Abstract
Background
COVID‐19 vaccinations in the United States are effective in preventing illness and hospitalization yet concern over post‐vaccination venous thromboembolism (VTE) risk has led to vaccine hesitancy.
Methods
The aim of this study was to compare VTE rates before and after COVID‐19 vaccination. COVID‐19 vaccinated patients ≥18 years between November 1, 2020 through November 1, 2021 were analyzed using electronic medical records across the Mayo Clinic enterprise. The primary outcome was imaging confirmed acute VTE (upper or lower deep vein thrombosis or pulmonary embolism) occurring 90 days before and after the date of first vaccine dose.
Results
A total of 792 010 patients with at least one COVID‐19 vaccination were identified (Pfizer, n = 452 950, Moderna, n = 290 607, and Janssen [Johnson & Johnson], n = 48 453). A total of 1565 VTE events occurred in the 90 days before (n = 772) and after (n = 793) COVID‐19 vaccination. VTE post‐vaccination occurred in 326 patients receiving Moderna (0.11%, incidence rate [IR] 4.58 per 1000p‐years), 425 patients receiving Pfizer (0.09%, IR 3.84 per 1000p‐years), and 42 receiving Janssen (0.09%, IR 3.56 per 1000p‐years). Compared to the pre‐vaccination timeframe, the adjusted hazard ratio (aHR) for VTE after the Janssen vaccination was 0.97 (95% confidence interval [CI] 0.63–1.50), aHR 1.02 (95% CI 0.87–1.19) for Moderna, and aHR 1.00 (95% CI 0.87–1.15) for Pfizer.
Conclusion
In this large cohort of COVID‐19 vaccinated patients, no increased risk for acute VTE post‐vaccination was identified for the authorized vaccines in the United States.
Keywords: COVID‐19, deep vein thrombosis, immunization, pulmonary embolism, SARS‐CoV‐2, vaccination, venous thromboembolism
Essentials
-
1.
COVID‐19 vaccine‐related venous thromboembolism (VTE) has led to vaccine hesitancy.
-
2.
VTE events before and after vaccination with Janssen, Pfizer, and Moderna were analyzed.
-
3.
No increased risk of acute VTE was observed in the 90 days post‐vaccination.
-
4.
COVID‐19 vaccination should not be withheld or delayed due to concerns for VTE.
Alt-text: Unlabelled Box
1. INTRODUCTION
The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, also known as COVID‐19, led to a race to develop effective vaccines. The United States has three authorized vaccines (BNT162b2—Pfizer‐BioNTech, mRNA‐1273—Moderna, and Ad. 26. COV2. S—Janssen/Johnson & Johnson) and these have been administered to millions of individuals. Vaccine safety beyond the initial clinical trials has been a major area of focus to maintain public trust and encourage high rates of vaccination. COVID‐19 infection itself is known to significantly increase short‐term venous thromboembolism (VTE)1 risk. In early 2021 reports of a rare thrombotic complication (vaccine‐induced immune thrombotic thrombocytopenia [VITT]2) were observed with the ChAdOx1 nCov‐19 (AstraZeneca) vaccine,3., 4., 5. which ultimately led many European nations to temporarily pause administration of this vaccine in March of 2021 for further review. A similar phenomenon was later recognized with the Janssen vaccine leading to a temporary pause in the administration of this vaccine in the United States in April 2021.6 While many of the thrombotic events described initially were in less typical locations (such as cerebral vein thrombosis and mesenteric vein thrombosis), speculation began to grow about the thrombotic risk for typical leg thrombosis and pulmonary embolism and in relation to other COVID‐19 vaccines.7
In this study, we evaluated the safety of COVID‐19 vaccinations related to VTE outcomes (deep vein thrombosis and pulmonary emboli) among the three Food and Drug Administration–authorized COVID‐19 vaccines in the United States using real‐world observational data, comparing the incidence of VTE between different vaccine manufacturers and within the 90 days before and after vaccination.
2. METHODS
2.1. Study population
Mayo Clinic enterprise patients at least 18 years or older were identified using an electronic search of a unified data platform of COVID‐19 vaccination records from November 1, 2020 through November 1, 2021. Mayo Clinic enterprise consists of numerous outpatient and inpatient locations utilizing a common medical record system, including three tertiary referral centers (Rochester, Minnesota; Phoenix, Arizona; and Jacksonville, Florida) and many regional Mayo Clinic Health System sites in Minnesota, Wisconsin, and Iowa. The date of the first COVID‐19 vaccination was used as the index date. This study was approved by the Mayo Clinic Institutional Review Board in Rochester, Minnesota. For requests of original data, please contact the corresponding author.
2.2. Data collection
Demographic variables including age at COVID‐19 vaccination, sex, race, and zip code were extracted from electronic records. Using the cohort of COVID‐19–vaccinated patients, electronic medical records were searched to identify all hospital admissions (elective or urgent/emergent) and emergency department (ED) visits, surgeries (requiring general or regional anesthesia) within 90 days before or after the index date, and pregnancy status and delivery dates. Laboratory records were searched to identify cases of laboratory‐confirmed COVID‐19 infection and testing for platelet factor 4 antibodies. International Classification of Diseases (ICD) 10 codes were used to evaluate for baseline comorbidities and to calculate a Charlson Comorbidity Index (CCI). Radiology text reports within 90 days before and after the index vaccination date were extracted (computed tomography [CT] scans of the chest and all venous Duplex ultrasounds of the upper or lower extremity).
2.3. Outcomes and analysis
Thromboembolic events were extracted from the electronic medical record in the 90 days before and after the index date through December 9, 2021. VTE was defined as upper or lower extremity deep vein thrombosis (DVT; proximal and distal) and pulmonary embolism (PE) and were analyzed from radiology text reports using highly accurate, previously validated natural language processing (NLP) algorithms.1., 8. VTE events within the 90 days after vaccination were compared to events occurring in the 90‐day window before vaccination using reversed person‐time analysis and the Kaplan‐Meier method. For patients with DVT and PE, the first event in each timeframe in closest proximity to the index date was used for the time to event analysis. Patients were censored at death or time of data extraction if follow‐up time had not reached 90 days. Cox proportional hazard models were used to compare rates of VTE in unadjusted and adjusted analyses. The rates of post‐vaccination thromboembolism were compared between vaccines using the Kaplan‐Meier method and multivariable Cox proportional hazard models adjusting for the CCI, age, sex, race, history of VTE or arterial thromboembolism, and history of atrial fibrillation. Sensitivity analyses were performed based on sex, age, geographic region, and among patients paneled to a Mayo Clinic primary care provider during the study timeframe. Statistical analyses were performed using JMP Pro version 14.1.
3. RESULTS
A total of 792 010 patients with at least one COVID‐19 vaccination were identified from November 1, 2020, to November 1, 2021. The most frequent vaccine administered was Pfizer (n = 452 950) followed by Moderna (n = 290 607) and Janssen (Johnson & Johnson; n = 48 453). The mean age of the overall cohort was 57 years (standard deviation 18.3) and 55% were female. A total of 1565 VTE events were identified by the NLP in the 90 days before and after COVID‐19 vaccination, 772 before and 793 after vaccination. Among the 793 patients with VTE after COVID‐19 vaccination, 489 had DVT (upper or lower extremity) and 426 had PE. The overall incidence rates (IR) of VTE were low, 4.1 per 1000 person‐years (p‐yrs) post‐vaccination compared to 4.0 per 1000p‐yrs pre‐vaccination. Patients with hospital admissions (elective or urgent/emergent) and/or ED visits occurred more frequently in the 90 days after vaccination (7.0% vs. 6.54%, P < .001). COVID‐19 infections occurred more commonly in the pre‐vaccination 90‐days (1.09% vs. 0.39%, P < .001). No difference was seen in the number of surgeries pre‐ versus post‐vaccination (2.38% vs. 2.40%, P = .28).
3.1. Pre/post‐vaccination thromboembolism
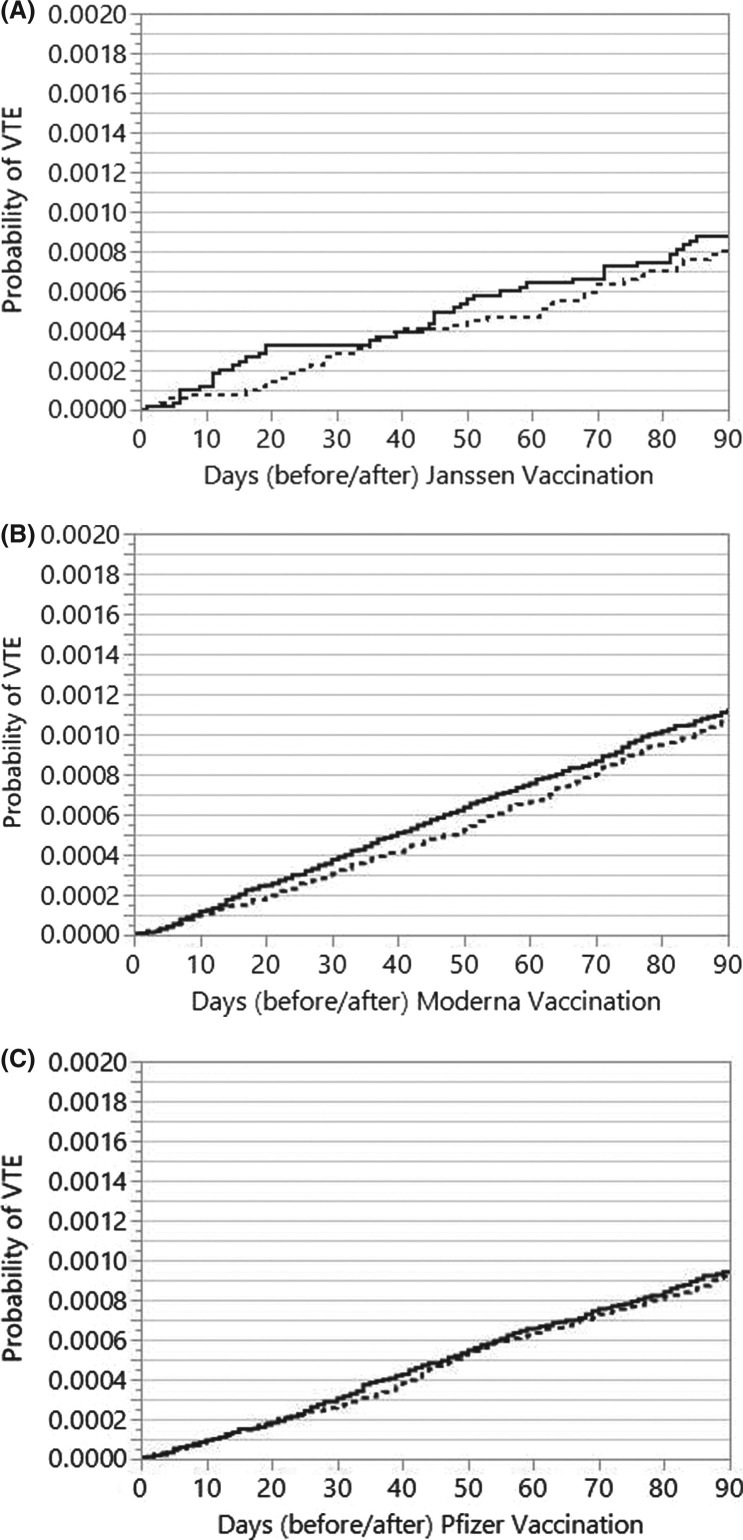
The time to VTE curves in the post‐ versus pre‐vaccination timeframes for each COVID‐19 vaccine are shown in Figure 1 . The unadjusted hazard ratio (HR) for VTE overall post‐vaccination was 1.04 (95% confidence interval [CI] 0.94–1.14). After the Janssen vaccination, the HR for VTE was 1.09 (95% CI 0.70–1.68), HR 1.05 (95% CI 0.90–1.23) for Moderna, and HR 1.02 (95% CI 0.89–1.17) for Pfizer. After multivariable adjustment for surgeries, hospitalizations, and COVID‐19 infections within 90 days, the adjusted HR (aHR) for VTE after the Janssen vaccination was 0.97 (95% CI 0.63–1.50), aHR 1.02 (95% CI 0.87–1.19) for Moderna, and aHR 1.00 (95% CI 0.87–1.15) for Pfizer. Sensitivity analysis performed in patients paneled to primary care providers (aHR 1.04, 95% CI 0.80–1.28) was similar to non‐paneled patients (aHR 0.94, 95% CI 0.79–1.12). Results were also similar for patients living in counties of the Rochester Epidemiology Project (aHR 1.08, 95% CI 0.94–1.24) or elsewhere (aHR 0.92, 95% CI 0.80–1.07). A sensitivity analysis was also performed excluding any patients with documented COVID‐19 infection and the results did not change significantly (Janssen: aHR 0.98, 95% CI 0.60–1.58; Moderna: aHR 0.91, 95% CI 0.77–1.07; Pfizer aHR 0.99, 95% CI 0.86–1.15).
FIGURE 1.
Crude rates of VTE before and after (A) Janssen (J&J), (B) Moderna, and (C) Pfizer vaccinations. VTE, venous thromboembolism. Dashed line is 90 days before vaccination and solid line is 90 days after vaccination
Due to the concern for VITT in younger women,9 the results were also stratified by sex and age (+/−60 years) and by vaccine manufacturer and there was no statistically significantly elevated risk of VTE within any of these subgroups with Janssen or Moderna vaccines. We did however observe an elevated post‐vaccination VTE risk in women less than 60 with Pfizer (aHR 1.53, 95% CI 1.07–2.19) whereas men less than 60 had a lower risk of VTE (aHR 0.70, 95% CI 0.50–0.97). On further evaluation of age in quartiles, a statistically significant risk in women was only present in the lowest age quartile (age 18–43) with the Pfizer vaccine, aHR 2.19 (95% CI 1.17–4.07). The incidence rates for VTE in young women in the pre‐ versus post‐vaccination 90 days were 1.07 and 1.77 per 1000 p‐yrs respectively. We further investigated this finding by identifying women who were pregnant in the study timeframe and found there was an increased number of vaccinations around the time of delivery and women with pregnancies were much more likely to receive vaccination with the Pfizer vaccine (Table 1 ). After removing these patients from the analysis, the results were no longer statistically significant (aHR 1.82, 95% CI 0.95–3.49). Within the overall group of women with current or recent pregnancies (any vaccine), the aHR for post‐vaccination VTE was 6.18 (95% CI 1.26–30.2). The lower overall sample size and event rates did not allow for further analyses such as stratification based on the trimester of pregnancy.
TABLE 1.
Baseline characteristics of COVID‐19 vaccinated patients
| Janssen (J&J) |
Moderna |
Pfizer |
P value | |
|---|---|---|---|---|
| N = 48 453 | N = 290 607 | N = 452 950 | ||
| Age, mean (SD) | 51.6 (16.4) | 59.2 (17.7) | 55.8 (18.6) | <.0001 |
| Male, n (%) | 24 852 (51.3) | 131 586 (45.3) | 199 063 (44.0) | <.0001 |
| Race, White, n (%) | 41 749 (86.2) | 255 140 (87.8) | 391 052 (86.3) | <.0001 |
| Two doses administered, n (%) | NA | 270 492 (93.1) | 428 313 (94.6) | |
| Interval between doses (days), median (IQR) | NA | 28 (28–28) | 21 (21–22) | |
| Charlson Comorbidity Index, mean (SD) | 1.11 (2.0) | 1.45 (2.3) | 1.33 (2.2) | |
| Dementia, n (%) | 751 (1.6) | 9557 (3.3) | 10 675 (2.4) | <.0001 |
| Diabetes | 4767 (9.8) | 36 679 (12.6) | 49 655 (11.0) | <.0001 |
| Diabetes complications, n (%) | 1433 (3.0) | 11 665 (4.01) | 16 085 (3.6) | <.0001 |
| Cancer, n (%) | 4643 (9.6) | 42 640 (14.7) | 59 916 (13.2) | <.0001 |
| Cancer w/ metastasis, n (%) | 639 (1.2) | 5271 (1.8) | 7251 (1.6) | <.0001 |
| Connective tissue disease, n (%) | 1815 (3.8) | 14 712 (5.1) | 21 212 (4.7) | <.0001 |
| Cerebrovascular accident, n (%) | 1166 (2.4) | 10 834 (3.7) | 14 587 (3.2) | <.0001 |
| Congestive heart failure, n (%) | 1669 (3.4) | 16 511 (5.7) | 21 584 (4.8) | <.0001 |
| HIV, n (%) | 1632 (3.4) | 8494 (2.9) | 15 085 (3.3) | <.0001 |
| Liver disease, n (%) | 3031 (6.3) | 20 939 (7.2) | 29 856 (6.6) | <.0001 |
| Liver disease, severe, n (%) | 329 (0.68) | 2957 (1.02) | 6246 (0.72) | <.0001 |
| Myocardial infarction, n (%) | 699 (1.4) | 5465 (1.88) | 7439 (1.64) | <.0001 |
| Paraplegia, n (%) | 278 (0.57) | 2284 (0.79) | 2750 (0.61) | <.0001 |
| Peptic ulcer disease, n (%) | 736 (1.5) | 5505 (1.9) | 8069 (1.8) | <.0001 |
| Peripheral vascular disease, n (%) | 1494 (3.1) | 13 371 (4.6) | 18 709 (4.1) | <.0001 |
| Pulmonary disease, n (%) | 6550 (13.5) | 43 521 (15.0) | 66 389 (14.7) | <.0001 |
| Renal disease, n (%) | 2879 (5.9) | 27 962 (9.6) | 37 618 (8.31) | <.0001 |
| Active or recent pregnancy, n (%) | 270 (0.56) | 1440 (0.50) | 4601 (1.02) | <.0001 |
| Ischemic stroke | 1006 (2.1) | 9405 (3.2) | 12 690 (2.8) | <.0001 |
| Atrial fibrillation | 1926 (4.0) | 20 082 (6.9) | 27 871 (6.2) | <.0001 |
| Venous thromboembolism | 1230 (2.5) | 10 005 (3.4) | 14 061 (3.1) | <.0001 |
| Heparin induced thrombocytopenia | 14 (0.03) | 169 (0.06) | 202 (0.04) | .005 |
| Hypertension | 1700 (3.5) | 10 076 (3.5) | 15 557 (3.4) | .59 |
| COVID‐19 infection (lab or ICD) | 3201 (6.6) | 12 453 (4.3) | 22 553 (5.0) | <.0001 |
| Arterial embolism | 162 (0.33) | 1300 (0.45) | 1785 (0.39) | <.0001 |
| REP County | 22 551 (46.6) | 113 653 (39.1) | 208 145 (46.0) | <.0001 |
| Mayo Clinic PCP | 25 852 (53.4) | 147 447 (50.7) | 256 320 (56.6) | <.0001 |
Abbreviations: ICD, International Classification of Diseases; HIV, human immunodeficiency virus; IQR, interquartile range; PCP, primary care physician; REP, Rochester Epidemiology Project; SD, standard deviation.
Concern about VTE risk post‐vaccination has been most pronounced in specific patient populations (those with prior VTE, previous COVID‐19 infection, and those with a history of heparin‐induced thrombocytopenia [HIT]) and therefore we performed additional pre/post analyses within these subgroups. In 25 296 patients with a history of VTE (any time before vaccination), there was a lower risk in the post‐vaccination compared to the pre‐vaccination timeframe (aHR 0.77, 95% CI 0.62–0.95). In 37 838 patients with COVID‐19 infection (ICD‐10 or laboratory confirmation) preceding the study timeframe (>90 days before index date) there was a higher risk of VTE in the pre‐vaccination 90 days compared to the post‐vaccination 90 days (aHR 1.67, 95% CI 1.15–2.41). In 385 patients with a history of HIT, there was no significant elevation in the post‐vaccination VTE risk (log‐rank P = .26); eight patients had VTE in the pre‐vaccination 90 days and four had VTE in the post‐vaccination 90 days. The results were also stratified by comorbidities and VTE rates were expectedly higher in patients with CCI > 1 (7.89 per 1000p‐yrs) compared to those with CCI = 0 (1.00 per 1000p‐yrs). However, comparing post‐vaccination versus pre‐vaccination rates within each group there was no evidence of increased post‐vaccination VTE risk (log‐rank P = .69 and P = .35 for those with CCI = 0 or CCI > 1, respectively). Among cancer patients, the rate of VTE was highest (IR 13.3 per 1000p‐yrs); however, again no increased risk was observed post‐vaccination compared to pre‐vaccination (log‐rank P = .35).
3.2. Post‐vaccination thromboembolism compared by vaccine manufacturer
Age, sex, and comorbidities were significantly different among patients receiving different vaccines (Table 1). Most patients receiving Pfizer (94.6%) or Moderna (93.1%) vaccines completed the recommended two doses at the appropriate time intervals within the study dates. The group of patients receiving Moderna vaccines had the highest mean CCI (1.45), followed by Pfizer (1.33) and then Janssen (1.11). The frequency of VTE post‐vaccination varied by vaccine type. VTE occurred in 326 patients receiving Moderna (0.11%, IR 4.58 per 1000p‐yrs), 425 patients receiving Pfizer (0.09%, IR 3.84 per 1000p‐yrs), and 42 receiving Janssen (0.09%, IR 3.56 per 1000p‐yrs). In multivariable Cox proportional hazard models (age, sex, race, CCI, atrial fibrillation, prior arterial or venous thromboembolism, surgeries within 90 days, admissions/ED visits within 90 days, and COVID‐19 infections within 90 days) there was no difference in the post‐vaccination VTE risk within 90 days comparing Pfizer to Moderna (aHR 0.88, 95% CI 0.76–1.02) and Janssen to Moderna (aHR 0.85, 95% CI 0.62–1.18). To further examine the previous finding of higher post‐vaccination (compared to pre‐vaccination) VTE in younger women receiving Pfizer, cross‐product terms for the variables sex, age, and vaccine were added to the model (examining only the post‐vaccination timeframe) to evaluate for effect measure modification and P values were all >0.1. In a separate multivariable Cox proportional hazard model specifically examining the effects of young women vaccinated with Pfizer there was no elevation in VTE risk (aHR 1.14, 95% CI 0.75–1.73) within the post‐vaccination 90 days after adjusting for all other variables.
Laboratory testing for heparin‐induced thrombocytopenia (platelet factor 4 [PF4] antibody) was performed in 149 patients in the 90 days after vaccination (n = 20, Janssen; n = 65, Moderna; n = 64, Pfizer). Positive testing by enzyme‐linked immunosorbent assay (ELISA) for HIT (optical density >0.4) was identified in nine patients (n = 4, Moderna; n = 5, Pfizer). Five patients had evidence of VTE (with recent heparin exposure), three were diagnosed with VTE concurrently with PF4 testing, and two with initial VTE had subsequent testing (day 11 and day 13). The timing of PF4 testing from initial vaccination ranged from day 38 to day 80 post‐vaccination. All patients with VTE had clear provoking factors and none were suspected to have VITT. Among patients without VTE, two had a known history of HIT (pre‐vaccination) and the other two were presumed HIT (with recent heparin exposure) without associated thrombosis.
4. DISCUSSION
This study represents the largest observational cohort to specifically evaluate VTE outcomes in the authorized COVID‐19 vaccines in the United States. Post‐vaccination thrombosis has led patients to question the safety of vaccination and has led to vaccine hesitancy and/or avoidance. Our results comparing post‐vaccination to pre‐vaccination rates demonstrate no significant elevation in VTE risk with COVID‐19 vaccinations overall and specifically by each vaccine manufacturer. A comparison of risk between the vaccines also demonstrates no significant differences in VTE rates after multivariable adjustment.
Initial evaluations of thrombotic events post‐vaccination were limited by smaller sample sizes.10 Subsequent data from the Vaccine Safety Datalink studied more than 6 million Pfizer and Moderna vaccine recipients evaluating a variety of possible post‐vaccination adverse events and found no statistically significant increased risk for VTE.11 This study compared a day 1–21 post‐vaccination (first or second vaccine) interval to a day 22–42 interval (after the most recent dose) to compare rates of adverse events. For the outcome of VTE, the rate was 0.95 in the at‐risk interval compared to 0.90 per 1000p‐yrs for an adjusted HR of 1.16 (95% CI 1.00–1.34). This result was not statistically significant due to adjustment of significance level in the setting of multiple hypothesis testing.
As it relates to the outcome of VTE and vaccination it is less clear whether this might be an immediate or a more delayed adverse event (either due to delayed recognition and diagnosis or delayed thrombotic risk). Therefore, the current study evaluated a period of 90 days post‐vaccination to capture more delayed adverse events and used time‐to‐event curves to visualize event rates over this time. The rate of VTE in the post‐vaccination 90 days in the current study was 4.1 per 1000 person‐years, representing an approximately 4‐fold higher risk than the at‐risk interval in the Vaccine Safety Datalink study. The overall higher risk in the current study can in part be explained by the higher mean patient age (57 vs. 49 years). Patients in the current study may have also had a higher number of comorbidities due to their interaction with the medical system; however, this is uncertain because the Vaccine Safety Datalink data did not have this baseline information. The safety of the Pfizer vaccine in data reported from Israel has similarly demonstrated the safety of the vaccine12 in a national cohort of 884 828 citizens compared to a matched unvaccinated control population. The rates of DVT and PE were reported separately in this study and were lower than our study and the Vaccine Safety Datalink study, likely the result of a much younger vaccinated population (median age 38). The risk within 42 days for DVT (HR 0.87, 95% CI 0.55–1.40) and PE (HR 0.56, 95% CI 0.21–1.15) compared to the control population was not significantly different. This study did not specifically examine risk within various subgroups by age or sex.
The finding of a minor elevation in risk within a subgroup of young women receiving the Pfizer vaccine in our study appears to be the result of the increased risk associated with pregnancy and the postpartum state, as removal of these patients from the analysis eliminated significant differences in pre‐ versus post‐vaccination risk in the cohort of younger women. Sultan et al.13 reported an incident rate ratio of 6.1 (95% CI 4.7–7.9) for VTE in pregnant women in the third trimester and a 22‐fold increased risk in the first 6 weeks postpartum. Limited data from other studies have demonstrated the safety of vaccination in pregnant women14., 15. but have not specifically evaluated VTE outcomes. Pregnant women were more likely to receive the Pfizer vaccine making our findings most pronounced within this group. When examining the risk for VTE in pregnant women only in the post‐vaccination timeframe, after multivariable adjustment we found no increased risk specifically within this group, therefore we do not believe that the finding from the pre/post analysis suggests a higher risk of vaccination among pregnant women. Instead, our findings within this pregnant population represent a limitation of the pre/post comparison method employed as later stages of pregnancy and early postpartum (enriched by study design in the post‐vaccination 90 days) have a higher VTE incidence than earlier stages of pregnancy.
Current guidance from the Centers for Disease Control and Prevention recommend mRNA‐based COVID‐19 vaccines over the Janssen vaccine under most circumstances.16 They further recommend avoidance of the Janssen vaccine in patients with a history of HIT and in anyone with history of VITT. Compared to previously published studies, the current study additionally evaluated VTE outcomes with the Janssen vaccine. Concerns regarding thrombotic risk even in the absence of frank thrombocytopenia with this vaccine escalated after rare reports of VITT.6., 17., 18. Our results demonstrate no significant elevation in VTE risk with the Janssen vaccine in the post‐vaccination 90 days compared to the pre‐vaccination 90 days. The sample size of this cohort of vaccinated patients was smaller, which limits the evaluation of an outcome as rare as VITT; however, we believe that our analysis provides additional reassurance that the Janssen vaccine does not induce a generalized hypercoagulable state and that VITT is not the “tip of the iceberg.”
Our analysis is limited by its observational method and possible loss of outcome ascertainment in the pre‐ or post‐vaccination windows. To evaluate the possible influence of inadequate event ascertainment, sensitivity analyses were performed by geographic regions and among patients paneled to a primary care provider for the entire study duration and demonstrated similar results. The primary outcome of VTE in this study was assessed using a highly accurate NLP algorithm that evaluates upper and lower extremity Duplex ultrasound reports for DVT and CT scans of the chest for acute or newly identified thrombotic events. Atypical thrombotic events such as cerebral vein thrombosis or portal/mesenteric would not be identified by these algorithms because they were designed and validated only for extremity‐related DVT (not intracranial or intra‐abdominal) and PE. This study only included patients with clinically confirmed COVID‐19 vaccination and did not rely on a control population in which vaccination status may be uncertain.
Our data confirm the results of other published observational studies using detailed information from electronic medical records and a different study design. COVID‐19 vaccination does not lead to an appreciably increased risk for acute VTE post‐vaccination overall or individually for any of the authorized vaccines available in the United States. Additionally, no significant differences were seen in the risk for acute VTE post‐vaccination when comparing each vaccine to each other. COVID‐19 vaccination should not be withheld or delayed due to concerns for VTE.
CONFLICTS OF INTEREST
Dr. Anand Padmanabhan reports financial relationships with: equity ownership (Retham Technologies), advisory board (Veralox Therapeutics), patents/royalty (Mayo Clinic, Versiti, Retham Technologies). Dr. Meera Sridharan has received consultant fees as part of an advisory board for Alexion Pharmaceutical. The other authors report no conflicts of interest. This paper is not under consideration elsewhere. Presented in abstract form at the American Society of Hematology annual meeting, Atlanta, GA December 2021; Blood (2021) 138 (Supplement 1): 291. https://doi.org/10.1182/blood‐2021‐151396
AUTHOR CONTRIBUTIONS
All authors were involved in the conception and design or analysis and interpretation of the data, drafting of the manuscript or revising it critically, and read and approved the final manuscript.
Footnotes
Manuscript handled by: Jean Connors
Final decision: Jean Connors, 24 March 2022
REFERENCES
- 1.Pasha A.K., McBane R.D., Chaudhary R., et al. Timing of venous thromboembolism diagnosis in hospitalized and non‐hospitalized patients with COVID‐19. Thromb Res. 2021;207:150–157. doi: 10.1016/j.thromres.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nazy I., Sachs U.J., Arnold D.M., et al. Recommendations for the clinical and laboratory diagnosis of vaccine‐induced immune thrombotic thrombocytopenia (VITT) for SARS‐CoV‐2 infections: communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021;19(6):1585–1588. doi: 10.1111/jth.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov‐19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz N.H., Sørvoll I.H., Michelsen A.E., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scully M., Singh D., Lown R., et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV‐19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi: 10.1056/NEJMoa2105385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks P, Schuchat A. Joint CDC and FDA statement on Johnson & Johnson COVID‐19 vaccine. Accessed January 3, 2022. https://www.fda.gov/news‐events/press‐announcements/joint‐cdc‐and‐fda‐statement‐johnson‐johnson‐covid‐19‐vaccine
- 7.Smadja D.M., Yue Q.‐.Y., Chocron R., Sanchez O., Louet A.L.‐.L. Vaccination against COVID‐19: insight from arterial and venous thrombosis occurrence using data from VigiBase. Eur Respir J. 2021;58(1):2100956. doi: 10.1183/13993003.00956-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhary R., Padrnos L., Wysokinska E., et al. Macrovascular thrombotic events in a mayo clinic enterprise‐wide sample of hospitalized COVID‐19–positive compared with COVID‐19–negative patients. Mayo Clin Proc. 2021;96(7):1718–1726. doi: 10.1016/j.mayocp.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.See I, Lale A, Marquez P, et al. Case series of thrombosis with thrombocytopenia syndrome following COVID‐19 vaccination—United States, December 2020–August 2021. Medrxiv. 2021. [DOI] [PMC free article] [PubMed]
- 10.Shah A., Challener D.W., O'Horo J.C., Badley A.D. Vaccination safety don't toss the champagne with the cork. Mayo Clin Proc. 2021;96(7):1712–1713. doi: 10.1016/j.mayocp.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein N.P., Lewis N., Goddard K., et al. Surveillance for adverse events after COVID‐19 mRNA vaccination. JAMA. 2021;326(14):1390–1399. doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barda N., Dagan N., Ben‐Shlomo Y., et al. Safety of the BNT162b2 mRNA Covid‐19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sultan A.A., West J., Tata L.J., Fleming K.M., Nelson‐Piercy C., Grainge M.J. Risk of first venous thromboembolism in and around pregnancy: a population‐based cohort study. Br J Haematol. 2012;156(3):366–373. doi: 10.1111/j.1365-2141.2011.08956.x. [DOI] [PubMed] [Google Scholar]
- 14.Wainstock T., Yoles I., Sergienko R., Sheiner E. Prenatal maternal COVID‐19 vaccination and pregnancy outcomes. Vaccine. 2021;39(41):6037–6040. doi: 10.1016/j.vaccine.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blakeway H., Prasad S., Kalafat E., et al. COVID‐19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1–236.e14. doi: 10.1016/j.ajog.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control. Interim clinical considerations for use of COVID‐19 vaccines currently approved or authorized in the United States [updated 2/11/2022]. Accessed February 22 2022. https://www.cdc.gov/vaccines/covid‐19/clinical‐considerations/covid‐19‐vaccines‐us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid‐19%2Finfo‐by‐product%2Fclinical‐considerations.html#considerations‐Janssen
- 17.Bussel J, Connors J, Cines D, et al. Thrombosis with thrombocytopenia syndrome (also termed Vaccine‐induced Thrombotic Thrombocytopenia). Accessed January 3 2022. https://www.hematology.org/covid‐19/vaccine‐induced‐immune‐thrombotic‐thrombocytopenia
- 18.FDA and CDC lift recommended pause on Johnson & Johnson (Janssen) COVID‐19 vaccine use following thorough safety review. Accessed January 3, 2022. https://www.cdc.gov/media/releases/2021/fda‐cdc‐lift‐vaccine‐use.html