Highlights
-
•
Online adaptive radiotherapy (oART) is achievable within twenty minutes.
-
•
Cone-beam computed tomography-guided oART is feasible in daily clinical practice.
-
•
The adapted plan was always preferred over the scheduled plan.
Keywords: Online adaptive radiotherapy, CBCT-guided radiotherapy, Prostate cancer
Abstract
Background and purpose
Studies have shown the potential of cone-beam computed tomography (CBCT)-guided online adaptive radiotherapy (oART) for prostate cancer patients in a simulation environment. The aim of this study was to evaluate the feasibility of the clinical implementation of CBCT-guided oART for prostate cancer patients.
Materials and methods
Between February and July 2020, eleven prostate cancer patients were treated with CBCT-guided oART using a fractionation scheme of 20 × 3 Gy to the prostate and 20 × 2.7/3.0 Gy to the seminal vesicles for more advanced stages. The on-couch adaptive workflow consisted of influencer (prostate, seminal vesicles, rectum, bladder) review, target review, scheduled (re-calculated) and adapted (re-optimized) plan generation, an independent QA procedure and treatment delivery. Treatment time, proportion of adapted fractions and reasons for plan adaptation were evaluated.
Results
Mean total treatment time (±SD) from CBCT acquisition to end of treatment delivery was 17.5 ± 3.2 min (range: 10.8–28.8 min). In all 220 fractions, the PTV coverage was increased for the adapted plan compared to the scheduled plan. The V60Gy of bladder and rectum were below the constraints (<5% and <3%) for both scheduled and adapted plans in 171 out of 220 fractions and for the adapted plan only in 30 out of 220 fractions. In 19 out of 220 fractions, the V60Gy of the bladder and/or rectum was above the constraint for the adapted plan.
Conclusions
The clinical implementation of CBCT-guided oART is feasible for prostate cancer patients. The adaptive workflow is possible within twenty minutes on average with a dedicated team.
1. Introduction
In radiotherapy, the two most important goals are optimal target dose for adequate tumor control and minimal normal tissue exposure to prevent the development of side effects. Over the years, it has been proven difficult to achieve these goals due to e.g. challenges in correcting for daily anatomical changes [1], [2], [3], [4], [5]. The correction of inter-fractional anatomical changes was limited to rigid-body matching of the planning and daily acquired images, also called image-guided radiotherapy (IGRT). Nevertheless, IGRT cannot completely account for residual non-rigid inter-fractional variations such as rotation, deformation or differential motion between organs [2], [4].
Online adaptive radiotherapy (oART), which aims to re-optimize the treatment plan every treatment fraction while the patient is in the treatment position on the treatment couch, is an important novel approach to account for inter-fractional anatomical changes and optimize the therapeutic ratio [4], [6], [7]. Magnetic resonance (MR)-guided oART has already demonstrated to be feasible within a clinical setting [8], [9], [10]. Despite the advantages of good soft-tissue contrast and real-time image guidance, MR-guided oART still requires significant additional time and resources [11].
Recently, cone-beam computed tomography (CBCT)-guided oART became commercially available. Because of artificial intelligence (AI) aided contouring and the highly automated plan re-optimization together integrated in a system, the promised adaptive time slots were forecasted to amount to only fifteen minutes [12]. In comparison, MR-guided oART still requires time slots of 40 min up to one hour [11], [13], [14], [15]. Due to the expected variability in rectal and bladder filling which induces inter- and intra-fractional anatomical changes of the target volume, CBCT-guided oART could be of additional interest for prostate cancer patients [16]. Other reasons are the relative ease of anatomy and the relatively large group of patients, since prostate cancer is worldwide the second most frequently diagnosed cancer in men [16], [17].
Several studies have already simulated CBCT-guided oART for prostate cancer patients in a test environment retrospectively [18], [19], [20]. Sibolt et al. simulated the treatment for various pelvic sites, including eight prostate cancer patients and evaluated plan quality, AI-segmentation accuracy and oART feasibility [18]. Moazzezi et al. simulated CBCT-guided oART and studied the accuracy of auto-segmentation and need for manual edits for 25 prostate cancer patients [19]. In addition, a recent study by Byrne et al. presented early results on the accuracy of auto-segmented contours, plan quality and treatment fraction timing for four retrospective and two clinical intact prostate cancer patients [20]. Whereas Byrne et al. presented also results of clinical patients, the number of clinical patients analyzed in their study was limited.
The purpose of this study was to evaluate the feasibility of the clinical implementation of CBCT-guided oART for prostate cancer patients as part of a routine clinical workflow. More specifically, we describe the implementation of the complete workflow and report its first clinical results.
2. Materials and Methods
2.1. Patients
Between February and July 2020, eleven consecutive biopsy-proven intact prostate cancer patients underwent CBCT-guided oART with Ethos therapy (Varian Medical Systems, Palo Alto, CA). These patients were eligible within the time slot we had available. Patients with nodal involvement were excluded. All patients were treated using a fractionation scheme of 20 times 3 Gy to the prostate. For patients with more advanced stages, an additional total dose of 54 Gy (four patients) or 60 Gy (three patients) was given to the (base of the) seminal vesicles using a simultaneous integrated boost technique. The institutional review board of Medisch Spectrum Twente approved the study and judged that the study does not fall under the scope of the Medical Research Involving Human Subjects Act (WMO).
2.2. Description of the system
Ethos therapy is a novel solution for CBCT-guided oART and has been commercially available since 2019. The system is based on an O-ring linear accelerator and sub-minute kV CBCT guidance with the possibility of improved image quality via iterative reconstruction algorithms [21]. A detailed description of the technical characteristics of the system that underlies the adaptive capabilities has been published elsewhere [12].
2.3. Treatment preparation workflow
Prior to the first oART treatment session, all eleven patients underwent preparation in accordance with the local standard clinical protocol. Planning CT and MRI scans were acquired with a comfortably full bladder and without any specific rectal preparation. Patients were instructed to void the bladder half an hour prior to the scans and treatment and drink subsequently 500 ml of water.
Bladder, rectum, prostate and seminal vesicles are the system-defined influencers for prostate cancer patients. These organs are called influencers since they highly impact the propagation of the clinical target volume (CTV) from the planning CT to the daily CBCT during the adaptive workflow on the couch. These system-defined influencers, CTV and other organs at risk (OARs) were contoured on the planning CT within the adaptive planning software. The MRI scan was rigidly registered to the planning CT scan and was only used as a guide to aid in the manual contouring of the CTV on the planning CT. Whereas the contouring of the influencers prostate and seminal vesicles was only limited to the organs, the contoured CTV could also comprise of any tumor extension beyond the organ. The CTV was expanded to a planning target volume (PTV) using a margin of 7 mm in the lateral and anterior-posterior direction and 8 mm in the cranial-caudal direction, that is in accordance with our applied CTV-PTV margins when treating prostate cancer patients using an IGRT workflow. For each patient, a nine field intensity-modulated radiotherapy pre-treatment reference plan was created on the planning CT.
2.4. Adaptive workflow on couch
The adaptive workflow can be divided into three main components: influencer contouring and review, target propagation and review and treatment plan adaptation, plan QA and review. After patient setup and CBCT acquisition, the influencers are segmented by an AI algorithm based on convolutional neural networks [12]. During influencer review, the AI generated contours of bladder, rectum, prostate and seminal vesicles are assessed and manually adjusted when necessary. After influencer review, the influencers are used to guide a structure-guided deformation algorithm that propagates the CTV from the planning CT to the daily CBCT anatomy [12]. Manual adjustment of the target is possible when deemed necessary. The registered MR images that were used during the initial contouring on the planning CT were also available as assistance during influencer and target review at each treatment on the treatment console [12]. Thirdly, two plans are generated based on the daily CBCT anatomy: the re-calculated pre-treatment reference plan (scheduled plan) and the re-optimized pre-treatment reference plan (adapted plan). Both plans are then evaluated and compared based on predefined plan objectives, prioritizing target coverage. Next, an independent dose check using a gamma passing criterion of 3%/2mm, 20% threshold is performed for QA of the adapted plan (Mobius3D, Varian Medical Systems, Palo Alto, CA). Parallel to the plan QA, a second CBCT is then acquired for position verification. If the CTV moves outside the PTV, the couch is rigidly shifted in three directions using the gold fiducials within the prostate, after which the chosen treatment plan is delivered. If the intra-fraction motion cannot be corrected for with a couch shift, the adaptive workflow will be completely restarted. The full multidisciplinary team is present during the oART treatment sessions, including two radiation technologists (RTTs), a radiation oncologist, a medical physicist and a technical physician.
2.5. Analysis
The oART treatment sessions of all patients were evaluated based on treatment duration from CBCT acquisition to the end of treatment delivery. Treatment duration analysis was divided into total treatment time and its subcomponents comprising of influencer, target and treatment plan generation and review. Treatment-related acute toxicities (within 3 months) were reported using the Common Terminology Criteria for Adverse Events version 5.0 [22]. Influencer and target contour edits were classified as clinically acceptable if minor edits were required or major edits were required to a small number (up to 10%) of slices. In addition, they were classified as clinically unacceptable if major edits were required to a large number (>10%) of slices, including deletion and recontouring of the structure. Moreover, the proportion of adapted fractions and clinical reasons for plan adaptation were examined, focusing on PTV60Gy coverage (V95%>99%) and rectum (V60Gy < 3%) and bladder (V60Gy < 5%) constraints.
3. Results
3.1. Treatment time
No deviations in the planned workflow or patient-related events during treatment were reported. Mean total treatment time (±SD) from CBCT acquisition to the end of treatment delivery was 17.5 ± 3.2 min (range: 10.8–28.8 min). Time statistics of the different workflow steps related to treatment adaptation are provided in Table 1.
Table 1.
Time statistics of the workflow steps related to treatment adaptation, including influencer, target and plan generation and review.
| Mean (minutes) | Stdev (minutes) | Minimum (minutes) | Maximum (minutes) | |
|---|---|---|---|---|
| Influencer generation and review | 6.5 | 2.5 | 1.9 | 15.0 |
| Target generation and review | 0.8 | 0.4 | 0.3 | 3.5 |
| Treatment plan generation and review | 4.6 | 1.4 | 2.2 | 11.1 |
3.2. Contour adjustments and intra-fraction motion
For all fractions, the influencers required clinically acceptable edits. For none of the fractions it was necessary to manually adjust the propagated targets or perform a couch shift based on the second CBCT just prior to treatment delivery.
3.3. Plan selection
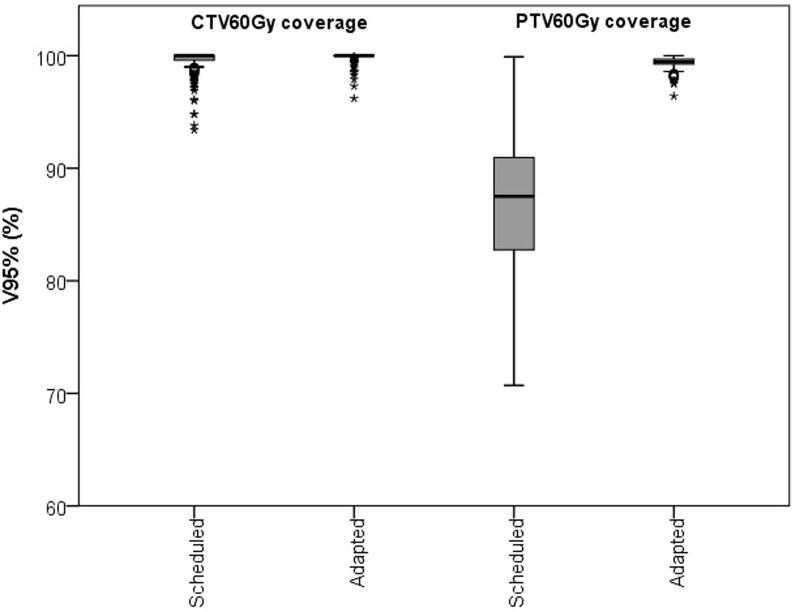
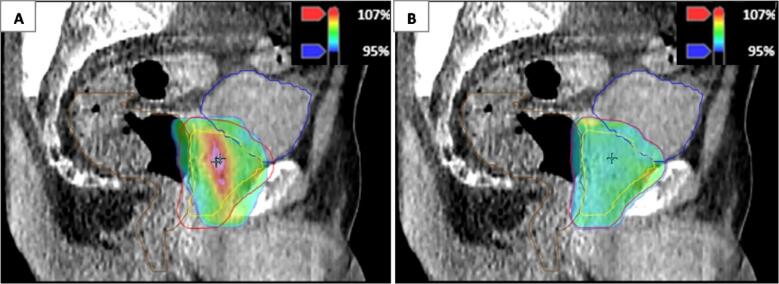
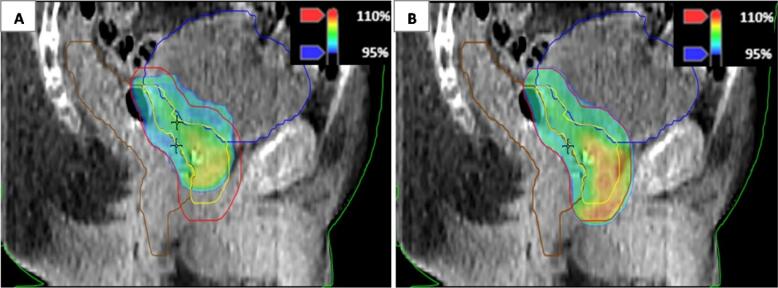
The adapted plan was chosen in all 220 fractions because of at least an increased PTV60Gy coverage with respect to the scheduled plan (Fig. 1). Mean V95% of PTV60Gy (±SD) was 86.7 ± 6.2% for the scheduled plans and 99.4 ± 0.5% for the adapted plans. The corresponding mean V95% of CTV60Gy (±SD) was 99.5 ± 1.0% and 99.9 ± 0.4% for the scheduled and adapted plans, respectively. The V60Gy of bladder and rectum were below the constraints for both scheduled and adapted plans in 171 out of 220 fractions (78%). In the remaining 49 fractions, the V60Gy of bladder, rectum, or a combination of both were above the constraints for the scheduled plan, adapted plan, or both. In 30 out of 220 fractions (14%), the V60Gy of bladder and rectum were below the constraints only in the adapted plan (see illustrative comparison in Fig. 2). Mean decrease (±SD) of V60Gy of bladder and rectum was 3.9 ± 1.8% (range: 0.6–9.5%) for the adapted plan compared to the scheduled plan. In 19 out of 220 fractions (9%), the V60Gy of the bladder, rectum, or a combination of both were above the constraint for the adapted plan (see illustrative comparison in Fig. 3). Mean V60Gy of bladder or rectum (±SD) was 4.7 ± 1.2% (range: 3.1–6.9%) for the adapted plan. In 10 of these fractions, the V60Gy constraints of bladder or rectum were also violated for the scheduled plan.
Fig. 1.
Boxplot showing the CTV60Gy and PTV60Gy coverage for the scheduled and the adapted plan. In the boxplot, the inner line denotes the median value, the box the interquartile range and the whiskers the minimum and maximum value excluding the outliers (data points >1.5 times the interquartile range away from the 75th or 25th percentile) that are presented as single markers.
Fig. 2.
Comparison of isodose distribution in color wash of scheduled (A) and adapted plan (B) for a patient for whom the coverage of the planning target volume (PTV) (red) was increased for the adapted plan compared to the scheduled plan, whereas both bladder and rectum doses were below the V60Gy constraints (<5% and <3%) for the adapted plan only. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Comparison of isodose distribution in color wash of scheduled (A) and adapted plan (B) for a patient for whom the increased coverage of the planning target volume (PTV) (red) in the adapted plan resulted in a violation of the V60Gy < 3% constraint of the rectum. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
We have successfully implemented CBCT-guided oART for prostate cancer patients. It is proven feasible to treat prostate cancer patients adaptively within twenty minutes on average with a dedicated team. In addition, the adapted plan was chosen over the scheduled plan in all cases.
Treatment-related acute toxicities were identified for 2 out of 11 patients. One patient experienced grade 1 proctitis and one patient grade 2 urinary urgency after treatment. For the other 9 patients, no treatment-related acute toxicities were reported. Our study was of course not designed to evaluate treatment-related toxicity. Nevertheless, we have no indication that our patients experienced increased toxicity. Further detailed studies in this respect are however indicated.
The mean total treatment time from CBCT acquisition to the end of treatment delivery was 17 min. Comparable results have been shown for (simulated) CBCT-guided oART treatments for other pelvis and head and neck cancer patients [18], [23]. Moreover, this result is also comparable to Byrne et al., in which the adaptive workflow for intact prostate cancer patients, from simulated completion of image acquisition to plan acceptance, was carried out in on average 15 min for four retrospective patients in the emulator. In addition, Byrne et al. reported a clinical treatment time, which they described as the time of opening the patient on the treatment machine to the time of closing the patient, which was on average 34 min for two intact prostate cancer patients [20]. Whereas their method of treatment time recording differs from ours, our used time slots were with twenty minutes much less. However, Byrne et al. did not divide the recorded treatment time into the subcomponents of the workflow.
We spent most of the time on influencer review, with a mean of 6.5 min. A relatively large range of 1.9 to 15.0 min was seen, which is due to anatomical variations that influence the performance of the AI contouring algorithm. As a result, the extent to which manual adjustments of the AI generated contours were necessary was highly variable between patients and fractions. Most limitations in the performance of the AI contouring algorithm were seen for patients with air in the rectum or small bowel. However, in this study the influencers required clinically acceptable edits for all fractions, which is in correspondence with the results of earlier studies [18], [19], [20].
In contrast to influencer review, on average less than one minute was spent on target review, mainly since no manual target adjustments were deemed necessary. This highlights the importance of accurate influencer review, which is in correspondence with observations of recent studies in which the same approach was used [18], [20], [23]. An explanation for the observation that no manual target adjustments were required, is that the influencers which are used for target propagation (prostate and seminal vesicles) were already edited in influencer review. This is in correspondence with the results of Byrne et al. in which the CTV of intact prostate cancer patients overlaps with influencers and as a result required less editing than the CTV of prostate bed patients [20].
The treatment plan generation and review took on average 4.6 ± 1.4 min with a range of 2.2 to 11.1 min. This relatively large range can be explained by the variable time spent on independent plan QA.
Possible reduction of the time spent on influencer review can be expected in the future. Willigenburg et al. trained RTTs to perform daily online contour adaptation for MR-Linac treatment of prostate cancer patients. They recently reported on shorter and less variable adaptation times in the patients that were treated at a later date compared to earlier treated patients [24]. Our center trained five RTTs to be advanced adapter parallel to the treatment of the initial patients. In gaining more experience with the adaptive workflow, we as well hope to reduce the treatment time. Overall, CBCT-guided oART is competitive with MR-guided oART, that still requires time slots of 40 min up to one hour [11], [13], [14], [15]. Moreover, the relatively short treatment time enables the large scale implementation of oART within a high-volume clinical practice, also for more conventional fractionation schemes, as opposed to ultra-hypofractionated schemes only. The MRI was only used as an assistance both during contouring of the target on the planning CT, as well as on the daily CBCTs. The current clinical workflow is, not only in our center but in many centers worldwide, primarily CT-based [25]. Moreover, studies on local control and side effects are as far as we know also based on a CT-based workflow [26], [27]. In theory, an MRI-only workflow may result in smaller targets, which subsequently might lead to less toxicity and side effects [28]. Future studies will no doubt shed light on this subject which should than also take local control into account.
The adapted plan was selected in all fractions, mainly to achieve maximization of the target coverage. The frequency of adapted plan selection is consistent with Byrne et al, in which the adapted plan was selected in 98.8% of fractions [20]. Interestingly, the reported frequency of adapted plan selection in this study was higher in comparison with Sibolt et al., in which the re-optimized treatment plan was selected in 83% of simulated treatment fractions [18]. A possible explanation for the difference with Sibolt et al. may be that their reported data also included prostate cancer patients with pelvic lymph nodes.
As was illustrated with the isodose distribution in color wash of the scheduled plans in Fig. 2, Fig. 3, inter-fractional variations such as bladder and rectum filling can result in underdosage of the target, which is in line with other studies on this subject [29], [30], [31]. With CBCT-guided oART, it was possible to correct for inter-fractional variations. As a result, the target coverage was increased for the adapted plans, that will probably further optimize the therapeutic ratio.
A limitation to this study is that we have analyzed the target and OAR doses for each fraction separately without accounting for the given dose of previously delivered fractions. Therefore, the accumulated dose of scheduled and adapted plans requires further investigation for more prostate cancer patients. Moreover, we have implemented CBCT-guided oART for intact prostate cancer patients only, due to the relative ease of the anatomy. In doing so, we have excluded e.g. prostate bed patients and prostate cancer patients with nodal involvement. We have gained experience with intact prostate cancer patients and in the near future we will expand to patient groups with more complex anatomy, e.g. the aforementioned patients or targets not overlapping with influencers.
A limitation of the CBCT-guided oART system is the lack of continuous intra-fraction monitoring. As an alternative, a second CBCT was acquired just prior to treatment delivery for position verification. The monitored intra-fraction motion of the CTV was within the currently used CTV-PTV margin of 7–8 mm. Therefore, a couch shift to correct for intra-fractional variations was not needed. The CTV-PTV margin of 7–8 mm was chosen since it is the CTV-PTV margin we used in non-oART patients and is a compensation for both inter- and intra-fraction motion. As oART enables correction for inter-fractional variations, the hypothesis is that the currently used CTV-PTV margin can be reduced. Further research on the effect of intra-fraction motion during the adaptive treatment is necessary to confirm this hypothesis.
In conclusion, the clinical implementation of CBCT-guided oART for prostate cancer patients is feasible within a high-volume clinical practice. The re-optimized adapted plan was always selected in favor of the recalculated scheduled plan and an adaptive treatment session was performed within twenty minutes on average.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Lisanne G.M. Zwart, Email: lisanne.zwart@mst.nl.
Francisca Ong, Email: f.ong@mst.nl.
Liselotte A. ten Asbroek, Email: l.zwolsman@mst.nl.
Erik B. van Dieren, Email: e.vandieren@mst.nl.
Siete A. Koch, Email: s.koch@mst.nl.
Anand Bhawanie, Email: a.bhawanie@mst.nl.
Elisabeth de Wit, Email: e.dewit@mst.nl.
Judith J. Dasselaar, Email: judith.dasselaar@mst.nl.
References
- 1.Bernier J., Hall E.J., Giaccia A. Radiation oncology: a century of achievements. Nat Rev Cancer. 2004;4:737–747. doi: 10.1038/nrc1451. [DOI] [PubMed] [Google Scholar]
- 2.Gupta T., Narayan C.A. Image-guided radiation therapy: physician’s perspectives. J Med Phys. 2012;37:174–182. doi: 10.4103/0971-6203.103602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The International Commission on Radiation Units and Measurements. ICRU Report 83 Prescribing, Recording and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT). Journal of the International Commission on Radiation Units and Measurements 2010;10. 10.1093/jicru/10.1.Report83.
- 4.Sonke J.J., Aznar M., Rasch C. Adaptive radiotherapy for anatomical changes. Semin Radiat Oncol. 2019;29:245–257. doi: 10.1016/j.semradonc.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Dawson L.A., Sharpe M.B. Image-guided radiotherapy: rationale, benefits, and limitations. Lancet Oncol. 2006;7:848–858. doi: 10.1016/S1470-2045(06)70904-4. [DOI] [PubMed] [Google Scholar]
- 6.Green O.L., Henke L.E., Hugo G.D. Practical clinical workflows for online and offline adaptive radiation therapy. Semin Radiat Oncol. 2019;29:219–227. doi: 10.1016/j.semradonc.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan D. Adaptive radiotherapy: merging principle into clinical practice. Semin Radiat Oncol. 2010;20:79–83. doi: 10.1016/j.semradonc.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Acharya S., Fischer-Valuck B.W., Kashani R., Parikh P., Yang D., Zhao T., et al. Online magnetic resonance image guided adaptive radiation therapy: first clinical applications. Int J Radiat Oncol Biol Phys. 2016;94:394–403. doi: 10.1016/j.ijrobp.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Henke L.E., Olsen J.R., Contreras J.A., Curcuru A., DeWees T.A., Green O.L., et al. Stereotactic MR-guided online adaptive radiation therapy (SMART) for ultracentral thorax malignancies: results of a phase 1 trial. Adv Radiat Oncol. 2019;4:201–209. doi: 10.1016/j.adro.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finazzi T., Palacios M.A., Haasbeek C.J.A., Admiraal M.A., Spoelstra F.O.B., Bruynzeel A.M.E., et al. Stereotactic MR-guided adaptive radiation therapy for peripheral lung tumors. Radiother Oncol. 2020;144:46–52. doi: 10.1016/j.radonc.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Van Herk M., McWilliam A., Dubec M., Faivre-Finn C., Choudhury A. Magnetic resonance imaging-guided radiation therapy: a short strengths, weaknesses, opportunities, and threats analysis. Int J Radiat Oncol Biol Phys. 2018;101:1057–1060. doi: 10.1016/j.ijrobp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Archambault Y., Boylan C., Bullock D., Morgas T., Peltola J., Ruokokoski E., et al. Making on-line adaptive radiotherapy possible using artificial intelligence and machine learning for efficient daily re-planning. Med Phys Int J. 2020;8:77–86. [Google Scholar]
- 13.Tetar S.U., Bruynzeel A.M.E., Lagerwaard F.J., Slotman B.J., Bohoudi O., Palacios M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys Imag Radiat Oncol. 2019;9:69–76. doi: 10.1016/j.phro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tocco B.R., Kishan A.U., Ma T.M., Kerkmeijer L.G.W., Tree A.C. MR-guided radiotherapy for prostate cancer. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.616291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunlop A., Mitchell A., Tree A., Barnes H., Bower L., Chick J., et al. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin Transl Radiat Oncol. 2020;23:35–42. doi: 10.1016/j.ctro.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim-Reinders S., Keller B.M., Al-Ward S., Sahgal A., Kim A. Online adaptive radiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:994–1003. doi: 10.1016/j.ijrobp.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 17.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 18.Sibolt P., Andersson L.M., Calmels L., Sjöström D., Bjelkengren U., Geertsen P., et al. Clinical implementation of artificial intelligence-driven cone-beam computed tomography-guided online adaptive radiotherapy in the pelvic region. Phys Imag Radiat Oncol. 2021;17:1–7. doi: 10.1016/j.phro.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moazzezi M., Rose B., Kisling K., Moore K.L., Ray X. Prospects for daily online adaptive radiotherapy via ethos for prostate cancer patients without nodal involvement using unedited CBCT auto-segmentation. J Appl Clin Med Phys. 2021;22:82–93. doi: 10.1002/acm2.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne M., Archibald-Heeren B., Hu Y., Teh A., Beserminji R., Cai E., et al. Varian ethos online adaptive radiotherapy for prostate cancer: early results of contouring accuracy, treatment plan quality, and treatment time. J Appl Clin Med Phys. 2022;23 doi: 10.1002/acm2.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai B., Laugeman E., Mazur T.R., Park J.C., Henke L.E., Kim H., et al. Characterization of a prototype rapid kilovoltage x-ray image guidance system designed for a ring shape radiation therapy unit. Med Phys. 2019;46:1355–1370. doi: 10.1002/mp.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NIH National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf; 2017 [accessed October 27, 2021].
- 23.Yoon S.W., Lin H., Alonso-Basanta M., Anderson N., Apinorasethkul O., Cooper K., et al. Initial evaluation of a novel cone-beam CT-based semi-automated online adaptive radiotherapy system for head and neck cancer treatment – a timing and automation quality study. Cureus. 2020;12 doi: 10.7759/cureus.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willigenburg T., De Muinck Keizer D.M., Peters M., Claes A., Lagendijk J.J.W., De Boer H.C.J., et al. Evaluation of daily online contour adaptation by radiation therapists for prostate cancer treatment on an MRI-guided linear accelerator. Clin Transl Radiat Oncol. 2021;27:50–56. doi: 10.1016/j.ctro.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salembier C., Villeirs G., De Bari B., Hoskin P., Pieters B.R., Van Vulpen M., et al. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother Oncol. 2018;127:49–61. doi: 10.1016/j.radonc.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Brand D.H., Tree A.C., Ostler P., Van der Voet H., Loblaw A., Chu W., et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomized, open-label, phase 3, non-inferiority trial. LancetOncol. 2019;20:1531–1543. doi: 10.1016/S1470-2045(19)30569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widmark A., Gunnlaugsson A., Beckman L., Thellenberg-Karlsson C., Hoyer M., Lagerlund M., et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomized, non-inferiority, phase 3 trial. LancetOncol. 2019;394:385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 28.Gunnlaugsson A., Persson E., Gustafsson C., Kjellén E., Ambolt P., Engelholm S., et al. Target definition in radiotherapy of prostate cancer using magnetic resonance imaging only workflow. Phys Imag Radiat Oncol. 2019;9:89–91. doi: 10.1016/j.phro.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X., Quan E.M., Li Y., Pan X., Zhou Y., Wang X., et al. A fully automated method for CT-on-rails-guided online adaptive planning for prostate cancer intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:835–841. doi: 10.1016/j.ijrobp.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Zelefsky M.J., Crean D., Mageras G.S., Lyass O., Happersett L., Ling C.C., et al. Quantification and predictors of prostate position variability in 50 patients evaluated with multiple CT scans during conformal radiotherapy. Radiother Oncol. 1999;50:225–234. doi: 10.1016/s0167-8140(99)00011-0. [DOI] [PubMed] [Google Scholar]
- 31.De Crevoisier R., Tucker S.L., Dong L., Mohan R., Cheung R., Cox J.D., et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:965–973. doi: 10.1016/j.ijrobp.2004.11.032. [DOI] [PubMed] [Google Scholar]