Abstract
Objective
The objective of this study is to present a novel clinical manifestation of infection with the Omicron variant of the SARS‐CoV‐2 virus affecting mainly young, vaccinated, and healthy adults. We describe a new group of COVID‐19 patients seeking emergency care with symptoms similar to the life‐threatening condition epiglottitis. Here, we present a case series and discuss management.
Methods
We performed a retrospective single‐center case study of patients diagnosed with COVID‐19 who were referred to the Ear, Nose, and Throat Emergency Department (ENT ED) between January 1 and January 23, 2022 with clinical symptoms such as acute odynophagia, severe sore throat, and fever. Ethical approval was obtained from the Swedish Ethical Review Authority (2020‐02579). Informed consent was obtained from all patients included in the study.
Results
Twenty patients meeting inclusion criteria were identified. Fifteen patients were fully vaccinated against COVID‐19. Four patients needed a short hospitalization for their symptoms. The most common diagnoses were COVID‐19‐associated acute viral laryngotracheitis and/or viral pharyngitis. Six patients presented with signs of secondary bacterial infection and were put on antibiotics.
Conclusion
Previous variants of SARS‐CoV‐2 infection affected predominantly the lower respiratory tract and were associated with loss of smell and taste in many patients. The Omicron variant seems to affect predominantly the upper airways and cause acute laryngitis without olfactory dysfunction. In some patients, the clinical manifestation is similar to the symptoms of epiglottitis. In such a case, a prompt examination of the larynx is the gold standard to exclude inflammatory edema in the upper airways. None of the patients described in this study developed epiglottitis. In this study, we discuss the management of acute odynophagia in COVID‐19 patients.
Keywords: COVID‐19, epiglottitis, laryngitis, Omicron, SARS‐CoV‐2
Introduction
Since the beginning of the COVID‐19 pandemic, an estimated 360 million cases and 5.6 million deaths have been reported due to SARS‐CoV‐2 infection [1]. While the vaccine immunity against the SARS‐CoV‐2 virus has been increasing worldwide, on November 11, 2021, the first case of Omicron—the novel variant of concern—was reported in Botswana and several days later in Hong Kong [2]. Since then, Omicron has rapidly spread across the globe, replacing the Delta variant and causing new infection waves in multiple countries.
Omicron has been proven to be a highly transmissible variant with doubling time of approximately 2–3 days [3, 4]. The exact clinical features and complications are still unknown. The preliminary data from the ZOE COVID Study run by ZOE and King's College of London indicate that Omicron symptoms come predominantly from the upper respiratory tract and include runny nose, sore throat, headache, fatigue, and sneezing [5]. The loss of smell and taste—one of the previously common symptoms of COVID‐19 infection—is reported to be rare during Omicron infection. Early data also suggest that Omicron infection is associated with a lower risk of hospitalization, serious disease, and death [6, 7].
In the last week of 2021, Omicron was the dominant variant in the region of Stockholm, Sweden [8]. At the same time as the Omicron variant became dominant, we experienced a high volume of young adults referred to our Ear, Nose, and Throat Emergency Department (ENT ED) with acute odynophagia, severe sore throat, and fever. Most of the patients were vaccinated against COVID‐19 and did not have any comorbidities. The most common diagnoses in those patients were COVID‐19‐associated acute viral laryngotracheitis and/or viral pharyngitis. Those patients did not present with symptoms from the lower respiratory tract and did not experience smell/taste loss. Most notably, this is a new group of patients seeking emergency care with symptoms similar to the life‐threatening condition epiglottitis. With an increasing number of Omicron cases, it is anticipated that the volume of patients with these symptoms could become overwhelming for EDs. We present a case series of 20 adults with COVID‐19 with acute odynophagia as the main symptom of Omicron infection. We also briefly describe the appropriate management of patients presenting with this clinical manifestation of COVID‐19.
Methods
Design
We conducted a retrospective single‐center case study, enrolling consecutive patients with a confirmed COVID‐19 diagnosis who were referred to the ENT ED at Karolinska University Hospital, Stockholm, due to acute odynophagia, severe sore throat, and fever between January 1 and January 23, 2022. Included motivations for referral to the ENT ED were as follows: severe sore throat, peritonsillitis, acute odynophagia, and epiglottitis.
Case confirmation
To be included in the study, patients were required to have a positive reverse transcription‐polymerase chain reaction (RT‐PCR) test detecting SARS‐CoV‐2 RNA in a nasal swab or a positive rapid antigen test performed by a healthcare professional and registered in the electronic medical documentation within 3 days before or following the visit at the ENT ED. Patients who did not meet this criteria were excluded from further analysis.
Five patients enrolled in the study were tested for COVID‐19 between January 3 and January 9 (week 1). Five patients were tested for COVID‐19 between January 10 and January 16 (week 2). Ten patients were tested for COVID‐19 between January 17 and January 23 (week 3). According to official statistics of the Public Health Agency of Sweden, among sequenced PCR samples, during week 1, 91% of cases were the Omicron variant. During week 2, 96.3% of cases were confirmed as Omicron. During week 3, 98.6% of cases were confirmed as Omicron [9]. Thus, with high probability—close to certainty—patients included in the study were infected with the Omicron variant of SARS‐CoV‐2.
Data collection
Medical records were reviewed and patients’ demographic and medical histories were collected. Laboratory information, vaccination status, and details of ENT assessment were available in the medical records. The following epidemiological variables were collected: gender, age, comorbidities, the status of vaccination against COVID‐19, and history of past COVID‐19 infection. The referral and notes following the ENT assessment were reviewed to extract symptoms of COVID‐19 infection in enrolled individuals. We searched for general symptoms such as fever, asthenia, cough, myalgia, headache, diarrhea, dyspnea, and ENT symptoms such as odynophagia, sore throat, otalgia, nasal obstruction, hoarseness of voice, self‐reported olfactory disorder, and self‐reported taste disorder. We used descriptive statistics to characterize the described cohort.
Follow‐up was performed by the main investigator (K.P.) in the form of telephone calls to enrolled patients. Information about the resolution of the infection and management of symptoms was collected.
Results
Demographic and epidemiologic characteristics of COVID‐19 patients
Twenty patients meeting inclusion criteria were identified. The mean age of patients was 32 ± 10 years. There were nine (45%) female and 11 (55%) male patients. Nineteen patients had the COVID‐19 diagnosis confirmed by a positive RT‐PCR test and one patient had the diagnosis confirmed by a positive antigen test performed by a healthcare professional. The majority of patients had no comorbidities (85%). The demographic data are summarized and presented in Table 1.
Table 1.
Epidemiological, clinical features, and vaccination status of COVID‐19 patients
| Characteristics | Patients (N = 20) |
|---|---|
| Age (Mean; SD) | 32 ± 10 |
| Gender (F/M) | 9 (45%)/11 (55%) |
| Comorbidities | 3 (15%) |
| Depression | 1 |
| Bipolar disorder | 1 |
| Hepatitis B | 1 |
| Healthy | 17 (85%) |
| Vaccination | |
| Fully vaccinated a | 15 (75%) |
| Partly vaccinated b | 1 (5%) |
| Unvaccinated | 4 (20%) |
Abbreviations: F/M, female/male.
At least two doses of COVID‐19 vaccine.
One dose of COVID‐19 vaccine.
Vaccination status of COVID‐19 patients
The summary of data on vaccination status is summarized in Table 1. Fifteen patients (75%) were fully vaccinated against COVID‐19. One fully vaccinated patient had previous COVID‐19 infection confirmed with a positive RT‐PCR test in 2020. There were four patients (20%) who were unvaccinated against COVID‐19 and did not have COVID‐19 previously. One patient was vaccinated with just one dose.
Clinical picture of COVID‐19‐associated acute odynophagia
All patients included in this case series had acute odynophagia and severe throat pain as a major complaint. Seven patients (35%) presented with hoarseness of voice as a part of clinical manifestation. Fifteen patients had fever at the time they presented at the ENT ED. In six patients, signs of secondary bacterial infection were identified, and thus treatment with antibiotics was immediately started. Four patients (20%) needed hospitalization for management of their symptoms, including one patient who presented with edema of the arytenoid region. None of the patients developed a clinical picture of epiglottitis and needed airway management. None of the patients presented with loss of smell or taste. All the patients recovered from their COVID‐19 infections.
The laryngeal findings in the majority of patients included general redness in the hypopharynx and larynx. As stated earlier, only one patient had a finding of edema in the arytenoid region that quickly resolved after administration of intravenous corticosteroids and epinephrine inhalation. The summary of laryngeal findings of the enrolled patients is presented in Table 2. Figure 1 shows representative laryngofiberscopic findings in one of the patients with the erythematous epiglottis and bilateral erythematous arytenoids with saliva pooling in the pyriform sinus.
Table 2.
A summary and laryngeal finding in patients presenting to Ear, Nose, and Throat (ENT) Emergency Department with acute odynophagia, severe sore throat, and fever during Omicron wave in Sweden
| No. | Sex | Age | Vaccinated (Y/N/P) | Vaccine doses (0‐3) | COVID‐19 before (Y/N) | Comorbidities | Motives for referral to ENT emergency department | Odynophagia (Y/N) | Throat sore (Y/N) | Hoarseness (Y/N) | Fever | Laryngoscopy findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | F | 28 | Y | 2 | N | None |
Peritonsillitis Odynophagia |
Y | Y | Y | Y | General redness in hypopharynx and larynx. No swelling of the epiglottis. Bacterial tonsillitis. |
| 2. | M | 22 | Y | 2 | N | None |
Peritonsillitis Odynophagia |
Y | Y | N | Y | General redness in hypopharynx and larynx. No swelling of the epiglottis. |
| 3. | M | 22 | N | 0 | N | Hepatitis B |
Epiglottitis Laryngotracheitis |
Y | Y | Y | Y | General redness in hypopharynx and oropharynx. No swelling of the epiglottis. |
| 4. | M | 46 | Y | 2 | Y | None |
Sore throat Fever Facial swelling |
Y | Y | N | Y | General redness in hypopharynx, larynx, and epiglottis. No swelling of the epiglottis. |
| 5. | F | 37 | Y | 2 | N | None | Odynophagia | Y | Y | N | N | Redness/edematous hypopharynx. Larynx and epiglottis without changes. |
| 6. | M | 21 | N | 0 | N | None | Severe sore throat | Y | Y | N | N | Redness/edematous hypopharynx. Larynx and epiglottis without changes. |
| 7. | F | 27 | Y | 2 | N | None | Epiglottitis | Y | Y | Y | Y | Redness/edematous hypopharynx. Redness in the larynx. Epiglottis without changes. |
| 8. | M | 35 | Y | 2 | N | None | Odynophagia | Y | Y | N | Y | Edematous arytenoid region with general redness in larynx and hypopharynx. |
| 9. | M | 48 | Y | 2 | N | None | Odynophagia | Y | Y | N | N | General redness in hypopharynx, larynx, and epiglottis. No swelling of the epiglottis. |
| 10. | F | 30 | P | 1 | N | None | Epiglottitis | Y | Y | Y | Y | General redness in the larynx and hypopharynx. Secretion in the larynx. |
| 11. | M | 28 | Y | 2 | N | None | Epiglottitis | Y | Y | N | Y | General redness in hypopharynx, larynx, and epiglottis. No swelling of the epiglottis. |
| 12. | F | 33 | Y | 2 | N | None |
Peritonsillitis Odynophagia |
Y | Y | Y | Y | General redness in hypopharynx, larynx, and epiglottis. No swelling of the epiglottis. |
| 13. | F | 25 | N | 0 | N | None | Epiglottitis | Y | Y | N | Y | General redness in hypopharynx, larynx, and epiglottis. No swelling of the epiglottis. Edematous hypopharynx. |
| 14. | F | 19 | Y | 2 | N | None | Odynophagia | Y | Y | N | Y | General redness in the hypopharynx and larynx. Epiglottis without redness and swelling. |
| 15. | F | 58 | Y | 3 | N | Depression | Odynophagia | Y | Y | N | Y | General redness in the hypopharynx and larynx. Epiglottis without redness and swelling. Redness in the subglottic region. |
| 16. | M | 30 | Y | 3 | N | None | Odynophagia | Y | Y | Y | N | General redness in the hypopharynx and larynx. Epiglottis without redness and swelling. |
| 17. | M | 39 | Y | 2 | N | None | Odynophagia | Y | Y | N | Y | General redness in the hypopharynx and larynx. Epiglottis without redness and swelling. |
| 18. | F | 37 | Y | 2 | N | None | Odynophagia | Y | Y | Y | Y | General redness in the hypopharynx and larynx. Epiglottis without redness and swelling. Edematous hypopharynx and ulcers in vocal cords. |
| 19. | M | 26 | N | 0 | N | None | Odynophagia | Y | Y | N | N | General redness in the hypopharynx and larynx. Epiglottis without redness and swelling. |
| 20. | M | 34 | Y | 2 | N | Bipolar disorder |
Sore throat Odynophagia |
Y | Y | N | Y | General redness in the hypopharynx and larynx. Epiglottis without redness and swelling. |
Fig. 1.
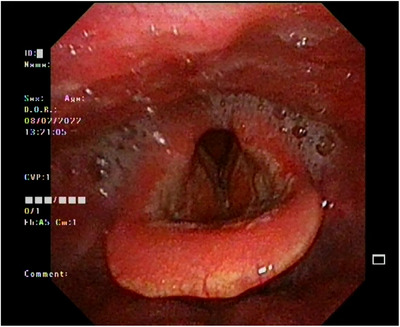
Laryngofiberscope findings. Erythematous epiglottis and bilateral erythematous arytenoids with saliva pooling in the pyriform sinus. No signs of epiglottic edema or upper airway obstruction.
Management
Treatments of odynophagia caused by viral or bacterial agents include treating the underlying infection. As there is no widely used specific treatment for COVID‐19, the first treatment choice is pain management including paracetamol and nonsteroidal anti‐inflammatory drugs (NSAIDs) such as ibuprofen or diclofenac. In addition, affected patients may use a local anesthetic containing lidocaine in the form of a spray or oral solution to numb the throat. Antibiotics are not recommended if there is no suspicion of secondary bacterial infection. Antibiotics have been shown to have a modest beneficial effect over placebo in reducing the symptom of sore throat [10]. In cases where antibiotics are indicated, penicillin V taken two or three times daily for 10 days is the treatment of choice.
Patients with severe symptoms may be given one dose of epinephrine in an inhaled form using a nebulizer to reduce airway inflammation. This can be repeated frequently. Epinephrine inhalation gives a quick effect and may relieve pain by reducing edema; however, its effect wears off quickly. Another alternative is a single dose of oral or intramuscular corticosteroids. Dexamethasone is the recommended corticosteroid due to its long‐lasting effects. Corticosteroids were shown to moderately reduce throat pain in several clinical trials [11].
Discussion
We report a case series of COVID‐19‐positive patients seeking the ENT ED due to acute odynophagia, severe sore throat, and fever. During the first weeks of the Omicron wave in Sweden, we experienced a high volume of young patients presenting with this clinical manifestation of infection with SARS‐CoV‐2. All patients developed COVID‐19‐associated acute laryngitis and/or pharyngitis. The clinical triad of symptoms these patients presented with increased the likelihood that they had the life‐threatening condition epiglottitis. Thus, a prompt clinical examination including laryngoscopy is necessary to make a diagnosis and guide management.
None of the patients described in this study presented with swollen/edematous epiglottis and required airway management. Only one patient presented with swelling of an arytenoid region. However, in the literature search we identified seven cases of acute epiglottitis with concomitant COVID‐19 infection [12, 13, 14, 15, 16, 17, 18], so this diagnosis should be considered and promptly excluded, especially if a patient is presenting with a typical clinical triad.
The clinical picture of SARS‐CoV‐2 infection with acute odynophagia, severe sore throat, and fever has become a common COVID‐19 manifestation of the Omicron variant. During the previous waves, symptoms such as cough, fever, and loss of taste or smell were more prominent. Those symptoms are currently rarely reported by Omicron‐infected patients [5]. The preliminary evidence suggests that Omicron gives milder symptoms compared to Delta, but it is still unclear whether the reduced severity is caused by the variant's characteristics or if it is due to the increasing vaccine immunity worldwide. Still, even though the preliminary evidence suggests that Omicron is milder, a significant percentage of patients need to be hospitalized to manage their symptoms. In the described cohort, 20% needed hospitalization for management of their symptoms. All those patients were fully vaccinated with at least two doses against COVID‐19.
In the described cohort, once the suspicion of epiglottitis was ruled out, patients were assigned with diagnoses such as acute viral laryngitis or acute viral pharyngitis based on the picture of the larynx and hypopharynx in laryngoscopy. In a few patients with signs of secondary bacterial superinfection, oral antibiotics were administered. The traditional treatment of acute laryngitis includes voice rest, analgesic therapy, and humidification [19]. According to the findings of a Cochrane Review investigating the benefits of antibiotic use for acute laryngitis in adults, antibiotics appear to have no benefit in treating this entity [20].
Conclusions
In conclusion, we present a case series of 20 patients with COVID‐19‐associated laryngotracheitis and pharyngitis who were admitted to our ENT ED during the SARS‐CoV‐2 Omicron wave. In those patients, the dominant symptoms were acute odynophagia, severe sore throat, and fever. The majority of patients were young, healthy, and vaccinated against COVID‐19. This clinical manifestation of COVID‐19 was uncommon during previous waves. Because a similar medical triad of symptoms is associated with the life‐threatening condition epiglottitis, a prompt examination of the larynx is recommended to exclude inflammatory edema in the upper airways. None of the patients with COVID‐19‐associated odynophagia presented with edema in the larynx and epiglottis causing airway obstruction. Still, 20% of the patients—despite being vaccinated against COVID‐19—needed to be hospitalized for their symptom management. The management of acute odynophagia in the absence of edema in the airways consists of high‐dose analgesics, NSAIDs, and local anesthetic to numb the lining of the mouth and throat. In severe cases, epinephrine inhalation and oral/intravenous corticosteroids may be needed to relieve the symptoms.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
Conceptualization, data curation, formal analysis, methodology, project administration, visualization, writing–original draft, and writing–review and editing: Krzysztof Piersiala. Conceptualization, investigation, project administration, writing–original draft, writing–review and editing: Lara Kakabas. Conceptualization, investigation, methodology, project administration, writing–original draft, writing–review and editing: Anna Bruckova. Conceptualization, methodology, project administration, supervision, writing–original draft, writing–review and editing: Magnus Starkhammar. Conceptualization, investigation, project administration, supervision, writing–original draft, and writing–review and editing: Lars Olaf Cardell.
Piersiala K, Kakabas L, Bruckova A, Starkhammar M, Cardell LO. Acute odynophagia: A new symptom of COVID‐19 during the SARS‐CoV‐2 Omicron variant wave in Sweden. J Intern Med. 2022;292:154–161.
References
- 1. WHO . WHO coronavirus (COVID‐19) dashboard. 2022. Available from: https://covid19.who.int/. Accessed 29 Jan 2022.
- 2. WHO . Update on omicron. 2021. Available from: https://www.who.int/news/item/28‐11‐2021‐update‐on‐omicron. Accessed 29 Jan 2022.
- 3. Grabowski F, Kochańczyk M, Lipniacki T. Omicron strain spreads with the doubling time of 3.2—3.6 days in South Africa province of Gauteng that achieved herd immunity to Delta variant. medRxiv. 2021: 10.1101/2021.12.08.21267494 [DOI] [Google Scholar]
- 4. Ferguson N, Ghani A , Cori A, Hogan A, Hinsley W, Volz E. Report 49: Growth, population distribution and immune escape of Omicron in England. Imperial College London; 2021. [Google Scholar]
- 5. Omicron and cold‐like symptoms rapidly taking over in London . [cited Dec 2021]. Available from: https://covid.joinzoe.com/post/omicron‐and‐cold‐like‐symptoms‐rapidly‐taking‐over‐in‐london.
- 6. Espenhain L, Funk T, Overvad M, Edslev SM, Fonager J, Ingham AC, et al. Epidemiological characterisation of the first 785 SARS‐CoV‐2 Omicron variant cases in Denmark, December 2021. Eurosurveillance 2021;26(50):2101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christie B. Covid‐19: early studies give hope omicron is milder than other variants. BMJ. 2021;375:n3144. [DOI] [PubMed] [Google Scholar]
- 8.Vecka 52, 2021 – Statistik om förekomst av misstänkta fall av SARS‐CoV‐2 virusvarianten omikron. Available from: https://www.folkhalsomyndigheten.se/smittskydd‐beredskap/utbrott/aktuella‐utbrott/covid‐19/statistik‐och‐analyser/sars‐cov‐2‐virusvarianter‐av‐sarskild‐betydelse/statistik‐om‐forekomst‐av‐misstankta‐fall‐av‐sars‐cov‐2‐virusvarianten‐omikron/vecka‐52‐2021/. Accessed 29 Jan 2022.
- 9.Share of SARS‐CoV‐2 sequences that are the omicron variant in Sweden. Available from: https://www.folkhalsomyndigheten.se/smittskydd‐beredskap/utbrott/aktuella‐utbrott/covid‐19/statistik‐och‐analyser/sars‐cov‐2‐virusvarianter‐av‐sarskild‐betydelse/. Accessed 29 Jan 2022.
- 10. Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2006;(4):CD000023. [DOI] [PubMed] [Google Scholar]
- 11. de Cassan S, Thompson MJ, Perera R, Glasziou PP, Del Mar CB, Heneghan CJ, et al. Corticosteroids as standalone or add‐on treatment for sore throat. Cochrane Database Syst Rev. 2020;5(5):CD008268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emberey J, Velala SS, Marshall B, Hassan A, Meghjee SP, Malik MJ, et al. Acute epiglottitis due to COVID‐19 infection. Eur J Case Rep Intern Med. 2021.8(3):002280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwamoto S, Sato MP, Hoshi Y, Otsuki N, Doi K. COVID‐19 presenting as acute epiglottitis: a case report and literature review. Auris Nasus Larynx. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fondaw A, Arshad M, Batool S, Robinson B, Patel T. COVID‐19 infection presenting with acute epiglottitis. J Surg Case Rep. 2020;2020(9):rjaa280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Renner A, Lamminmäki S, Ilmarinen T, Khawaja T, Paajanen J. Acute epiglottitis after COVID‐19 infection. Clin Case Rep. 2021;9(7):e04419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith C, Mobarakai O, Sahra S, Twito J, Mobarakai N. Case report: epiglottitis in the setting of COVID‐19. IDCases. 2021;24:e01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cordial P, Le T, Neuenschwander J. Acute epiglottitis in a COVID‐19 positive patient. Am J Emerg Med. 2022;51:427.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alqaisi S, Almomani A, Alwakeel M, Akbik B. 239: epiglottitis case report: a unique presentation of COVID‐19 infection. Crit Care Med. 2021;49(1):105. [Google Scholar]
- 19. Dworkin JP. Laryngitis: types, causes, and treatments. Otolaryngol Clin North Am. 2008;41(2):419–36, ix. [DOI] [PubMed] [Google Scholar]
- 20. Reveiz L. Cardona AF. Antibiotics for acute laryngitis in adults. Cochrane Database Syst Rev. 2015;(5):CD004783. [DOI] [PMC free article] [PubMed] [Google Scholar]


