacteria species known to be depleted in COVID‐19 patients at baseline, week 2, week 4 and week 5 in the SIM01 and controls groups. (c) Relative abundance of bacteria species known to be enriched in COVID‐19 patients at baseline, week 2, week 4 and week 5 in SIM01 and control groups. P values were determined by Friedman test, Post‐hoc: Wilcoxon signed‐rank test with Bonferroni correction. [Color figure can be viewed at wileyonlinelibrary.com]
To further demonstrate whether SIM01 administration is essential to restoring gut dysbiosis in patient with COVID‐19, we defined beneficial and pathogenic bacteria according to the fecal metagenomic analysis of microbial profile in COVID‐19 patients and non‐COVID‐19 subjects as previously published. 2 The abundance of beneficial bacterial species depleted in COVID‐19 was significantly increased at weeks 2, 4 and 5 (P < 0.01 vs baseline, Friedman test, Fig. 3b) whereas the abundance of pathogenic bacterial species enriched in COVID‐19 was significantly decreased at weeks 4 and 5 (P < 0.01 vs baseline, Friedman test, Fig. 3c). No such changes were observed in the control group (Fig. 3a–c). To avoid any selection bias, 10 patients were randomly selected in the SIM01 group to repeat the metagenomic analysis by subsampling for 100 times. The mean and 95% confidence interval (CI) were computed for 100 times of subsampling. Significant changes in metagenomic analysis including increase in total probiotics species, increase in bacteria species depleted in COVID−19 patients, decrease of bacteria species enriched in COVID−19 patients and increase of Bifidobacterium adolescentis , after SIM01 treatment, could be detected 80% of 100 times of subsampling (Friedman test, P < 0.05). The original mean from all 25 subjects was within 95% CI and comparable to the mean of 100 times of subsampling (Fig. S3).
Nasopharyngeal SARS‐CoV‐2 viral load
Thirty‐four of the 35 patients had SARS‐CoV‐2 nucleic acid detected in nasopharyngeal samples at hospitalisation. We found that treatment with SIM01 showed a significant negative correlation with SARS‐CoV‐2 viral load over time (Fig. 4) but not in the control group.
Figure 4.
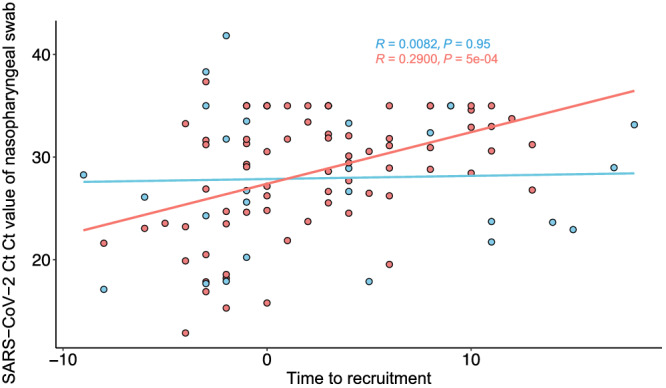
CT values of SARS‐CoV‐2 in nasopharyngeal samples were correlated along time within SIM01 group and control groups using Kendall rank correlation. Group:  , Control;
, Control;  , SIM01. [Color figure can be viewed at wileyonlinelibrary.com]
, SIM01. [Color figure can be viewed at wileyonlinelibrary.com]
Correlations between microbial functions and plasma inflammation markers
We investigated which microbial functions primarily drove the difference in control and SIM01 group by assessing gut microbiota functional capacity using HUMANN2 (v0.11.1). Both the control group and SIM01 group showed enrichment in pathways related to l‐lysine, l‐threonine and l‐methionine biosynthesis and l‐isoleucine biosynthesis, branched amino acid biosynthesis at week 5 compared with baseline. Moreover, patients receiving SIM01 showed enrichment in pathways related to l‐lysine biosynthesis, 5‐aminoimidazole ribonucleotide biosynthesis and pyruvate fermentation at week 5 compared with baseline. We then correlated the differential pathways with inflammatory markers (IL‐6, MCP‐1, M‐CSF, TNF‐α, IL‐1RA, IL‐18, CXCL‐10, and MIG), the super pathway of Clostridium acetobutylicum acidogenic fermentation was negatively correlated with MCP‐1 whereas pathways for glycogen and 4‐deoxy‐L‐threo‐hex‐4‐enopyranuronate degradation were negatively correlated with IL‐6 (Table S2). We then linked the relative abundance of three Bifidobacterium species to the identified inflammatory markers to assess whether SIM01 had any anti‐inflammatory action. We found that the relative abundance of B. adolescentis showed a significant negative correlation with plasma TNF‐α and that Bifidobacterium longum showed a significant negative correlation with plasma MIG in the SIM01 arm at week 5 (Fig. 5).
Figure 5.
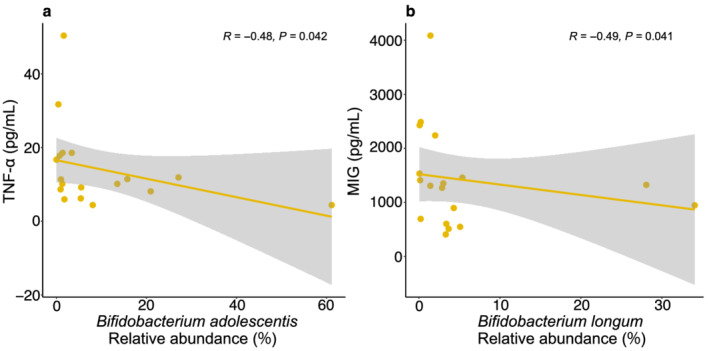
The correlations between plasma cytokine level and relative abundance of Bifidobacterium at week 5 were tested using Spearman's rank correlation coefficient. (a) Correlation between plasma TNF‐α and Bifidobacterium adolescentis . (b) Correlation between plasma MIG and Bifidobacterium longum . [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
To the best of our knowledge, this is the first study showing that a novel microbiome formula, SIM01, could hasten antibody formation against SARS‐CoV‐2. We have also demonstrated that treatment with SIM01 significantly reduced plasma pro‐inflammatory markers, restored depleted beneficial commensals, and was associated with a reduction in nasopharyngeal viral load in COVID‐19 patients. Increasing evidence suggests that gut microbiota is involved in the humoral immune response and could shape the B cell repertoire. 20 Our finding provides further support that modulation of gut microbiota contributes to enhancing the recovery from SARS‐CoV‐2 infection.
For the first time, we have shown that a novel combination of probiotics could enhance antibody formation in patients with COVID‐19. Currently, there are no human studies on the effect of probiotics on antibody formation against SARS‐CoV‐2 infection. It is generally believed that probiotics modulate the functions of dendritic cells, macrophages, and T and B lymphocytes, and regulate immunomodulatory functions through activation of toll‐like receptors. 21 A previous study reported that a combination of Lactobacillus and Bifidobacterium could raise plasma IgG, IgG1, and IgG3 levels in subjects receiving influenza vaccine. 22 However, whether this observation correlated with improvement in gut dysbiosis was unknown. We found that enhanced antibody response against SARS‐CoV‐2 correlated with enrichment of bacteria used in our probiotic formula. Our results have provided further evidence that modulation of gut microbiota can improve host immune function against COVID‐19.
Measurement of plasma pro‐inflammatory cytokines has clinical significance in COVID‐19. Patients with COVID‐19 often suffer from the consequence of cytokine storm, leading to severe multi‐organ damage. 23 Probiotics and prebiotics offer a plausible way to alleviate cytokine storm through modulation of Th1/Th2 immune responses in virus infections. 24 In this regard, we measured key immune markers that were shown to be elevated in COVID‐19. 23 In line with previous evidence, our study showed that SIM01 formula was associated with a significant reduction in pro‐inflammatory immune markers of IL‐6, MCP‐1, M‐CSF, TNF‐α and IL‐1RA compared with controls. This supports our hypothesis that treatment with SIM01 has a potent anti‐inflammatory action in hospitalised COVID‐19 patients.
Effective delivery and colonization of probiotic bacteria in the gut is essential to restoring gut dysbiosis. Using metagenomics sequencing, we could demonstrate enrichment of the bacterial species of SIM01 in the gut. Importantly, we found that certain commensal bacteria depleted in COVID‐19 patients 1 , 2 were significantly increased after receiving SIM01. At higher taxonomic level, patients receiving SIM01 had an increase of Actinobacteria and Firmicutes. Our results indicated that SIM01 not only replenished Bifidobacteria alone but also favored the co‐existence of other commensal bacteria. This could be achieved by modulation of host immunity or by a cross‐feeding effect between probiotics Bifidobacteria with butyrate‐producing colonic bacteria such as F. prausnitzii via the production of acetate. 25 On the other hand, we also found that harmful bacterial species enriched in COVID‐19 1 , 2 were significantly decreased after receiving SIM01. Suppression of opportunistic pathogens such as E. coli and Bacteroides spp. could be achieved by competing for nutrients or secretion of certain metabolites. Overall, our data suggested that SIM01 restored the commensal bacteria and suppressed opportunistic pathogens in COVID‐19 patients.
Our study had a number of strengths. Firstly, this is among the first clinical studies showing the beneficial effects of probiotic bacteria on the immune response of SARS‐CoV‐2 infection. Enhancement of antibody formation and suppression of proinflammatory cytokines have potential clinical significance. Secondly, using metagenomics sequencing we were able to demonstrate colonization of the bacterial species in SIM01. The latter helps restore gut dysbiosis associated with SARS‐CoV‐2. Prolonged dysbiosis may contribute to delayed complications such as long COVID. 2 , 5
Our study had limitations. Firstly, the non‐randomized study design might have inadvertently introduced bias in patient selection. The latter may account for the imbalance in the baseline bacteria species due to uncontrolled differences in diet, co‐morbidities or the use of other concomitant drugs, and a larger controlled study will be important to confirm these findings. Secondly, though we did not observe a significant increase in Bifidobacterium bifidum and B. longum after SIM01 administration, it is difficult to discern from the current study if the lack of increase affects its efficacy. Others have shown that B. bifidum had a protective effect on a murine model of colitis despite its poor ability of colonization, 26 and only approximately 30% of subjects achieved stable colonization of B. longum after oral administration in humans. 27 Future mechanistic studies will be important to elucidate the importance of individual bacteria species within SIM01, optimal duration of treatment, and the magnitude of colonization in the gut necessary for reported benefits.
Nevertheless, among patients hospitalised with COVID‐19, this proof‐of‐concept study suggested that the use of SIM01 hastens antibody formation, reduces nasopharyngeal viral load and pro‐inflammatory response, and improves gut dysbiosis. Modulation of gut microbiota offers hope to boost immunity against COVID‐19. A large‐scale randomized trial is underway to confirm our observations.
Supporting information
Figure S1. Relative abundance of probiotic species contained in SIM01.
Figure S2. The impact of SIM01 on the gut microbial diversity at phylum and order level.
Figure S3. Mean and 95% CI for 100 times of subsampling.
Table S1. The impact of SIM01 on the gut microbiota at species level at Week 5.
Table S2. Correlations between microbial functions and plasma inflammation markers.
Acknowledgments
The authors thank Whitney Tang, Amy Li, and Kitty Cheung for technical and logistical assistance and Wenye Xu, Alan Chu, Chun Pan Cheung, and Qin Liu for samples collection and processing.
Zhang, L. , Xu, Z. , Mak, J. W. Y. , Chow, K. M. , Lui, G. , Li, T. C. M. , Wong, C. K. , Chan, P. K. S. , Ching, J. Y. L. , Fujiwara, Y. , Chan, F. K. L. , and Ng, S. C. (2022) Gut microbiota‐derived synbiotic formula (SIM01) as a novel adjuvant therapy for COVID‐19: An open‐label pilot study. Journal of Gastroenterology and Hepatology, 37: 823–831. 10.1111/jgh.15796.
Declaration of conflict of interest: Prof. Siew Ng and Prof. Francis Chan report serving as founder member for GenieBiome Limited. Prof. Siew Ng also has served as advisory board member for Pfizer, Ferring, Janssen, Abbvie and speaker for Ferring, Tillotts, Menarini, Janssen, Abbvie, and Takeda. She has received research grants from Olympus, Ferring and Abbvie. Prof. Francis Chan has served as the Director & Board member of CUHK Medical Centre Limited and First Director of the Board of Directors for CUHK Multi‐Scale Medical Robotics Centre Limited. He also has served as advisor and lecture speaker for Eisai Co. Ltd, AstraZeneca, Pifzer Inc, Takeda Pharmaceutical Co., Takeda (China) Holdings Co. Ltd. Prof. Siew Ng, Prof. Francis Chan, and Dr Zhilu Xu are inventors of patent applications for “Composition for Improving Immunity” (CN202010657312.5, CN202011259564.9, CN202110223880.9, PCT/CN2021/090531, and TW110115155). Prof. Siew Ng and Prof. Francis Chan are inventors of patent applications for “Therapeutic and Diagnostic Use of Microorganisms for COVID‐19” (US63/016,759, US63/015,310, US63/064,821, PCT/CN2021/090488, and TW110115153). No other potential conflict of interest relevant to this article was reported. No other potential conflict of interest relevant to this article was reported.
Financial support: This study was supported by a generous donation from The DH Chen Foundation.
Clinical Trial Registry: The trial registration number is NCT04581018. Date of registration: October 9, 2020 (retrospectively registered).
Contributor Information
Francis K L Chan, Email: fklchan@cuhk.edu.hk.
Siew C Ng, Email: siewchienng@cuhk.edu.hk.
References
- 1. Zuo T, Zhang F, Lui GCY et al. Alterations in gut microbiota of patients with COVID‐19 during time of hospitalization. Gastroenterology 2020; 159: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yeoh YK, Zuo T, Lui GC et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID‐19. Gut 2021; 70: 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gu S, Chen Y, Wu Z et al. Alterations of the gut microbiota in patients with coronavirus disease 2019 or H1N1 influenza. Clin. Infect. Dis. 2020; 71: 2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang H, Ai JW, Yang W et al. Metatranscriptomic characterization of COVID‐19 identified a host transcriptional classifier associated with immune signaling. Clin. Infect. Dis. 2020: ciaa663. 10.1093/cid/ciaa663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Y, Gu S, Chen Y et al. Six‐month follow‐up of gut microbiota richness in patients with COVID‐19. Gut 2021: gutjnl‐2021‐324090. 10.1136/gutjnl-2021-324090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klaenhammer TR, Kleerebezem M, Kopp MV, Rescigno M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012; 12: 728–734. [DOI] [PubMed] [Google Scholar]
- 7. King S, Glanville J, Sanders ME, Fitzgerald A, Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta‐analysis. Br. J. Nutr. 2014; 112: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Puyenbroeck K, Hens N, Coenen S et al. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: a randomized, double‐blind, placebo‐controlled trial in healthy elderly nursing home residents. Am. J. Clin. Nutr. 2012; 95: 1165–1171. [DOI] [PubMed] [Google Scholar]
- 9. National Health Committee of the People's Republic of China . National Administration of Traditional Chinese Medicine Diagnostic and therapeutic guidance for 2019 novel coronavirus disease (version 5). http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf
- 10. Aachary AA, Prapulla SG. Xylooligosaccharides (XOS) as an emerging prebiotic: microbial synthesis, utilization, structural characterization, bioactive properties, and applications. Comprehensive Reviews in Food Science and Food Safety 2011 2011; 10: 2–16. [Google Scholar]
- 11. Amaretti A, Bernardi T, Leonardi A, Raimondi S, Zanoni S, Rossi M. Fermentation of xylo‐oligosaccharides by Bifidobacterium adolescentis DSMZ 18350: kinetics, metabolism, and beta‐xylosidase activities. Appl. Microbiol. Biotechnol. 2013; 97: 3109–3117. [DOI] [PubMed] [Google Scholar]
- 12. Barczynska R, Jurgonski A, Slizewska K, Juskiewicz J, Kapusniak J. Corn starch dextrin changes intestinal microbiota and its metabolic activity in rats fed a basal and high‐fat diet. British Food Journal 2019; 121: 2219–2232. [Google Scholar]
- 13. Hughes C, Davoodi‐Semiromi Y, Colee JC et al. Galactooligosaccharide supplementation reduces stress‐induced gastrointestinal dysfunction and days of cold or flu: a randomized, double‐blind, controlled trial in healthy university students. American Journal of Clinical Nutrition 2011; 93: 1305–1311. [DOI] [PubMed] [Google Scholar]
- 14. Van Laere KMJ, Hartemink R, Bosveld M, Schols HA, Voragen AGJ. Fermentation of plant cell wall derived polysaccharides and their corresponding oligosaccharides by intestinal bacteria. J. Agric. Food Chem. 2000; 48: 1644–1652. [DOI] [PubMed] [Google Scholar]
- 15. Wronkowska M, Soral‐Smietana M, Biedrzycka E. Utilization of resistant starch of native tapioca, corn and waxy corn starches and their retrograded preparations by Bifidobacterium. Int. J. Food Sci. Nutr. 2009; 59: 80–87. [DOI] [PubMed] [Google Scholar]
- 16. Wu J, Liu J, Zhao X, Liu CY, Wang W, Wang DW et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID‐19) in Jiangsu province: a multicenter descriptive study. Clin. Infect. Dis. 2020; 71: 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marina P, Beatriz PG, Roberto PB, Oteo J, Hernan MA, Mayte PO et al. Prevalence of SARS‐CoV‐2 in Spain (ENE‐COVID): a nationwide, population‐based seroepidemiological study. Lancet 2020; 396: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lai CKC, Chen ZG, Lui G et al. Prospective study comparing deep‐throat saliva with other respiratory tract specimens in the diagnosis of novel coronavirus disease (COVID‐19). J Infect Dis 2020; 222: 1612–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014; 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li H, Limenitakis JP, Greiff V et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature 2020; 584: 274–278. [DOI] [PubMed] [Google Scholar]
- 21. Yan F, Polk DB. Probiotics and immune health. Curr. Opin. Gastroenterol. 2011; 27: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rizzardini G, Eskesen D, Calder PC et al. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB‐12(R) and Lactobacillus paracasei ssp. paracasei, L. casei 431(R) in an influenza vaccination model: a randomised, double‐blind, placebo‐controlled study. Br. J. Nutr. 2012; 107: 876–884. [DOI] [PubMed] [Google Scholar]
- 23. Lucas C, Wong P, Klein J et al. Longitudinal analyses reveal immunological misfiring in severe COVID‐19. Nature 2020; 584: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu J, Zhang L, Lin W, Tang W, Chan FKL, Ng SC. Probiotics, prebiotics and dietary approaches during COVID‐19 pandemic. Trends Food Sci Technol 2021; 108: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Riviere A, Selak M, Lantin D, Leroy F, Vuyst LD. Bifidobacteria and butyrate‐producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016; 7: 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grimm V, Radulovic K, Riedel CU. Colonization of C57BL/6 Mice by a potential probiotic Bifidobacterium bifidum strain under germ‐free and specific pathogen‐free conditions and during experimental colitis. PLoS One 2015; 10: e0139935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maldonado‐Gomez MX, Martinez I, Bottacini F et al. Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host Microbe 2016; 20: 515–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Relative abundance of probiotic species contained in SIM01.
Figure S2. The impact of SIM01 on the gut microbial diversity at phylum and order level.
Figure S3. Mean and 95% CI for 100 times of subsampling.
Table S1. The impact of SIM01 on the gut microbiota at species level at Week 5.
Table S2. Correlations between microbial functions and plasma inflammation markers.


