Abstract
Campylobacter jejuni is a pathogenic, microaerophilic, gram-negative, mesophilic bacterium. Three strains isolated from humans with enteric campylobacteriosis were able to survive at high population levels (107 cells ml−1) as viable-but-nonculturable (VBNC) forms in microcosm water. The VBNC forms of the three C. jejuni strains were enumerated and characterized by using 5-cyano-2,3-ditolyl tetrazolium chloride–4′,6-diamino-2-phenylindole staining. Cellular volume, adenylate energy charge, internal pH, intracellular potassium concentration, and membrane potential values were determined in stationary-phase cell suspensions after 48 h of culture on Columbia agar and after 1 to 30 days of incubation in microcosm water and compared. A notable increase in cell volume was observed with the VBNC state; the average cell volumes were 1.73 μl mg of protein−1 for the culturable form and 10.96 μl mg of protein−1 after 30 days of incubation in microcosm water. Both the internal potassium content and the membrane potential were significantly lower in the VBNC state than in the culturable state. Culturable cells were able to maintain a difference of 0.6 to 0.9 pH unit between the internal and external pH values; with VBNC cells this difference decreased progressively with time of incubation in microcosm water. Measurements of the cellular adenylate nucleotide concentrations revealed that the cells had a low adenylate energy charge (0.66 to 0.26) after 1 day of incubation in microcosm water, and AMP was the only nucleotide detected in the three strains after 30 days of incubation in microcosm water.
The viable-but-nonculturable (VBNC) physiological state of bacteria was first described by environmental microbiologists. Since then, the VBNC state has been found in numerous human pathogens, including Escherichia coli (64), Salmonella enteritidis (49), Vibrio cholerae (10), Legionella pneumophila (24), and Campylobacter jejuni (48). Standard culture methods cannot detect VBNC cells efficiently, although the cells remain potentially pathogenic under favorable conditions (44, 45, 57). The viability of VBNC cells is thus routinely studied by optical microscopic methods. Direct viable counting, which is based on cellular elongation in the presence of DNA gyrase inhibitors, was first described by Kogure et al. (30). Another way to measure residual metabolic activity in cells is to measure the cellular accumulation of insoluble formazan crystals from different tetrazolium salts; 2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyltetrazolium chloride and 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) are the most frequently used salts (54, 66). Because of the fluorescent nature of its reduced formazan form, CTC is the compound that is used most frequently to determine viable cell (i.e., respiring cell) counts (47).
C. jejuni is now recognized as a leading human food-borne pathogen (40, 55). During the last few years, notable increases in human enteric campylobacteriosis have been reported in many countries (58). The VBNC state of C. jejuni cells is still a matter of controversy; some authors consider this physiological state a degenerating form (34), and other authors claim that the VBNC state is a dormant state and that the organism is able to grow again under favorable conditions (57). Temperature is a very important factor in the loss of culturable properties. Medema et al. (34) showed that the ability to culture cells was lost within 3 days when the cells were incubated at 25°C. Jones et al. (26) suspended various strains of C. jejuni in sterile surface water and incubated them at 4°C. The cells were in the VBNC state after 18 to 28 days, depending on the strain. Measurements of the metabolic activities of these cell suspensions demonstrated that cells incubated at 25 or 37°C metabolized the low levels of nutrients added to the cell suspensions faster than cells incubated at 4°C metabolized the nutrients. Thus, the number of culturable cells decreased more slowly when preparations were incubated at 4°C (48). In addition, microcosm water systems are characterized by important nutrient depletion and hypoosmotic conditions, which probably induce the VBNC state. However, VBNC C. jejuni cells have also been found in C. jejuni cell suspensions incubated in a rich culture medium. Hazeleger et al. (22) observed the VBNC state with various C. jejuni strains within 6 weeks of inoculation into brain heart infusion incubated at 4°C. A lack of culturability in natural oligotrophic environments has also been described for bacteria in soil (61). The potential infectivity of nonculturable forms of C. jejuni (26) and the transition between the VBNC state and active cells provide an interesting example of a strategy for survival of bacteria under adverse conditions.
Homeostasis of bacterial cells has been largely documented in response to osmotic shocks, such as those obtained after salt addition in the food industry. Potassium, a major cation of cells, is involved in maintenance of cell turgor pressure, in enzyme activation, and in internal pH homeostasis (2). Bacteria exposed to hyperosmotic shock conditions and bacteria exposed to hypoosmotic stress take up and loose large amounts of potassium, respectively (13, 14, 46). Related modification of the cell volume is sometimes observed in addition to modifications of the internal pH, the membrane potential, the proton motive force, or the adenylate energy charge (AEC) of the cells (3, 12, 35, 59, 65).
In a previous work the authors described the occurrence of the VBNC state in a collection of 36 C. jejuni strains. Three of these strains, strains Bf, 79, and 85, maintained metabolic activity for an extended period (30 days) when they were suspended in microcosm water. The effects of factors such as temperature, pH, and NaCl concentration on the VBNC state were studied (9) by staining preparations with CTC-DAPI (4′,6-diamino-2-phenylindole, dihydrochloride solution) in order to detect and enumerate C. jejuni VBNC cells (8). Morphological changes in the VBNC state of these three strains were monitored by scanning electron microscopy. In this study, we physiologically characterized VBNC cells of C. jejuni Bf, 79, and 85. The cell volumes, internal potassium and sodium concentrations, internal pH changes, and AECs of cells of the three strains were compared. Measurements were obtained for late-log-phase cell suspensions and for VBNC cell suspensions of C. jejuni after 15 to 30 days of starvation in microcosm water.
MATERIALS AND METHODS
Chemicals.
All chemicals were obtained from Merck (Nogent-sur Marne, France) and were analytical grade. Dow Corning type 508V70 silicone oil was purchased from Prolabo (Fontenay-sous-Bois, France), [carboxyl-14C]benzoic acid and [carboxyl-14C]dextran were acquired from Isotopchim (Ganagobie-Peyruis, France), and tritium-labelled water was obtained from Dupont NEN (Les Ulis, France). [3H]inulin and tetra[3H]phenylphosphonium bromide were acquired from Amersham Life Sciences (Les Ulis, France). The scintillation liquid used was Universol ES obtained from ICN (Orsay, France), and the scintillation vials used were Wheaton low-40K glass vials obtained from Bioblock (Strasbourg, France). The protein assay kit was obtained from Bio-Rad (Munich, Germany). Nigericin was purchased from Sigma (La Verpillère, France).
Bacterial strains and culture conditions.
The following three C. jejuni strains of human origin were used in this work: strains Bf, 79, and 85. These strains were grown on Columbia agar (Unipath, Basingstoke, England) supplemented with 5% lysed horse blood for 24 h at 37°C under a microaerobic atmosphere (Campypack; Becton Dickinson, Cockeysville, Md.). All of the strains were identified as members of C. jejuni subsp. jejuni by workers at the Laboratory Centre for Disease Control, Ottawa, Ontario, Canada. Two subcultures were grown under similar conditions before cells were suspended in microcosm water.
Starvation.
The microcosm water method described by Rollins and Colwell (48) was used to obtain nonculturable cells (starved cells) of C. jejuni. After growth on Columbia agar, cells were collected and suspended in bottles containing 500 ml of filter-sterilized (pore size, 0.2 μm; Nalgene) surface water adjusted to pH 6.0 ± 0.1 with a solution containing 0.1 mol of NaOH liter−1. The bottles were then incubated at 4°C with gentle shaking (100 rpm) for up to 30 days.
Spread plate counting.
The culturability of cells was determined by spread plate counting on Columbia agar containing 5% lysed horse blood as previously described by Federighi et al. (15). When the concentration of culturable cells was less than 300 cells ml−1, 0.1-ml portions of microcosm water were added to 10 petri dishes containing Columbia agar supplemented with 5% lysed horse blood. The numbers of CFU were determined after 48 h of incubation under a microaerobic atmosphere at 42°C. When the culturable cell counts were below the detection limit, culturability was assessed by an enrichment method in which Preston buffer (Oxoid, Dardilly, France) was used. A 1-ml portion of each microcosm was added to 9 ml of Preston buffer along with 450 μl of lysed horse blood, and the preparation was incubated for 24 h at 37°C. A 0.2-ml portion of this enrichment broth was then plated onto two Columbia agar plates, and the plates were incubated for 2 to 5 days at 42°C. A lack of residual culturable cells in C. jejuni VBNC cell suspensions was verified by filtering 10 ml of microcosm water and placing the filter on Columbia agar as described previously.
VBNC cell enumeration.
Samples of C. jejuni suspensions were removed at different times from microcosm water suspensions and were stained with the CTC and DAPI as described by Cappelier et al. (8). A 500-μl portion of brain heart infusion (Biokar, Beauvais, France) and 100 μl of a 0.05-g liter−1 solution of pyruvic acid (Sigma Chemical Co., St. Louis, Mo.) were added to 0.5 ml of the bacterial suspension to stimulate cell respiration. CTC (Polysciences, Warrington, Pa.) was diluted in water to a final concentration of 5 mmol liter−1, and the mixture was incubated for 4 h at 37°C under a microaerobic atmosphere. Cells were then harvested by filtration through a black isopore polycarbonate membrane filter (pore size, 0.2 μm; diameter, 25 mm; Millipore, Watford, Ireland) and covered with a 5-μg ml−1 DAPI (Molecular Probes, Eugene, Oreg.) solution for 5 min for counterstaining. Counts were obtained randomly by using 20 microscopic fields per filter. For each sample, two filters were counted. The viable cell counts (cells containing CTC formazan crystals) and the total cell counts (cells stained by DAPI; i.e., viable and nonviable cells) were determined for the three strains. The results were expressed as the number of bacteria per milliliter of original sample, as previously described by Federighi et al. (15).
Flow cytometry monitoring.
Flow cytometry measurements were obtained with a Facscan flow cytometer (Becton Dickinson, Pont-de-Claix, France). One hundred microliters of a bacterial suspension was diluted in 1 ml of phosphate-buffered saline (pH 7.0). Samples were analyzed at 488 nm with an argon laser. Forward light scattering was measured in the stationary phase and in microcosm water cell suspensions. The measurements were processed by using the Lysis II software, and the data were collected and used to construct as histograms.
Preparation of cell suspensions.
After up to 30 days of starvation in microcosm water, late-exponential-phase cultures (0.5 or 1 liter) of the three strains of C. jejuni were centrifuged for 30 min at 13,000 × g. The pellets were resuspended in 2 to 10 ml of the resulting supernatant in order to obtain cell suspensions having average protein concentrations of 1 to 10 mg ml−1, as determined by the method of Lowry et al. (32). The cell suspensions of the three strains of C. jejuni were used to determine (i) intracellular volumes, (ii) internal pH values, (iii) membrane potentials, (iv) intracellular metal ion concentrations, and (v) cellular adenine nucleotide concentrations.
Intracellular volume measurement.
Intracellular volume was measured with radioactively labeled probes by the method of Rottenberg (50). A 100-μl (37-kBq) portion of a stock solution of [carboxyl-14C]dextran (370 kBq of [14C]dextran per ml dissolved in distilled water) and 20 μl (74 kBq) of 3H2O (7.4 MBq g−1) were added to each 2-ml cell suspension (4 mg of protein ml−1), and the preparations were mixed and incubated for 10 min with gentle shaking. Three 300-μl samples were then added to Eppendorf tubes containing 100 μl of perchloric acid (1 mol liter−1) and a 300-μl layer of type 508V70 silicone oil (density = 1.03), as previously described by Miguelez and Gilmour (35). The preparations were then centrifuged at 13,000 × g for 15 min so that the cells passed through the oil layer and collected on the bottom of the tube. The amounts of radioactivity in the supernatant above the oil layer and in the pellet were then determined. Scintillation vials containing the double-labelled samples were counted with a Betamatic liquid scintillation counter obtained from Kontron Instruments (Montigny-le-Bretonneux, France) by using a manual optimized 14C-3H double-labelling program. Nonspecific binding of radioactive probes to cells and debris was measured after French press treatment of cell suspensions at 240 MPa, and the values obtained were compared to values obtained after cell suspensions were boiled for 20 min.
Intracellular pH measurement.
The intracellular pH values of dense cell suspensions (4 mg of protein ml−1) were determined by measuring the internal accumulation of a weak acid, as described by Booth et al. (5). A 100-μl (37-kBq) portion of a stock solution of [carboxyl-14C]benzoic acid (1.3-GBq mmol−1 solution in water) was added to each 2-ml dense cell suspension along with 200 kBq of [3H]inulin (111-GBq mmol−1 stock solution in water), which was included as a marker for extracellular water. Incubation, harvesting of the supernatant and pellet, and counting of radioactivity were performed as described above for internal volume measurements. The internal pH values of C. jejuni cell suspensions were also measured with the fluorescent probe BCECF [2′,7′-bis(2-carboxyethyl)-5-(and-6)carboxyfluorescein] by using a model 625 fluorimeter (Kontron) and a method described by Graber et al. (18) and Futseather et al. (17). A pure BCECF solution in dimethyl sulfoxide was added to C. jejuni cell suspensions (108 cells ml−1) to obtain a final BCECF concentration of 5 μmol liter−1. Cell suspensions were allowed to equilibrate for 2 h at 4°C before centrifugation and resuspension in microcosm water (1 mg of protein ml−1). One hundred microliters of each cell suspension was added to 1 ml of microcosm water along with 100 μl of a 1.2-mol liter−1 KCl stock solution (final KCl concentration, 100 μmol liter−1). Internal pH values were immediately measured with excitation at wavelengths of 490 to 450 nm and emission at a wavelength of 535 nm. The ratio of fluorescence at 490 nm to fluorescence at 450 nm was related to the internal pH of each cell suspension, as previously described by Tsujimoto et al. (60) and Noel et al. (39). The ionophore nigericin (final concentration, 5 μmol liter−1) and a 1.2-mol liter−1 KCl solution (final KCl concentration, 100 μmol liter−1) were added to 100 μl of each C. jejuni cell suspension, which resulted in elimination of pH gradients across the cell membrane. The intracellular pH was thus fixed at the external pH, which was measured with a pH meter (model 525 WTW digital pH meter; Prolabo, Paris, France). Titration of cell suspensions with small amounts of NaOH (0.5 mol liter−1) or HCl (0.5 mol liter−1) resulted in a calibration curve for the fluorescence ratio as a function of the pH, from which the intracellular pH of each sample was determined.
Membrane potential measurement.
Membrane potentials were measured with cell suspensions (4 mg of cell protein/ml). Tetra[3H]phenylphosphonium bromide was used as the radioactive probe for membrane potential measurements, as described previously by Bakker et al. (4). Aliquots (100 μl; 185 kBq) of a stock solution of tetra[3H]phenylphosphonium bromide (1.4-TBq mmol−1 ethanolic solution) were added to 2-ml dense cell suspensions along with 100 μl of an unlabelled tetraphenylphosphonium bromide stock solution (final concentration, 10 mmol liter−1) to prevent nonspecific binding of the radioactive probe to the cells. Incubation, harvesting of the supernatant and pellet, and counting of the radioactivity were performed as described above for internal volume measurements. The data were compared with data obtained under similar experiment conditions after preliminary cell treatment with EDTA by a method described by Booth et al. (5) for gram-negative bacteria. Cells (10 mg of protein ml−1) were first harvested and washed once in a solution containing 120 mmol of Tris-HCl buffer (pH 8.0) per liter. EDTA-KOH (pH 7.0) was then added to a final concentration of 1 mmol liter−1, and the preparation was incubated for 2 min with gentle agitation. The cells were then centrifuged at 10,000 × g for 15 min at room temperature. The cell pellet was washed by resuspension and centrifugation in a solution containing 5 mmol of Tris per liter and 5 mmol of MES (4-morpholineethanesulfonic acid)-HCl buffer per liter (pH 7.0) and then centrifuged again and resuspended in the microcosm water system.
K+ concentration determination.
The potassium concentrations in C. jejuni cell suspensions were determined by flame atomic absorption spectroscopy as described by Schönheit and Perski (53). Aliquots (10 ml) of cell suspensions were added to polycarbonate tubes along with 2 ml of type 508V70 silicone oil (d = 1.03), and the preparations were centrifuged at 13,000 × g for 30 min. The pellets were resuspended in 2 ml of a solution containing 1.4 mol of H2SO4 per liter and mineralized for 4 h at 120°C. The pellets were then diluted 1:25, and the supernatants were diluted 1:50 with a solution containing 6 mmol of CsCl per liter in H2SO4 (0.1 mol liter−1). The potassium concentrations in both pellets and supernatants were estimated at 766.5 nm by using a Pye-Unicam SP9 apparatus (Philips, Paris, France). The cellular potassium content of the microcosm water was determined immediately after cells were suspended in microcosm water and 1, 15, and 30 days after cells were suspended in microcosm water. Nonspecific binding of potassium to cells and debris was measured after French press treatment of cell suspensions at 240 MPa, and the values obtained were compared to values obtained after cell suspensions were boiled for 20 min.
Determination of cellular adenine nucleotide contents.
Adenine nucleotides were extracted by the method of Walker-Simmons and Atkinson (62). Samples (1 ml of cell suspension) were rapidly added to 0.2 ml of ice-cold HClO4. The cell extract was kept in ice for 20 min and then frozen at −70°C. The extract was thawed after 24 h and centrifuged at 13,000 × g for 5 min. Aliquots (1 ml of supernatant) were removed and neutralized with 0.35 ml of a solution containing 2.6 mol KOH liter−1 and 0.58 mol KHCO3 liter−1. After 10 min in ice, the neutralized extract was centrifuged at 13,000 × g for 5 min to remove the KClO4 precipitate, and 1 ml of supernatant was removed, immediately frozen at −70°C, and lyophilized. The freeze-dried extract was resuspended in 0.2 ml of ultrapure water before analysis. Extracellular adenine nucleotide contents were also determined by centrifuging 1.2 ml of a dense bacterial suspension at 13,000 × g for 5 min. Aliquots (1 ml of supernatant) were treated as described above. Intracellular nucleotides were quantified by determining the difference between the total and extracellular nucleotide contents. The adenine nucleotides were analyzed by reversed-phase high-performance liquid chromatography performed with a Waters system (St Quentin-en-Yvelines, France) equipped with a model 616 quaternary pump, a model 717 plus autoinjector, and a model 486 UV-visible detector. Chromatography was performed with on a Hypersil octadecyl silane column (250 by 4.6 mm; thickness, 5 μm) protected by an integrated precolumn at room temperature; and the flow rate was 1.2 ml min−1. A gradient system based on the system of Crescentini and Stocchi (11) was used for analysis. Buffer A consisted of 100 mM KH2PO4 adjusted to pH 6.00 with KOH, and buffer B consisted of 15% methanol in buffer A. A 9-min isocratic step with buffer A was followed by a linear increase up to 100% buffer B for 5 min, and then buffer B was used for 9 min. The initial conditions were restored in 2 min. Samples (50 μl) were injected, and eluates were monitored at 260 nm. Quantification was carried out by measuring peak heights with Millenium 2010 software, version 2.1.
Protein contents of dense cell suspensions.
The protein contents of the cell suspensions described above were determined by using the method of Lowry et al. (32); bovine serum albumin was used as the standard.
RESULTS
Production of VBNC cells of C. jejuni.
Microscopic counting of total and viable cells, as well as CFU, was used to monitor conversion of C. jejuni cells to the VBNC state in microcosm water. The metabolic activity of cells was determined by the CTC assay. The surviving cells of the three strains of C. jejuni were counted. The total cell counts (approximately 108 cells ml−1) did not change during the 30 days of incubation in microcosm water. The cell count data for the three strains revealed that there was a loss of culturability of 1 log during the first day of incubation in microcosm water. A sudden decrease of in the culturability of cells was observed after 5 to 7 days of incubation in microcosm water, depending on the strain. With strains 79 and 85 the VBNC state was reached after 14 days of starvation, and with strain Bf the VBNC state was reached after 16 days in microcosm water, as indicated by decreases in concentration to less than 1 CFU per ml. In the VBNC state, approximately 107 cells per ml contained CTC formazan crystals, indicating that metabolically active cells (i.e., VBNC cells) were present. The protein contents of C. jejuni 79 and 85 VBNC cell suspensions did not change during 30 days of incubation in microcosm water, while during the first 15 days of incubation of C. jejuni Bf the protein content decreased 50%.
Flow cytometry monitoring.
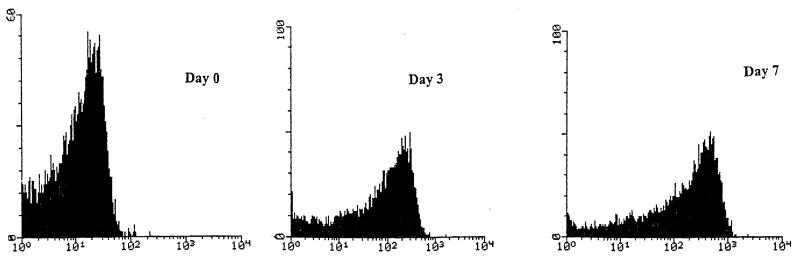
Flow cytometry measurements revealed that there was an increase in the forward light scattering intensity (Fig. 1). A notable change was observed during the first few days in microcosm water. The peak channel values were 2 × 101 relative fluorescence units (RFU) at zero time and 3 × 102 RFU on day 3. Small changes occurred on the following days, and the peak channel value was 5 × 102 RFU after 7 or 15 days of starvation. Thus, the forward light scattering signal indicated that there was an increase in C. jejuni cell size during this period of starvation.
FIG. 1.
Changes in cell size during conversion of culturable cells to VBNC cells for three strains of C. jejuni in cell suspensions in the microcosm water system. The forward light scattering histograms for days 0, 3, and 7 demonstrate that there was a progressive increase in cell size as the number of VBNC cells in the microcosm water system increased.
Measurement of cell water volumes in fresh cultures and VBNC cell suspensions of C. jejuni.
The culturable cells of the three strains of C. jejuni had small internal water volumes; the average content of the cells was 1.73 μl of water mg of protein−1 (Table 1). For all the three strains there was a notable increase in the cell volume in the VBNC state when the values were calculated as microliters of water per milligram of cell protein. The increases in cell volume from the culturable state to the VBNC state were approximately the same for all three strains.
TABLE 1.
Comparison of cell volumes of three strains of C. jejuni in cell suspensions in the late log phase (after 48 h of growth on Columbia agar plates) and in the VBNC state (after 15 and 30 days of incubation in microcosm water)
| C. jejuni strain | Cell vol (μl mg of cell protein−1) ona:
|
||
|---|---|---|---|
| Day 0 (culturable cells) | Day 15 (VBNC cells) | Day 30 (VBNC cells) | |
| Bf | 1.85 ± 0.41 | 5.07 ± 0.72 | 10.60 ± 0.39 |
| 85 | 1.04 ± 0.50 | 5.07 ± 0.16 | 11.24 ± 0.31 |
| 79 | 2.31 ± 0.79 | 3.87 ± 0.25 | 11.06 ± 0.83 |
Triplicate measurements were obtained for three different suspensions of each C. jejuni strain. The values are means ± standard deviations.
Internal pH values of culturable and VBNC C. jejuni cells.
Stationary-phase (48-h-old) C. jejuni cultures transferred from the Columbia culture medium (final pH, 7.3 ± 0.1) to the microcosm water system (pH 6.0 ± 0.1) were able to maintain a positive ΔpH (internal pH − external pH) of 0.6 to 0.9 pH unit during the first day of incubation. The internal pH values of VBNC cell suspensions of each of the three strains (107 VBNC cells ml−1) decreased within 15 days to values very close to the external pH values, and the average ΔpH was approximately 0.15 pH unit. After 30 days in microcosm water, the internal pH was similar to the external pH (pH 5.85) for strains 79 and Bf, and strain 85 was able to maintain a ΔpH of 0.15 pH unit (Table 2).
TABLE 2.
Internal pH changes in suspensions of culturable and VBNC cells of three C. jejuni strains in the microcosm water system
| C. jejuni strain | Internal pH in cell suspension ona:
|
||
|---|---|---|---|
| Day 0 (culturable cells) | Day 15 (VBNC cells) | Day 30 (VBNC cells) | |
| Bf | 6.73 ± 0.10 | 6.30 ± 0.21 | 5.85 ± 0.35 |
| 85 | 6.75 ± 0.19 | 6.13 ± 0.08 | 6.05 ± 0.21 |
| 79 | 6.63 ± 0.18 | 6.00 ± 0.11 | 5.75 ± 0.05 |
Two experiments were performed, and duplicate measurements were obtained with each technique. The values are means ± standard deviations.
Measurement of the membrane potential of culturable and VBNC C. jejuni cell suspensions.
Modifications of the membrane potentials of cells were determined after 1, 15, and 30 days of incubation in microcosm water. VBNC cells had a low membrane potential in the microcosm water system (Table 3). An average membrane potential of 66 ± 14 mV (negative inside) was obtained for the three strains of C. jejuni in the stationary phase. Progressive decreases in the membrane potential were observed with increases in the time of suspension in the microcosm water system (Table 3). The mean membrane potential decreased to −35 mV after 1 day of incubation in the microcosm water system and was at approximately the same level 15 days later. After 30 days in the microcosm water, the membrane potential was near zero.
TABLE 3.
Membrane potentials for three strains of C. jejuni in culturable and VBNC cell suspensions in the microcosm water system
| C. jejuni strain | Membrane potential (mV) ona:
|
|||
|---|---|---|---|---|
| Day 0 (culturable cells)b | Day 1 | Day 15 (VBNC cells) | Day 30 (VBNC cells) | |
| Bf | 54 ± 1 | 35 ± 5 | 31 ± 8 | 5 ± 1 |
| 85 | 66 ± 3 | 23 ± 3 | 34 ± 1 | 14 ± 1 |
| 79 | 79 ± 1 | 47 ± 1 | 50 ± 1 | 2 ± 1 |
Three measurements were obtained. The values are means ± standard deviations.
Measurements were obtained immediately after cells were suspended in the microcosm water system.
Intracellular K+ concentrations in late-log-phase and VBNC cell suspensions of C. jejuni.
The cellular K+ contents of cells in microcosm water were determined immediately after cells were suspended in microcosm water and after 1, 15, and 30 days of incubation in microcosm water. A notable decrease in the internal K+ content of cells was observed during the first day of incubation in microcosm water. A much slower decrease in the cellular K+ content of VBNC cells was observed after 15 and 30 days of incubation (Table 4). During the latter period, cells with high residual potassium contents exhibited much higher potassium losses.
TABLE 4.
Internal potassium contents of three strains of C. jejuni in culturable and VBNC cell suspensions in the microcosm water system
| C. jejuni strain | Internal K+ concn in cells (mmol liter−1) ona:
|
|||
|---|---|---|---|---|
| Day 0 (culturable cells)b | Day 1 | Day 15 (VBNC cells) | Day 30 (VBNC cells) | |
| Bf | 115.0 ± 3.1 | 65.3 ± 1.2 | 21.2 ± 0.7 | 2.3 ± 0.4 |
| 85 | 124.0 ± 2.5 | 65.0 ± 2.8 | 6.3 ± 0.2 | 1.5 ± 0.3 |
| 79 | 170.1 ± 5.2 | 70.5 ± 2.8 | 3.6 ± 0.2 | 1.7 ± 0.5 |
Three measurements were obtained. The values are means ± standard deviations.
Measurements were obtained immediately after cells were suspended in the microcosm water system.
AECs of C. jejuni cell suspensions.
Stationary-phase cells of C. jejuni suspended in the water microcosm system exhibited different adenine nucleotide charges depending on the strain. After suspension in the microcosm water system, strains 85 and 79 had low AECs (0.32 and 0.40, respectively) compared to strain Bf, which had an AEC of 0.66 (Table 5). After 15 days in the microcosm water, the ATP and ADP levels were below the detection threshold of the method used. By contrast, the internal AMP concentrations varied from 0.2 to 0.4 nmol mg of protein−1.
TABLE 5.
AECs of three strains of C. jejuni in culturable and VBNC cell suspensions in the microcosm water system
| C. jejuni strain | Day | Intracellular adenine nucleotide concn (nmol mg of protein−1)
|
AEC | ||
|---|---|---|---|---|---|
| ATP | ADP | AMP | |||
| Bf | 0a | 0.47 ± 0.007 | 0.13 ± 0.003 | 0.21 ± 0.005 | 0.66 |
| 15b | NDc | ND | 0.26 ± 0.006 | ||
| 30b | ND | ND | 0.26 ± 0.005 | ||
| 85 | 0a | 0.19 ± 0.004 | 0.13 ± 0.004 | 0.68 ± 0.015 | 0.32 |
| 15b | ND | ND | 0.29 ± 0.006 | ||
| 30b | ND | ND | 0.39 ± 0.008 | ||
| 79 | 0a | 0.32 ± 0.007 | 0.13 ± 0.003 | 0.51 ± 0.010 | 0.40 |
| 15b | ND | ND | 0.13 ± 0.003 | ||
| 30b | ND | ND | 0.19 ± 0.004 | ||
Culturable cells.
VBNC cells.
ND, below detection level.
DISCUSSION
Our results show that C. jejuni cells suspended in sterile filtered surface water enter a VBNC state. This C. jejuni VBNC state was described first by Rollins and Colwell (48) and then by Saha et al. (51), Stern et al. (57), Hazeleger et al. (22), and Bovill and Mackey (6). Whether a strain enters this VBNC state depends on the strain. In a study of seven C. jejuni strains, Medema et al. (34) found the VBNC state in only one strain. Our strains came from a set of 36 C. jejuni strains of human origin, and only 3 of these 36 strains (the strains used in our study) were able to enter the VBNC state (data not shown). In this study, C. jejuni cells entered the VBNC state in approximately 15 days when they were incubated at 4°C in surface water.
Changes in cell size in oligotrophic environments and in starvation microcosms have been described for different bacteria. Starvation routinely leads to a reduction in the size of the cells and to ultramicrobacteria which are not retained by 0.22-μm-pore-size polycarbonate membrane filters. Alcaligenes, Aeromonas, Vibrio, and Pseudomonas ultramicrocells have been collected from a marine estuary (33). The size of the cells of a psychrophilic marine Vibrio strain was reduced to 1 μm after 2 weeks of starvation (41). Scanning electron microscopy observations demonstrated that C. jejuni 85 VBNC cells were shorter and thicker than C. jejuni 85 culturable cells. This was not the case for the other two strains of C. jejuni; most VBNC cells of strains 79 and Bf were spiral shaped and were very similar to culturable cells in microcosm water, except that they were longer (15). This is consistent with the results of other studies of bacteria which found few differences in size between cells in natural oligotrophic environments and cells grown under eutrophic conditions (36). Other physiological changes in C. jejuni cells in oligotrophic environments are consistent with the results of previous studies of proton motive force, membrane potential, and potassium movement in hypoosmotic medium. Such changes are often described as a strategy to minimize cell maintenance requirements (42). In 1970 Boylen and Ensign (7) described a 30% decrease in the dry weight of Arthrobacter cells after 30 days of starvation, although all of the cells remained viable. This was accompanied by loss of 60% of the cellular RNA and loss of 20% of the proteins during the first few days of starvation. With our C. jejuni suspensions, we observed no protein losses in the VBNC state (day 15 to day 30 in microcosm water). In addition, the VBNC state was accompanied by notable morphological changes that resulted in an increase in the cell water volume. The decrease in the potassium content of C. jejuni cells after 1 day of starvation was much higher than the values routinely obtained after a hypoosmotic shock (14). However, the culturability of cells did not change. Previous studies of Clostridium perfringens demonstrated that loss of more than 70% of the total potassium content did not affect cell viability (19). In previous physiological studies in which a similar method was used to obtain measurements, rapid decreases in the proton motive force were observed in Staphylococcus epidermidis and Streptococcus cremoris, and these decreases were thought to be due to increased numbers of nonviable cells (23, 43). The transmembrane potential (Δψ) decreased rapidly to zero during the first 1 h of lactose starvation in S. cremoris (43). In a similar way, a large proton motive force and Δψ were maintained by Vibrio fluvialis for only 24 h of starvation (56). The authors explained that this was due in part to a more alkaline internal pH (pH 8.5) than intracellular pH of nonstarving bacteria (pH 8.0). The internal pH of C. jejuni starving cells was lower than the pH values measured immediately after cells were suspended in microcosm water, but ΔpH values of 0.2 to 0.3 pH unit were still maintained after 15 days of starvation. These values are much lower than values obtained for starving cells of Thiobacillus acidophilus, for which ΔpH values of 2 to 3 pH units was obtained during 200 h of starvation. As in our cell suspensions, this proton motive force was not detectable after extended periods of starvation (67).
Numerous studies of AECs in microbial cells have revealed AECs ranging from 0.81 to 0.94 in growing or stationary-phase cells (16, 21, 31). Previous studies of Escherichia coli demonstrated that maintenance is still possible at AECs of 0.5 to 0.8 and that there is a loss of viability at AECs below 0.5 (37). In Rhodospirillum rubrum, AECs as low as 0.32 were observed in resting-cell suspensions (52). Our measurements revealed an AEC of approximately 0.5 after 1 day of incubation in microcosm water system, which is in good agreement with previous measurements of AECs of either senescent populations or associations of actively growing and dead or dying cells (21, 27). This finding was confirmed by the rapid decrease in cell culturability during the first week that C. jejuni cells were suspended in microcosm water, although no loss of viability was observed during the first day of incubation in water. The AEC of VBNC cells is no more representative of the real physiological state of cells (38). The average concentration of ATP in growing or resting bacterial cells is 2 mmol liter−1. This corresponds to 0.3 to 16 fg per cell after 24 h of culture (25). After 1 day of incubation in microcosm water, the intracellular ATP level in C. jejuni suspensions was 0.24 mmol liter−1 or approximately 50 fg per cell. A review of AEC values during starvation demonstrated that many bacteria are not able to retain their adenine nucleotide pools during starvation (28). Measurements of ATP concentrations in marine isolates revealed average levels of 0.5 to 6.5 fg of ATP per cell. In starving cells the concentrations were one-fifth these values (20). Starvation experiments performed with Pseudomonas and Arthrobacter suspensions revealed notable decreases in the cellular ATP content, and the average values were as low as 0.1 fg CFU−1 (63); Amy et al. found 0.6 fg of ATP per viable cell after 36 days of starvation in a marine Vibrio sp. (1). Although we could not measure the real level of ATP present in C. jejuni cells, we can discuss the maximum amount of ATP which could have been present in cells based on the method used. This maximum amount corresponds to 6.5 pmol of ATP in C. jejuni VBNC cells at a concentration of 107 cells ml−1 after 30 days of starvation in the microcosm water system. This indicates that in our samples each cell contained less than 0.33 fg of ATP. This value is in good agreement with values determined previously for starving cell suspensions of T. acidophilus (67), which showed that the ATP contents of VBNC cell suspensions progressively decreased to zero.
The levels of the VBNC cells of the three strains of C. jejuni were high in the microcosm water system. Physiological measurements for cells in the VBNC state revealed numerous similarities with previous measurements obtained for starving cell suspensions of members of other genera. Compared to values generally obtained for growing or resting-cell suspensions, the values described above demonstrated that there was a progressive decrease in the ability of cells to maintain internal homeostasis. However, this did not result in cell death after 30 days of incubation in microcosm water, as C. jejuni was still able to revert to a culturable pathogenic state, as demonstrated by experiments performed with a newborn mice model (15).
ACKNOWLEDGMENTS
We are indebted to Véronique Moretto for preparing VBNC suspensions and for scanning electron microscope observations, to Albert Rossero for measuring C. jejuni cell size by flow cytometry, to Annette Ronse and Florence Jugiau for helpful technical support, and to H. Leclerc for valuable discussions.
This research was funded by a grant from Régions Nord-Pas-de-Calais and Pays-de-Loire.
REFERENCES
- 1.Amy P S, Pauling C, Morita R Y. Starvation-survival processes of a marine Vibrio. Appl Environ Microbiol. 1983;45:1041–1048. doi: 10.1128/aem.45.3.1041-1048.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakker E P. Cell K+ and K+ transport systems in prokaryotes. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press; 1993. pp. 205–224. [Google Scholar]
- 3.Bakker E P. Low affinity K+ uptake systems. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press; 1993. pp. 253–276. [Google Scholar]
- 4.Bakker E P, Rottenberg H, Caplan S R. An estimation of the light-induced electrochemical potential difference of protons across the membrane of Halobacterium halobium. Biochim Biophys Acta. 1976;440:557–572. doi: 10.1016/0005-2728(76)90042-6. [DOI] [PubMed] [Google Scholar]
- 5.Booth I R, Mitchell W J, Hamilton W A. Quantitative analysis of proton-linked transport systems. The lactose permease of Escherichia coli. Biochem J. 1979;182:687–696. doi: 10.1042/bj1820687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bovill R A, Mackey B M. Resuscitation of “non-culturable” cells from aged cultures of Campylobacter jejuni. Microbiology. 1997;143:1575–1581. doi: 10.1099/00221287-143-5-1575. [DOI] [PubMed] [Google Scholar]
- 7.Boylen C W, Ensign J C. Long-term starvation-survival of rod and spherical cells of Arthrobacter crystallopoites. J Bacteriol. 1970;103:569–577. doi: 10.1128/jb.103.3.569-577.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappelier J M, Lazaro B, Rossero A, Fernandez-Astorga A, Federighi M. Double staining CTC/DAPI to detect and count viable but nonculturable Campylobacter jejuni cells. Vet Res. 1997;28:547–555. [PubMed] [Google Scholar]
- 9.Cappelier J M, Federighi M. Demonstration of viable but nonculturable state for Campylobacter jejuni. Rev Med Vet. 1998;149:319–326. [Google Scholar]
- 10.Colwell R R, Brayton P R, Grimes D J, Roszak D B, Hug S A, Palmer L M. Viable but non-culturable Vibrio cholerae and related pathogens in the environment: implications for release of genetically engineered microorganisms. Bio/Technology. 1985;3:817–820. [Google Scholar]
- 11.Crescentini G, Stocchi V. Fast reversed-phase high-performance liquid chromatographic determination of nucleotides in red blood cells. J Chromatogr. 1984;290:393–399. doi: 10.1016/s0021-9673(01)93592-7. [DOI] [PubMed] [Google Scholar]
- 12.Dinnbier U, Limpinsel E, Schid R, Bakker E P. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch Microbiol. 1988;150:348–357. doi: 10.1007/BF00408306. [DOI] [PubMed] [Google Scholar]
- 13.Epstein W. Osmoregulation by potassium transport in Escherichia coli. FEMS Microbiol Rev. 1986;39:73–78. [Google Scholar]
- 14.Epstein W, Kim B S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971;108:639–643. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Federighi M, Tholozan J L, Cappelier J M, Tissier J P, Jouve J L. Evidence of non-coccoid viable but non-culturable Campylobacter jejuni cells in microcosm water by direct viable count, CTC-DAPI double staining, and scanning electron microscopy. Food Microbiol. 1998;15:539–550. [Google Scholar]
- 16.Franzen J S, Binkley S B. Comparison of the acid-soluble nucleotides in Escherichia coli at different growth rates. J Biol Chem. 1961;236:515–519. [PubMed] [Google Scholar]
- 17.Futseather C M, Kjeldstad B, Johnsson A. Measurement of the intracellular pH of Propionibacterium acnes: comparison between the fluorescent probe BCECF and 31P-NMR spectroscopy. Can J Microbiol. 1993;39:180–186. doi: 10.1139/m93-025. [DOI] [PubMed] [Google Scholar]
- 18.Graber M L, Dilillo D C, Friedman B L, Pastoriza-Munoz E. Characteristics of fluoroprobes for measuring intracellular pH. Anal Biochem. 1986;156:202–212. doi: 10.1016/0003-2697(86)90174-0. [DOI] [PubMed] [Google Scholar]
- 19.Guerlava, P., S. Nolf, and J. L. Tholozan. 1998. Rapid cooling, moderate heat treatment and nisin addition influence cell homeostasis of Clostridium perfringens type A. Int. J. Food Microbiol. 195–203. [DOI] [PubMed]
- 20.Hamilton R D, Holm-Hansen O. Adenosine triphosphate content of marine bacteria. Limnol Oceanogr. 1967;12:319–324. [Google Scholar]
- 21.Harrison D E F, Maitra P K. Control of respiration and metabolism in growing Klebsiella aerogenes. The role of adenine nucleotides. Biochem J. 1969;112:647–656. doi: 10.1042/bj1120647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hazeleger W, Arkesteil C, Toorop-Bouma A, Beumer R. Detection of coccoid form of Campylobacter jejuni in chicken products with the use of the polymerase chain reaction. Food Microbiol. 1994;24:273–281. doi: 10.1016/0168-1605(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 23.Horan N J, Midgley M, Dawes E A. Effects of starvation on transport, membrane potential and survival of Staphylococcus epidermidis under anaerobic conditions. J Gen Microbiol. 1981;127:223–230. doi: 10.1099/00221287-127-2-223. [DOI] [PubMed] [Google Scholar]
- 24.Hussong D, Colwell R R, O’Brien M, Weiss E, Pearson A D, Weiner R M, Burge W D. Viable Legionella pneumophilla not detected by culture on agar media. Bio/Technology. 1987;5:947–950. [Google Scholar]
- 25.Jakubczak E, Leclerc H. Mesure de l’ATP bactérien par bioluminescence: étude critique des méthodes d’extraction. Ann Biol Clin. 1980;38:297–304. [PubMed] [Google Scholar]
- 26.Jones D M, Sutcliffe E M, Curry A. Recovery of viable but non-culturable Campylobacter jejuni. J Gen Microbiol. 1991;137:2477–2482. doi: 10.1099/00221287-137-10-2477. [DOI] [PubMed] [Google Scholar]
- 27.Karl D M. Cellular nucleotide measurements and application to the microbial loop. Microbiol Rev. 1980;44:739–796. doi: 10.1128/mr.44.4.739-796.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowles C J. Microbial metabolic regulation by adenine nucleotide pools. Symp Soc Gen Microbiol. 1977;27:241–283. [Google Scholar]
- 29.Kobs M. A procedure for preparing microscopic organisms for SEM. Eur Microsc Anal. 1995;35:25. [Google Scholar]
- 30.Kogure K, Sindu V, Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979;25:415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- 31.Liao C L, Atkinson D E. Regulation at the phosphoenolpyruvate branchpoint in Azotobacter vinelandii: pyruvate kinase. J Bacteriol. 1971;106:37–44. doi: 10.1128/jb.106.1.37-44.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;163:265–275. [PubMed] [Google Scholar]
- 33.MacDonnell M T, Hood M A. Isolation and characterization of ultramicrobacteria from a Gulf Coast estuary. Appl Environ Microbiol. 1982;43:566–571. doi: 10.1128/aem.43.3.566-571.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medema G J, Schets F M, van de Giessen A W, Havelaar A H. Lack of colonization of 1-day-old chicks by viable, non-culturable Campylobacter jejuni. J Appl Bacteriol. 1992;72:512–516. doi: 10.1111/j.1365-2672.1992.tb01868.x. [DOI] [PubMed] [Google Scholar]
- 35.Miguelez E, Gilmour D J. Regulation of cell volume in the salt tolerant bacterium Halomonas elongata. Lett Appl Bacteriol. 1994;19:363–365. [Google Scholar]
- 36.Moriarty D J W, Bell R T. Bacterial growth and starvation in aquatic environments. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 25–53. [Google Scholar]
- 37.Morita R Y. Estimating nutrient bioavailability for microbes. In: Reddy C A, Chakrabarty A M, Demain A L, Tiedje J M, editors. Bacteria in oligotrophic environments. New York, N.Y: Chapman and Hall; 1997. pp. 135–192. [Google Scholar]
- 38.Morita R Y. Physiology of starvation-survival. In: Reddy C A, Chakrabarty A M, Demain A L, Tiedje J M, editors. Bacteria in oligotrophic environments. New York, N.Y: Chapman and Hall; 1997. pp. 274–350. [Google Scholar]
- 39.Noel J, Tejedor A, Vinay P, Laprade R. Fluorescence measurements of intracellular pH on proximal tubule suspensions: the need for a BCECF sink. Renal Physiol Biochem. 1989;12:371–387. doi: 10.1159/000173215. [DOI] [PubMed] [Google Scholar]
- 40.Notermans S, Hoogenboom-Verdegaal H. Existing and emerging foodborne diseases. Int J Food Microbiol. 1992;15:197–205. doi: 10.1016/0168-1605(92)90049-9. [DOI] [PubMed] [Google Scholar]
- 41.Novitsky J A, Morita R Y. Morphological characterization of small cells resulting from nutrient starvation in a psychrophilic marine Vibrio. Appl Environ Microbiol. 1976;32:619–622. doi: 10.1128/aem.32.4.617-622.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oliver J D. Formation of viable but nonculturable cells. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 239–272. [Google Scholar]
- 43.Otto R, Vije J, ten Brink B, Klont B, Konings W N. Energy metabolism in Streptococcus cremoris during lactose starvation. Arch Microbiol. 1985;141:348–352. [Google Scholar]
- 44.Rahman I, Shahamat M, Kirchman P A, Russek-Cohen E, Colwell R R. Methionine uptake and cytopathogenicity of viable but nonculturable Shigella dysenteriae type 1. Appl Environ Microbiol. 1994;60:3573–3578. doi: 10.1128/aem.60.10.3573-3578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ravel J, Knight I T, Monohan C E, Hill R T, Colwell R R. Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: growth or resuscitation? Microbiology. 1995;141:377–383. doi: 10.1099/13500872-141-2-377. [DOI] [PubMed] [Google Scholar]
- 46.Richey B, Cayley D S, Mossing M C, Kolka C, Anderson C F, Farrar T C, Record M T., Jr Variability of the intracellular ionic environment of Escherichia coli. Differences between in vitro and in vivo effects of ion concentration on protein-DNA interactions and gene expression. J Biol Chem. 1987;262:7157–7164. [PubMed] [Google Scholar]
- 47.Rodriguez G G, Philipps D, Ishigmo K, Ridgway H F. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992;58:1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rollins D M, Colwell R R. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in natural, aquatic environment. Appl Environ Microbiol. 1986;58:531–538. doi: 10.1128/aem.52.3.531-538.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roszak D B, Grimes D J, Colwell R R. Viable but non-recoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol. 1984;30:334–338. doi: 10.1139/m84-049. [DOI] [PubMed] [Google Scholar]
- 50.Rottenberg H. The measurement of membrane potential and ΔpH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- 51.Saha S K, Saha S, Sanyal S C. Recovery of injured Campylobacter jejuni cells after animal passage. Appl Environ Microbiol. 1991;57:3388–3389. doi: 10.1128/aem.57.11.3388-3389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schön G. Der Einfluss der Kulturbedingungen auf den ATP-, ADP-, and AMP-Spiegel bei Rhodospirillum rubrum. Arch Mikrobiol. 1969;66:348–364. [PubMed] [Google Scholar]
- 53.Schönheit P, Perski H J. ATP synthesis driven by a potassium diffusion potential in Methanobacterium thermoautotrophicum is stimulated by sodium. FEMS Microbiol Lett. 1983;15:69–74. [Google Scholar]
- 54.Severin E, Stellmach J, Nachtigal H M. Fluorimetric assay of redox activity in cells. Anal Chim Acta. 1985;170:341–346. [Google Scholar]
- 55.Skirrow M B. Foodborne illness: Campylobacter. Lancet. 1990;336:921–923. doi: 10.1016/0140-6736(90)92282-m. [DOI] [PubMed] [Google Scholar]
- 56.Smigielski A J, Wallace B J, Marshall K C. Changes in membrane functions during short-term starvation of Vibrio fluvialis strain NCTC 11328. Arch Microbiol. 1989;151:336–347. [Google Scholar]
- 57.Stern N J, Jones D M, Wesley I V, Rollins D M. Colonization of chicks by nonculturable Campylobacter jejuni spp. Lett Appl Microbiol. 1994;18:333–336. [Google Scholar]
- 58.Taylor D N. Campylobacter infections in developing countries. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni. Current topics and future trends. Washington, D.C: American Society for Microbiology; 1992. pp. 20–30. [Google Scholar]
- 59.Tsapis A, Kepes A. Transient breakdown of the permeability barrier of the membrane of Escherichia coli upon hypoosmotic shock. Biochim Biophys Acta. 1977;469:1–12. doi: 10.1016/0005-2736(77)90320-0. [DOI] [PubMed] [Google Scholar]
- 60.Tsujimoto K, Semadeni M, Huflejt M, Packer L. Intracellular pH of halobacteria can be determined by the fluorescent dye 2′,7′-bis(carboxyethyl)-5(6)-carboxyfluorescein. Biochem Biophys Res Commun. 1988;155:123–129. doi: 10.1016/s0006-291x(88)81058-1. [DOI] [PubMed] [Google Scholar]
- 61.van Elsas J D, van Overbeek L S. Bacterial response to soil stimuli. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 55–79. [Google Scholar]
- 62.Walker-Simmons M, Atkinson D E. Functional capacities and the adenylate energy charge in Escherichia coli under conditions at nutritional stress. J Bacteriol. 1977;130:376–683. doi: 10.1128/jb.130.2.676-683.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webster J J, Hampton G J, Wilson J T, Ghiorse W C, Leach F R. Determination of microbial cell numbers in subsurface samples. Groundwater. 1985;23:17–25. [Google Scholar]
- 64.Xu H S, Roberts N, Singleton F L, Attwell R W, Grimes D J, Colwell R R. Survival and viability of non-culturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol. 1982;8:313–323. doi: 10.1007/BF02010671. [DOI] [PubMed] [Google Scholar]
- 65.Yamasaki K, Moriyama Y, Futai M, Tsuchiya T. Uptake and extrusion of K+ regulated by extracellular pH in Escherichia coli. FEBS Lett. 1980;120:125–127. doi: 10.1016/0014-5793(80)81061-1. [DOI] [PubMed] [Google Scholar]
- 66.Zimmerman R, Iturriaga R, Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978;36:926–939. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zychinsky E, Matin A. Effect of starvation on cytoplasmic pH, proton motive force, and viability of an acidophilic bacterium, Thiobacillus acidophilus. J Bacteriol. 1983;153:371–374. doi: 10.1128/jb.153.1.371-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]