Abstract
COVID‐19 is a systemic disease characterized by vascular damage, increased inflammation, and hypercoagulability. Acral ischemic lesions occur as a result of increased inflammation and cutaneous thrombosis. Skin lesions can sometimes be the only symptom of COVID‐19. In this respect, recognizing acro‐ischemic lesions, which are skin lesions, may help in the early diagnosis of the disease and in predicting the prognosis. In patients with skin lesions without typical symptoms, the diagnosis of COVID‐19 should be kept in mind. Herein, we describe five patients affected by COVID‐19 which developed acro‐ischemic lesions.
Keywords: acro‐ischemia, COVID‐19, leukocytoclastic vasculitis, livedo, penis necrosis, skin necrosis, thrombosis
1. INTRODUCTION
In December 2019, unexplained viral pneumonia cases began to be reported in Wuhan province of China. The agent was identified as a new coronavirus pathogen (SARS‐CoV‐2) and the disease was named Coronavirus disease 2019 (COVID‐19). In March 2020, the pandemic was declared by World Health Organization. 1 , 2 This rapidly spreading deadly virus has affected the health system in many countries adversely. Healthcare professionals had to fight with many complications caused by the disease while trying to keep people affected by the pandemic alive.
Although COVID‐19 was first defined as a respiratory problem, it was later understood to be a systemic disease. 3 The disease symptoms cover a wide range. While it may be asymptomatic or like flu, it may also progress with a clinic that leads to multiple organ failure. 4 , 5 The primary targets of the virus are vascular endothelium cells, pneumocytes, and immune cells. 6 Endothelial function is impaired by the infection and the inflammation of the endothelium with SARS‐COV‐2. The resulting endothelial dysfunction results in the loss of fibrinolytic feature and disposition to thrombosis. 7 , 8 Micro‐ and macro‐thrombosis in vessels may cause problems that may lead to multi‐organ failure. The resulting thromboinflammation state is associated with the weight of the disease. 6
The number of studies on skin findings of COVID‐19 disease is gradually increasing in literature. Although there is no consensus on naming the lesions, acral ischemic lesions seen in COVID‐19 disease are grouped in two: (1) pseudo chilblain (pseudo perniosis) (2) livedo or necrosis. Pseudo chilblain is mostly seen in young people and in the late periods of the disease, and it continues for an average of 12, 7 days. It involves first the feet and then the hands. 9 Livedo or necrosis is mostly seen in patients with advanced age and comorbidity and in patients whose clinic has a severe course. It is thought that it occurs secondary to acral ischemia and microvascular occlusion that occurs as a result of the worsening in the patient's general health or coagulation anomalies that occur. It is very rare among skin lesions due to COVID‐19 (6%).
In this study, we aimed to bring to literature five COVID‐19 patients who developed acro‐ischemic skin lesions (livedo, necrosis).
We included patients who were evaluated with acro‐ischemic findings in our tertiary care hospital between March 2020 and November 2021 and whose diagnosis of COVID‐19 was confirmed by RT‐PCR on a nasopharyngeal swab sample. Informed consent was obtained from all our patients or relatives. Variables such as demographics, comorbidities, clinical presentations, diagnosis, and treatment data were gathered.
2. CASE 1
A 61‐year‐old female patient who had necrosis and pain on the fingers, painful purpuric rashes on feet and legs and occasional hemorrhagic bullae (Figure 1A,B,C,D) was admitted to the emergency department of our hospital on October 29, 2020. There were no features in her anamnesis other than hypertension. It was learned that she never had complaints such as fever, cough, shortness of breath and malaise before. The patient's chest computerized tomography (CT) was found to be compatible with COVID‐19. COVID‐19 polymerase chain reaction (PCR) test studied from nasopharyngeal specimen was negative. Her test on November 10 was positive. The patient's wounds started from the soles of the feet and progressed to both feet and cruris. Incisional biopsy from cruris showed leukocytoclastic vasculitis (LCV). Hyperparakeratosis in the epidermis, ulceration, erythrocyte extravasation in the dermis, fibrinoid necrosis in the small vessels including adipose tissue veins and recanalized thrombus in medium‐sized veins, polymorphonuclear leukocytes in the vessel wall, and leukocytoclasia around vessels were observed. Covid PCR was negative in the tissue sample taken. The opened bullae ulcerated and then turned into complete skin necrosis on November 12 (Figure 1E,F,G,H). No blood flow in the left anterior tibial artery was detected in Doppler ultrasonography (USG). It started as livedoid lesions on the hands and bruising on the finger tips on October 10. Bruising on the fingers resulted in necrosis while liveoid lesions on the hand regressed on October 29 (Figure 1I,J). Total necrosis developed in the distal phalanx ends of the patient's right hand 1, 2, and 3 and left hand 1 and 2. But upper extremity Doppler USG was found to be normal. The patient who did not accept the amputation recommendation was not intervened. Necrotized tissues in the feet and cruris were debrided. The tissue defect formed as a result of debridement was closed with a skin graft.
FIGURE 1.
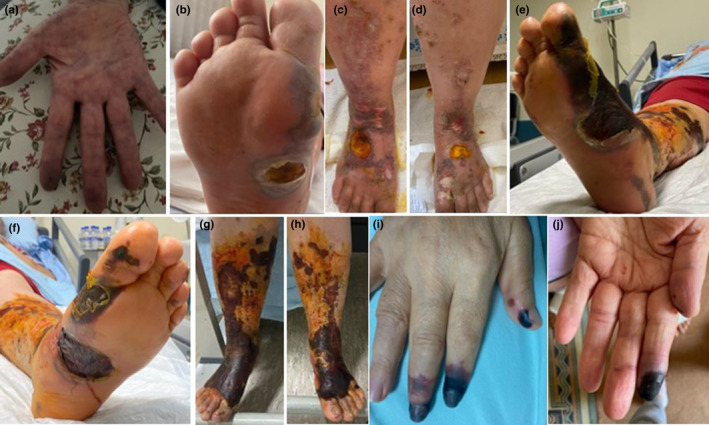
Clinical views of case 1. (A) livedoid lesion on the right hand (1st day). (B) hemorrhagic bulla on the sole of the right foot (9th day). (C,D) lesion that started as palpable purpura and ended with necrotic bullae on the lower extremities (16th day). (E,F,G,H) skin necrosis on the lower extremities (32th day). (I,J) necrosis on distal of the right and left hand finger (19th day). (The day of onset of symptoms was accepted as day 1)
3. CASE 2
A 82‐year‐old female patient referred with complaints of fever, cough, and malaise. The patient's chest CT was found to be compatible with viral pneumonia findings. PCR test was found to be positive. The patient was using metoprolol for arrhythmia and leflunomide for rheumatoid arthritis. She was admitted to hospital on 25th day after the COVID‐19 PCR test positivity with complaints of coldness and bruising in the right lower extremity. CT angiography showed complete occlusion of the right popliteal artery and bilateral deep femoral arteries. Occluded areas were observed distal to the left popliteal artery. Flow was restored with embolectomy. Skin necrosis was occurred in the right lower leg and ankle (Figure 2A, B). Right foot 1st and 2nd toe tips necrosis was occurred (Figure 2C). Skin necrosis was debrided and grafted with skin graft. Partial necrosis of the big toe tip healed secondary.
FIGURE 2.
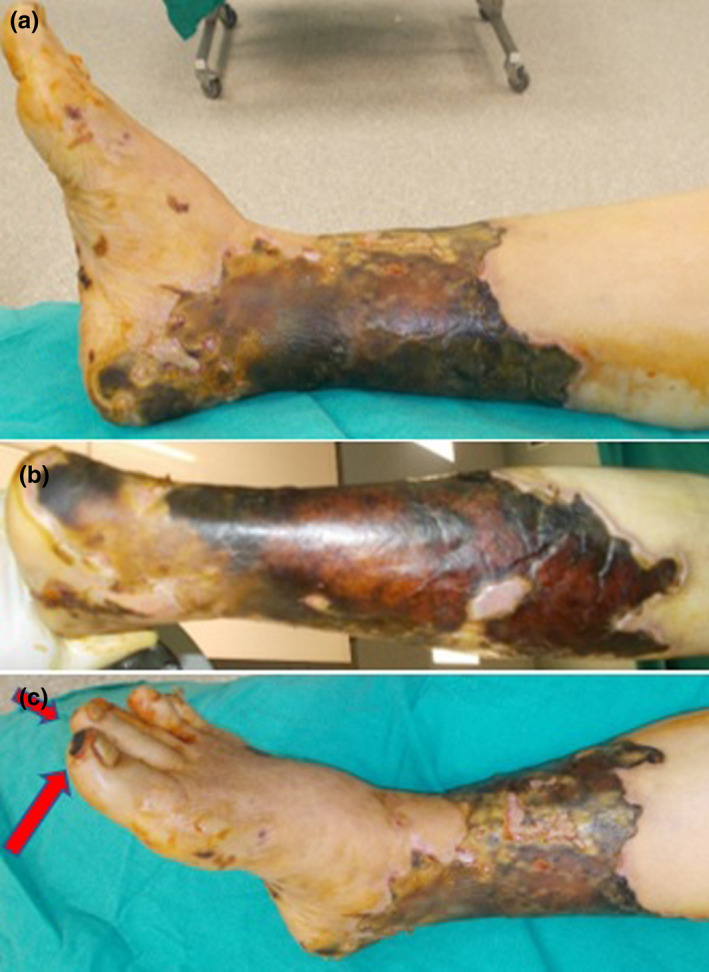
Clinical views of case 2. (A,B) circular skin necrosis on the right lower extremity. (C) necrosis on the right foot first and second toe tips necrosis
4. CASE 3
A 8‐year‐old male patient that was admitted to hospital with complaints of fever and shortness of breath was hospitalized and treated. The patient who had Down syndrome and who had been diagnosed with acute lymphocytic leukemia (ALL) for two years was using levetiracetam for seizures. The patient whose general condition worsened was intubated. Necrosis developed on the left hand second finger and distal of right first toe. (Figure 3A,B). COVID‐19 PCR test made 15 days later was found to be positive. Chest CT was found to be compatible with pneumonia. Low velocity monophasic flow was detected in bilateral radial, ulnar, posterior tibial, and dorsalis pedis arteries in Doppler USG.
FIGURE 3.
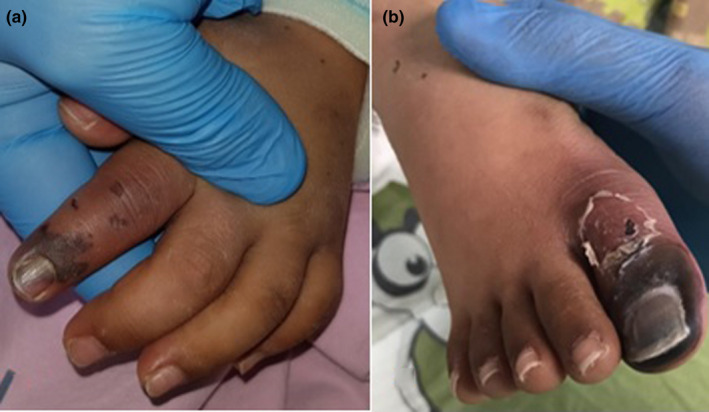
Clinical views of case 3. (A,B) necrosis on the left hand second finger and distal of right first toe
5. CASE 4
A 71‐year‐old male patient was admitted to our hospital with complaints of fever and malaise. COVID‐19 PCR test of the patient who had diabetes mellitus (DM) and chronic renal failure (CRF) was found to be positive. Chest CT was compatible with viral pneumonia. Right hand 1st, 2nd, 3rd, and 5th finger distal phalanx tips and glans penis tips had necrosis Doppler USG was normal. Distal phalanx tips of the right hand first and second fingers were amputated and left open and the necrotic parts of the glans penis were debrided (Figure 4A,B,C).
FIGURE 4.
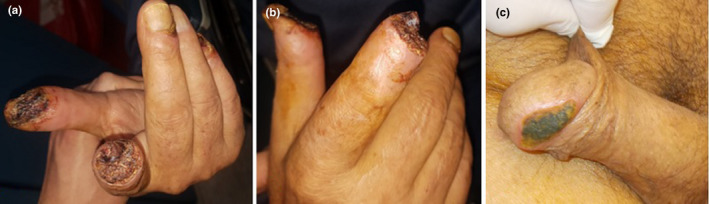
Clinical views of case 4 (A,B) Postoperative image of the necrotic right hand fingers 1,2,3, and 5. (C) necrosis of the glans penis
6. CASE 5
A 61‐year‐old male patient was admitted to the hospital with complaints of fever, cough, and malaise. COVID‐19 PCR test was positive. Our case who had HT and DM history in his anamnesis referred to the emergency service on the 15th day of his illness with the development of cyanosis, coldness and pain in the distal 2, 3, 4, and 5th fingers of the right hand. Hypoechoic thrombus material allowing partial flow in the ulnar artery lumen in the 1/3 distal part of the right forearm and monophasic flow in the distal right ulnar artery were detected in Doppler USG. Although there was a temporary improvement with low molecular weight heparin (LMWH) and iloprost treatment, embolectomy was performed because there was an increase in cyanosis, but the thrombus could not be removed. Necrosis developed in the finger tip (Figure 5).
FIGURE 5.
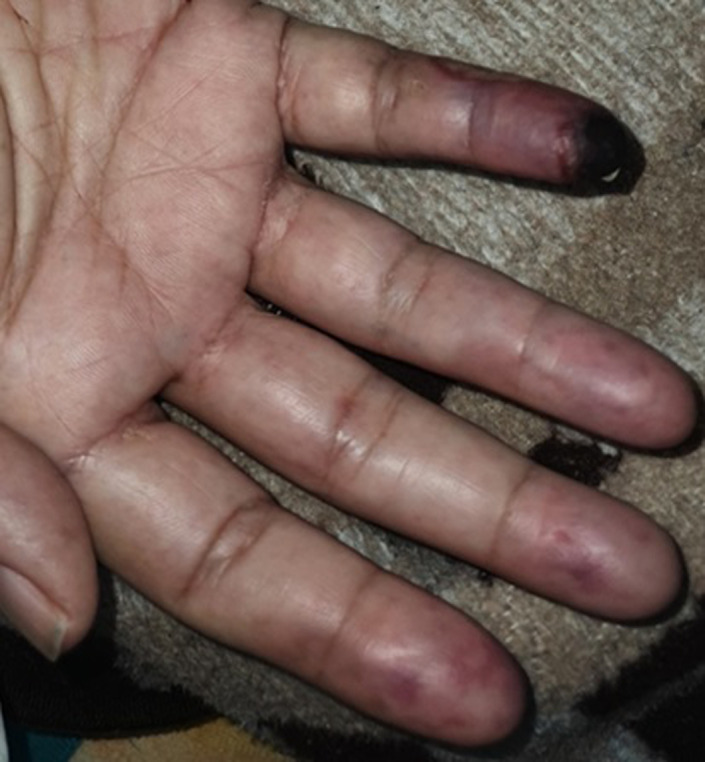
Clinical view of case 5, necrosis on the distal of right hand fifth finger
7. DISCUSSION
We present five COVID‐19 patients with acro‐ischemic lesions in the hope that they may be helpful in diagnosing the disease and predicting prognosis. One of the patients was a child, the other four patients were older than 60 years of age and all of the patients had at least one comorbid disease. Except for the child patient, the patients did not have a severe clinic. All of our patients were found to have PCR test positivity. Following the regression and disappearance of the livedoid lesions in the extremities, necrosis developed on finger tips. While all of our patients had finger/toe necrosis, one of our patients also had penile necrosis and two of our patients also had skin necrosis (Table 1). Radiological imaging revealed signs of thrombosis in the extremity vessels, except for one patient. One of our patients had thrombosis in the right popliteal artery leading to complete occlusion (Table 1). Leukocytoclastic vasculitis was found in the pathological examination of one of our patients who was presented with acro‐ischemic findings and skin rashes without typical COVID‐19 symptoms.
TABLE 1.
Patient's demographic, medical information, and timeline showing the clinical course of patients (onset of symptoms, occurrence of necrosis, time of diagnosis of covid‐19). The day of onset of symptoms was accepted as Day 1
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
|
Age/Gender Comorbidity |
61/F HT |
82/F RA |
8/M ALL, Down Syndrome |
71/M DM, CRF |
61/M HT, DM |
| Diagnosis methods and time |
19th day: Chest CT (+) 31st day: PCR (+) |
3th day: PCR and Chest CT (+) |
36th day: PCR and Chest CT (+) |
2nd day: PCR and Chest CT (+) |
5th day: PCR (+) |
|
COVID−19 symptoms and onset day |
Asymptomatic |
1st day Fever, cough, malaise |
1st day Fever, shortness of breath |
1st day Fever, malaise |
1st day Fever, cough, malaise |
|
Others symptoms and onset day |
1st day; Pain in hands and feet, bruising on finger tips, livedoid lesions on the hands (Figure 1A) 9th day; hemorrhagic bulla on the sole of the feet (Figure 1B) 16th day; painful purpuric rashes on feet and legs and occasional hemorrhagic bullae (Figure 1c, d) |
28th day Pain, coldness, and bruising in the right lower extremity |
21st day Bruising on finger and toe tips |
10th day Pain in hands and feet Bruising on finger tips Livedoid lesions on the hands |
15th day Pain, coldness and livedoid lesion on hands, bruising on finger tips |
| Necrotic lesions and onset day | |||||
| Finger/toe necrosis |
19th day Right hand 1,2 and 3th; left hand 1 and 2nd fingers (Figure 1I, J) |
39th day Right foot 1st and 2nd toe tips (Figure 2C) |
31st day Left hand 2nd finger (Figure 3A) Right foot 1st toe (Figure 3B) |
18th day Right hand 1,2,3 and 5th fingers (Figure 4A,B) |
28th day Right hand 2,3,4 and 5th fingers (Figure 5) |
| Skin necrosis |
33th day Bilateral lower leg and feet(Figure 1E,F,G,H) |
45th day Right lower leg (Figure 2A,B,C) |
– | – | – |
| Penis necrosis | – | – | – | 18th day Glans penis (Figure 4C) | – |
|
Extremity vascular evaluation Doppler or CT angio |
Doppler No blood flow in the left anterior tibial artery. |
CT angio Right popliteal artery and bilateral deep femoral arteries, totally occluded; no blood flow Left popliteal artery, occluded; partial blood flow |
Doppler bilateral radial and ulnar arteries, low velocity monophasic flow right dorsalis pedis and posterior tibial artery, low velocity monophasic flow |
Doppler Normal |
Doppler hypoechoic thrombus material was observed in the lumen of the right ulnar artery, which allowed partial blood flow |
| Laboratory findings | |||||
|
Lenfosit (1.3–3.5/ 103ul) |
0.47 | 0.40 | 0.43 | 1.4 | 2.82 |
|
Ferritin (22–322 ng/ml) |
396 | 44 | – | 201 | – |
|
CRP (0–0.5 mg/dl) |
13.8 | 2.4 | – | – | 11.74 |
|
PCT (0–0.5 ng/ml) |
0.11 | – | – | 0.28 | – |
|
PLT (145–366 103/ml) |
490 | 111 | 143 | 234 | 506 |
|
PT (10–14sn) |
11.2 | 28 | 15.6 | 13.9 | – |
|
D–dimer (0–0.55 mg/ml) |
2.24 | 5.63 | – | 1.78 | – |
Abbreviations: ALL, acute lymphocytic leukemia; CRF, chronic renal failure; CRP, C reactive protein; CT, computerized tomography; DM, diabetes mellitus; F, female; HT, hypertension; M, male; PCR, polymerase chain reaction PCT, procalcitonin; PLT, platelet; PT, prothrombin time; RA, rheumatoid arthritis.
The most common symptoms of COVID‐19 disease are fever, dry cough, headache, malaise, and muscle pain. Most of the patients have bilateral pneumonia. All of our patients were found to have involvement in chest tomography except one. The gold standard in the diagnosis of COVID‐19 is the PCR examination of swab samples taken from the throat. 10 PCR tests were positive in all of our patients.
SARS‐CoV‐2 uses angiotensin converting enzyme 2 receptors to infect the endothelium and causes inflammatory reaction. Inflammatory markers such as C reactive protein (CRP) and procalcitonin (PCT) increased in our patients (Table 1). This causes microvascular damage and abnormal activation of the coagulation system, resulting in increased vascular inflammation and thrombosis. 11 D‐dimer increases in our patients showed an increased tendency to coagulation (Table 1). Thromboses can be arterial, venous, and microvascular. Although thromboses are mostly reported to be venous, arterial thromboses were found in our patients. All of our patients were given low molecular weight heparin (LMWH), which has anti‐inflammatory properties and anticoagulant properties and which has taken its place in routine treatment. 12 , 13 However, in one study, in 90% of COVID‐19 patients with thrombotic complications developed thrombosis despite the use of LMWH. Although prophylaxis is thought to decrease thrombotic events and be effective on mortality, it has been observed that it does not eliminate this risk completely. 14 Rethrombosis developed even in patients who received intense anticoagulant therapy. 15 It has been reported that LMWHs used in patients with acro‐ischemia decrease D‐dimer and fibrinogen levels but does not cause a change in their clinic. 16 Although LMWH was used in all our patients, no significant improvement was observed in their clinics. Our results are in parallel with the literature.
Acral ischemic lesions occur as a result of increased inflammation and cutaneous thrombosis. 17 Pernio, one of the acral ischemic lesions, occurs due to increased inflammation. However, livedoid lesions occur due to cutaneous thrombosis and may also develop as precursor lesions of COVID‐19. 9 , 18 It is reported that acro‐ischemic lesions (livedo‐necrosis) and thromboses develop more frequently in individuals who are older, who have comorbid diseases and severe clinic. 9 , 17 One of our patients was an 8‐year‐old child patient and all other patients were 60 years old and older. All of our patients had comorbid diseases (Table 1). In their study, Karagounis et al. published 21 patients purpura and/or necrotic ulceration in the ear, face, distal extremities, and genitalia. It was reported that 19 of these patients were intubated and 17 had a triggering factor (constant contact and pressure of medical instruments) that caused lesion. 19 Two of our patients developed skin necrosis, while one developed penile necrosis, and none were found to have a cause that could trigger necrosis. Unlike studies which reported that acro‐ischemic lesions and thromboses were more frequent in patients with severe clinic 9 , 17 , 18 and associated with high mortality rates, 20 none of our patients were intubated except our child patient. This difference can be interpreted as skin lesions may be seen in different clinical severity in different genetic structures and geographies. Our results mostly supported the results of Sachdeva et al. 21 who reported that there was no correlation between COVID‐19 severity and the lesions. All but one of the patients had signs of thrombosis in Doppler USGs (Table 1). In this respect, it is possible to mention a correlation between acro‐ischemic findings and thrombosis.
In one of our patients, necrosis of the penis was detected along with finger necrosis. To the best of our knowledge, this is the second covid‐19 related penile necrosis case in the literature after the publication of Sarkis et al. 22
It is reported that necrotic lesions are more common in the distal leg areas where hydrostatic pressure is high. 23 In two of our patients who had skin necrosis, necrosis was in the distal of the leg in line with literature. Livedoid lesions seen in COVID‐19 patients are reported to be transient, 18 and they indicate vascular occlusion. 21 In parallel with the literature, livedoid lesions on the palms and pernio‐like lesions on the fingers were observed in our patients who had finger necrosis. While livedoid lesions regressed within 10–14 days, the circulation on the finger tips deteriorated gradually and resulted in necrosis. We believe that livedo may be a precursor lesion which may result in necrosis in terms of showing acral ischemia.
One of the different skin lesions associated with COVID‐19 is LCV. It is a vasculitis that involves small vessels and has many etiological factors (infection, autoimmune diseases, malignancies, and drugs). It is characterized by immune complex deposition in postcapillary venules, fibrinoid necrosis, and leukocytoclasia. It presents with symmetrically distributed palpable purpura which mostly involves the lower leg. In our patient (case 1), the lesion which started as purpura, turned into necrotic bullae that merged with each other as it progressed. The pathology of the incisional biopsy taken was LCV. We can see different results in literature when we examine COVID‐19 patients with LCV. In a study by Mayor et al., when PCR from nasopharyngeal swab was negative, unlike the previous study, PCR from tissue sample was positive in the study by Gomez et al. 24 , 25 Our study is not in parallel with both studies. While the PCR from nasopharyngeal swab was negative when biopsy was taken, PCR from tissue sample was negative too. Our patient did not have any complaints suggesting COVID‐19 such as fever, shortness of breath, and malaise. The chest CT taken when admitted for rashes was found to have COVID‐19 pneumonia findings, and the patient was found to have PCR positivity 12 days later. In both studies, the lesions showed 1 month after COVID‐19‐like complaints. In our patient, lesions occurred 3 days before COVID‐19 diagnosis. This situation was explained in literature as that skin lesions associated with COVID‐19 may occur before, during and after COVID‐19 diagnosis. Almost 70% of the lesions start after COVID‐19 diagnosis. 21 Consistent with the literature, 60% of our patients (3 patients) had skin lesion after diagnosis and 40% (2 patients) had skin lesion before diagnosis (Table 1).
Skin lesions can sometimes be the only symptom of COVID‐19. In this respect, recognizing acro‐ischemic lesions, which are skin lesions, may help in the early diagnosis of the disease and in predicting the prognosis. In patients with skin lesions without typical symptoms, the diagnosis of COVID‐19 should be kept in mind.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
ETHICAL APPROVAL
Written informed consent was obtained from each patient for publication of this study and associated digital images.
Ozbey R, Algan MF. Acro‐ischemic lesions in COVID‐19 patients: A case series. J Cosmet Dermatol. 2022;21:1822–1829. doi: 10.1111/jocd.14893
Funding information
There is no funding for this study
Contributor Information
Rafet Ozbey, Email: rafetozbey@gmail.com.
Mehmet Fatih Algan, Email: mfatihalgan@hotmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. World Health Organization. Coronavirus Disease 2019 (COVID‐19) Situation Report—63 . World Health Organization. 2020. www.who.int/docs/default‐source/coronaviruse/situationreports/20200323‐sitrep‐63‐covid‐19.pdf?sfvrsn=d97cb6dd_2. Accessed April 6, 2020.
- 2. Baydemır M. Investıgatıon of the effect of corona precautions on transmission prevention of upper respiratory tract infections and statistical comparison. International Research in Medical and Health Sciences. 2021;4(3):25‐32. [Google Scholar]
- 3. Katneni UK, Alexaki A, Hunt RC, Schiller T, DiCuccio M, Buehler PW, Ibla JC, Kimchi‐Sarfaty C. Coagulopathy and thrombosis as a result of severe COVID‐19 infection: a microvascular focus. Thromb Haemost. 2020;120(12):1668‐1679. doi: 10.1055/s-0040-1715841. Epub 2020 Aug 24. PMID: 32838472; PMCID: PMC7869056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Virol. 2020;127:104371. 10.1016/j.jcv.2020.10437112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verma V, Vishwakarma RK, Verma A, Nath DC, Khan HTA, Navaneetham K. Time‐to‐death approach in revealing chronicity and severity of COVID‐19 across the world. PLoS One. 2020;15(5):e0233074. 10.1371/journal.pone.023307413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit CareMed. 2020;48(9):1358‐1364. 10.1097/ccm.0000000000004458. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brake S, Barnsley K, Lu W, et al. Smoking upregulates angiotensin‐ Converting enzyme‐2 receptor: a potential adhesion site for novel coronavirus SARS‐ CoV‐2 (Covid‐19). JMC. 2020;9(3):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111(20):2605‐2610. 10.1161/CIRCULATIONAHA.104.510461. Epub 2005 May 16 PMID: 15897343. [DOI] [PubMed] [Google Scholar]
- 9. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71‐77. 10.1111/bjd.19163. Epub 2020 Jun 10. PMID: 32348545; PMCID: PMC7267236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(1): 23‐30. 10.2807/1560-7917.ES.2020.25.3.2000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033‐2040. 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becker RC. COVID‐19 update: Covid‐19‐associated coagulopathy. Journal of Thrombosis and Thrombolysis. 2020;50(1):54‐67. 10.1007/s11239-020-02134-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muñoz‐Rivas N, Abad‐Motos A, Mestre‐Gómez B, et al. Infanta Leonor Thrombosis Research Group. Systemic thrombosis in a large cohort of COVID‐19 patients despite thromboprophylaxis: a retrospective study. Thromb Res. 2021;199:132‐142. 10.1016/j.thromres.2020.12.024. Epub 2021 Jan 7. PMID: 33503547; PMCID: PMC7787910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomez‐Arbelaez D, Ibarra‐Sanchez G, Garcia‐Gutierrez A, et al. COVID‐19‐related aortic thrombosis: a report of four cases. Ann Vasc Surg. 2020;67:10‐13. 10.1016/j.avsg.2020.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ ischemia. Zhinghua Xue Xue Za Zhi. 2020;41:E006. [DOI] [PubMed] [Google Scholar]
- 17. Carrascosa JM, Morillas V, Bielsa I, Munera‐Campos M. Cutaneous Manifestations in the Context of SARS‐CoV‐2 Infection (COVID‐19). Actas Dermosifiliogr. 2020;111(9):734‐742. 10.1016/j.adengl.2020.10.001. Epub 2020 Aug 31. PMID: 32882184; PMCID: PMC7560260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID‐19: transient livedo reticularis. J Am Acad Dermatol. 2020;83(2):700. 10.1016/j.jaad.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karagounis TK, Shaw KS, Caplan A, Lo Sicco K, Femia AN. Acrofacial purpura and necrotic ulcerations in COVID‐19: a case series from New York City. Int J Dermatol. 2020;59(11):1419‐1422. 10.1111/ijd.15181. Epub 2020 Sep 23. PMID: 32966592; PMCID: PMC7537226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tan SW, Tam YC, Oh CC. Skin manifestations of COVID‐19: a worldwide review. JAAD Int. 2021;19:119‐133. 10.1016/j.jdin.2020.12.003. Epub 2020 Dec 16. PMID: 33479703; PMCID: PMC7754879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID‐19: report of three cases and a review of literature. J Dermatol Sci. 2020;98(2):75‐81. 10.1016/j.jdermsci.2020.04.011. Epub 2020 Apr 29. PMID: 32381430; PMCID: PMC7189855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sarkis P, Sarkis J, Alkassis M, Assaf J, El Gharib K. Penile ischaemia secondary to COVID‐19: why should the dermatologist be concerned? J Eur Acad Dermatol Venereol. 2021;35(8):e487‐e489. 10.1111/jdv.17287. Epub 2021 May 2. PMID: 33866610; PMCID: PMC8251477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID‐19‐associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118‐1129. 10.1016/j.jaad.2020.06.1016. Epub 2020 Jul 2. PMID: 32622888; PMCID: PMC7331510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayor‐Ibarguren A, Feito‐Rodriguez M, Quintana Castanedo L, Ruiz‐Bravo E, Montero Vega D, Herranz‐Pinto P. Cutaneous small vessel vasculitis secondary to COVID‐19 infection: a case report. J Eur Acad Dermatol Venereol. 2020;34(10):e541‐e542. 10.1111/jdv.16670. Epub 2020 Jul 3. PMID: 32441876; PMCID: PMC7280661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camprodon Gómez M, González‐Cruz C, Ferrer B, Barberá MJ. Leucocytoclastic vasculitis in a patient with COVID‐19 with positive SARS‐CoV‐2 PCR in skin biopsy. BMJ Case Rep. 2020;13(10):e238039. 10.1136/bcr-2020-238039. PMID: 33122236; PMCID: PMC7597471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


