Summary
There has been increasing concern about the long‐term impact of coronavirus disease 2019 (COVID‐19) as evidenced by anecdotal case reports of acute‐onset parkinsonism and the polysomnographic feature of increased rapid eye movement sleep electromyographic activity. This study aimed to determine the prevalence and correlates of dream‐enactment behaviours, a hallmark of rapid eye movement sleep behaviour disorder, which is a prodrome of α‐synucleinopathy. This online survey was conducted between May and August 2020 in 15 countries/regions targeting adult participants (aged ≥18 years) from the general population with a harmonised structured questionnaire on sleep patterns and disorders, COVID‐19 diagnosis and symptoms. We assessed dream‐enactment behaviours using the Rapid Eye Movement Sleep Behaviour Disorder Single‐Question Screen with an additional question on their frequency. Among 26,539 respondents, 21,870 (82.2%) answered all items that were analysed in this study (mean [SD] age 41.6 [15.8] years; female sex 65.5%). The weighted prevalence of lifetime and weekly dream‐enactment behaviours was 19.4% and 3.1% and were found to be 1.8‐ and 2.9‐times higher in COVID‐19‐positive cases, respectively. Both lifetime and weekly dream‐enactment behaviours were associated with young age, male sex, smoking, alcohol consumption, higher physical activity level, nightmares, COVID‐19 diagnosis, olfactory impairment, obstructive sleep apnea symptoms, mood, and post‐traumatic stress disorder features. Among COVID‐19‐positive cases, weekly dream‐enactment behaviours were positively associated with the severity of COVID‐19. Dream‐enactment behaviours are common among the general population during the COVID‐19 pandemic and further increase among patients with COVID‐19. Further studies are needed to investigate the potential neurodegenerative effect of COVID‐19.
Keywords: coronavirus disease 2019 (COVID‐19), dream‐enactment behaviour, neurodegeneration, prevalence, rapid eye movement sleep behaviour disorder (RBD)
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) is a global pandemic and has resulted in >270 million confirmed cases and 5.3 million deaths worldwide up to December 2021 (WHO COVID‐19 Dashboard, 2020). The COVID‐19 pandemic has extensive direct and indirect adverse effects on mood, sleep, and dreams/nightmares, not only among patients with COVID‐19 but also in the general population (Chung et al., 2021; Fränkl et al., 2021; Morin et al., 2021; Salari et al., 2020). Evidence from SARS‐CoV and Middle East respiratory syndrome CoV revealed a neurotropic nature of the infection (Li, Bai, & Hashikawa, 2020). Previous findings on long‐term consequences of SARS‐CoV showed that 40% of the survivors suffered from active psychiatric illness and chronic fatigue syndrome 4 years after the infection (Lam et al., 2009). Thus, it is conceivable that the same betacoronavirus clade (SARS‐CoV‐2) may also associate with neuropsychiatric symptoms/disorders, both during the acute and long COVID‐19 (referred to as “long COVID”) stages (Boldrini, Canoll, & Klein, 2021; Brundin, Nath, & Beckham, 2020).
Accumulating evidence indicate that olfactory impairment is a common presenting symptom of COVID‐19, affecting up to 80% of the cases (von Bartheld, Hagen, & Butowt, 2020). Importantly, olfactory impairment is often regarded as a heralding feature of neurodegeneration including Parkinson's disease (PD) (Heinzel et al., 2019). Interestingly, there has been a few confirmed cases with acute onset of parkinsonism supported by neuroimaging evidence of dopamine dysfunction shortly after SARS‐CoV‐2 infection (Cohen et al., 2020; Faber et al., 2020; Mendez‐Guerrero et al., 2020). Although it is intriguing that parkinsonism could develop in such an acute manner and with the age‐onset of parkinsonism being much younger (35–53 years) than the typical age of onset (Cohen et al., 2020; Faber et al., 2020; Mendez‐Guerrero et al., 2020), these findings forewarn of a possible surge of neurodegeneration, including PD, during the long COVID‐19 stage (Brundin et al., 2020).
Rapid eye movment (REM) sleep behaviour disorder (RBD) is a distinct parasomnia characterised by recurrent dream‐enactment behaviours (DEBs) and REM sleep without atonia. RBD is regarded as the most specific precursor of α‐synucleinopathies, such as PD, as >90% of patients with RBD will convert to clinically diagnosed α‐synucleinopathies within 15 years (Galbiati et al., 2019; Postuma et al., 2019). Thus, a major consideration is on how the SARS‐CoV‐2 potentially influences the development of RBD features that has a close relationship with parkinsonism. A recent study found that 36% (four out of 11) of patients with COVID‐19 presented with REM sleep without atonia on polysomnographic recording (Heidbreder et al., 2021), which suggested that SARS‐CoV‐2 might potentially trigger RBD features.
To the best of our knowledge, there is a lack of epidemiological results of DEBs, which is a hallmark of RBD and their correlates during the COVID‐19 pandemic in both the general population and patients with COVID‐19. We conducted an international online survey, the International COVID‐19 Sleep Study (ICOSS), to investigate the impact of the COVID‐19 pandemic on sleep and daily rhythms in adults (Chung et al., 2021; Fränkl et al., 2021; Merikanto et al., 2021; Morin et al., 2021). In the present study, we aimed to determine the prevalence and correlates of DEBs during the pandemic.
2. METHODS
2.1. Study procedure and participants
This was a cross‐sectional online survey (ICOSS study) conducted during the COVID‐19 pandemic between May and August 2020 with 15 participating countries/regions: Austria, Brazil, Canada, Jilin (China), Finland, France, Germany, Hong Kong (China), Italy, Japan, Norway, Poland, Sweden, UK, and USA. The study protocol related to study objectives, questionnaires included, and study procedures have been published (Partinen et al., 2021).
2.2. Standard protocol approvals, registrations, and patient consents
The potential participants aged ≥18 years were invited to complete an online survey in their own language via local and national news, social media, and newsletters at universities and hospitals. The survey was completed anonymously. The participants who voluntarily agreed to participate in the survey gave their online consent for the purpose of this study. Each international principal investigator obtained approval or exemption from their local Ethics Committee prior to initiation of the survey.
2.3. Questionnaires
We used the following items from the ICOSS core questionnaire (Partinen et al., 2021):
Demographic characteristics (e.g., age, sex, education level, ethnics) data were collected in structured questions. By collecting smoking data, participants were asked about the frequency (“Never or less than once per month,” “Less than once per week,” “1–2 days per week,” “3–5 days per week,” and “Every day or almost daily”) of smoking (cigarettes, cigars, cigarillos) or use of snuff tobacco. We dichotomised the answers into “less than weekly” and “weekly use.” Drinking of alcohol beverages was defined as participants who drank one or more bottle/can of beer, strong beer, cider, wine, or spirits per week. Caffeine consumption was defined as participants who drank one or more cup of coffee, tea, cola drinks, or energy drinks per day. Physical activity level was measured by asking participants on how many hours per week they did physically workout. The workout options included walking, jogging/cycling, running, and other (e.g., swimming) with frequencies ranged from “Not at all” to “Altogether 4 h or more per week” for each workout option.
A COVID‐19 diagnosis confirmed by a “yes” answer to the question, “Have you had COVID‐19?” Participants answering “I don't know” (18.2%) were coded as having no self‐reported COVID‐19 disease. Among those reporting COVID‐19, we further surveyed the severity of the disease by allowing respondents to rate the severity of disease (“mild,” “moderate,” “severe,” and “life threatening”) and whether they were hospitalised for the disease (Chung et al., 2021). As only few participants had “life threatening” COVID‐19, we put this category into “severe” group to facilitate the data analysis. We also surveyed the presence of the series of COVID‐19 symptoms: chills, cough, excessive sleepiness, fatigue, fever, gastrointestinal symptoms, headache, loss of taste and smell, muscle pain, running nose, shortness of breath, difficulty breathing, and sore throat.
The RBD Single‐Question Screen (RBD1Q) was employed to assess lifetime DEBs (Postuma et al., 2012). In detail, respondents were asked, “Have you ever been told, or suspected yourself, that you seem to ‘act out your dreams’ while asleep (for example, punching, flailing your arms in the air, making running movements, etc.)?” (Postuma et al., 2012). The sensitivity and specificity were 93.8% and 87.2% respectively when RBD1Q was used in clinical setting to differentiate patients with RBD from controls (Postuma et al., 2012). Besides the RBD1Q, we also surveyed the frequency of the DEBs symptoms on a 5‐point scale: “never or <1/month”, “<1/week”, “1–2 nights/week”, “3–5 nights/week”, and “every night or almost every night.” We further dichotomised the frequency into “weekly” and “non‐weekly.” This may help to capture recurrent weeks DEBs, which would suggest a higher likelihood of harbouring genuine RBD features.
The STOP (Snoring, Tiredness, Observed apnea, high blood Pressure) questionnaire to screen for obstructive sleep apnea (OSA) (Chung et al., 2021; Chung et al., 2008).
The Patient Health Questionnaire for Depression and Anxiety (PHQ‐4) to measure anxiety and depressive symptoms (Kroenke, Spitzer, Williams, & Lowe, 2009).
A two‐item self‐report measure to evaluate post‐traumatic stress disorder (PTSD) symptoms (Lang et al., 2012).
Sense of smell was assessed by two questions. The first question was “How do you rate your own sense of smell (olfactory sense)?” and the seven responses were set and ranged from “extremely good and sensitive” to “cannot smell almost anything.” The second question asked about the sense of smell related to COVID‐19. The responses included: “no change,” “worse than before infection”, “worsened and returned back”, “cannot smell almost anything.” We classified the responses “worse than before infection” and “cannot smell almost anything” as olfactory impairment.
Physical activity level was assessed by calculating weekly time spent on doing walking, jogging or cycling, running, or others (i.e., swimming). The options ranged from none to 4 h or more on a 5‐point scale.
Other questions related to insomnia symptoms, sleep pattern, circadian preferences, and OSA symptoms were reported separately (Chung et al., 2021; Fränkl et al., 2021; Merikanto et al., 2021; Morin et al., 2021).
2.4. Statistical analysis
The data of this study are presented as mean ± standard deviation (SD) if they fitted normal distribution or number (%) when appropriate. Cases with missing data were excluded in the analyses. The data were weighted by using the post‐stratification weighting method. The weighting index for age and sex was calculated separately based on the age and sex distribution of the population in each country/region (World Population Prospects 2019, United Nations, 2021), and then multiplied together to get the weighting index for each participant.
Chi‐square tests were employed to test the difference in the prevalence of lifetime DEBs and weekly DEBs among different subgroups. To estimate the prevalence of DEBs across different countries/regions, we conducted further meta‐analysis. To explore the correlates for DEBs, binary logistic regression models were fitted with DEBs as the dependent variable and each single factor as independent variables after adjusting for age and sex. As the score for physical activity level was not validated, we put the four items into the Principal Components Analysis to generate one score that represents the level of physical activities. To investigate the association of COVID‐19 symptoms with DEBs among COVID‐19‐positive participants, we used logistic regression models among participants with COVID‐19 diagnosis. The odds ratio (OR) and the related 95% confidence interval (CI) for each independent variable was calculated. We further performed the Holm's Sequential Bonferroni Procedure to deal with familywise error rates for multiple comparisons among various COVID‐19 symptoms.
We performed further sensitivity analysis after excluding data from Brazil and the USA, which had the highest prevalence of DEBs, respectively. All the analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22.0 (IBM Corp.) and Stata (StataCorp. 2021, Release 17). A two‐tailed p < 0.05 was considered statistically significant.
3. RESULTS
A total of 26,539 participants responded to the ICOSS survey. Among them, 21,870 (82.2%) had complete data of DEBs and COVID‐19 diagnosis. The participants with missing data were older (mean [SD] age 42.7 [16.0] versus 41.6 [15.8] years, p < 0.001) and were more often females (72.7% versus 65.5%, p < 0.001) than those with valid data. The weighted prevalence of DEBs and weekly DEBs was 19.4% and 3.1% in the study sample, respectively (Table 1).
TABLE 1.
Demographic characteristics and stratified prevalence of lifetime and weekly dream‐enactment behaviours
| n(%) | Prevalence of lifetime DEBs | Prevalence of weekly DEBs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Raw, % | p | Weighted, % | p | Raw, % | p | Weighted, % | p | ||
| Overall sample | 21,870 (100) | 21.0 | 19.4 | 3.4 | 3.1 | ||||
| Strata for age (years) | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| 18–39 | 10,894 (50.7) | 24.9 | 25.2 | 3.9 | 3.9 | ||||
| 40–49 | 3,774 (17.6) | 19.4 | 20.5 | 3.5 | 3.8 | ||||
| 50–59 | 3,207 (14.9) | 19.4 | 20.2 | 3.2 | 3.1 | ||||
| >60 | 3,619 (16.8) | 13.1 | 12.9 | 1.9 | 2.0 | ||||
| Missing data | 376 (1.7) | ||||||||
| Strata for sex | 0.08 | <0.001 | 0.11 | <0.001 | |||||
| Male | 7,545 (34.5) | 21.6 | 20.9 | 3.6 | 3.5 | ||||
| Female | 14,288 (65.5) | 20.6 | 18.0 | 3.2 | 2.7 | ||||
| Missing data | 37 (0.1) | ||||||||
| Education, university level or above | <0.001 | 0.002 | 0.43 | 0.79 | |||||
| Yes | 14,126 (64.6) | 20.3 | 18.9 | 3.4 | 3.2 | ||||
| No | 7,458 (34.1) | 22.4 | 20.7 | 3.2 | 3.1 | ||||
| Missing data | 286 (1.3) | ||||||||
| Ethnicity | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Caucasian/White | 9,222 (42.2) | 22.0 | 19.7 | 4.0 | 3.6 | ||||
| Asian | 9,023 (41.3) | 17.7 | 16.9 | 1.4 | 1.5 | ||||
| African, Hispanic, and others | 3,293 (15.1) | 27.2 | 26.7 | 6.9 | 6.6 | ||||
| Missing data | 332 (1.5) | ||||||||
Abbreviation: DEBs, dream‐enactment behaviours.
Age was inversely associated with the prevalence of both DEBs and weekly DEBs (lifetime DEBs ranged from 25.2% in participants aged 18–39 years to 12.9% in participants aged >60 years, p < 0.001; and weekly DEBs ranged from 3.9% in those aged 18–39 to 2.0% in those >60 years, p < 0.001). Male sex showed higher prevalence of DEBs (20.9% versus 18.0%, p < 0.001) and weekly DEBs (3.5% versus 2.7%, p < 0.001) than female sex. Higher education level was associated with a lower prevalence of lifetime DEBs (university level: yes 18.9% versus no 20.7%, p < 0.001) but not weekly DEBs. Participants who were African, Hispanic, and other ethnicities (26.7%) reported the highest prevalence of lifetime DEBs, followed by Caucasian (19.7%) and Asian (16.9%) participants (p < 0.001; Table 1). A similar trend was found for weekly DEBs (6.6% versus 3.6% versus 1.5%, p < 0.001; Table 1).
The prevalence of lifetime DEBs ranged from the lowest in Jilin, China (6.1%) to the highest in Brazil (33.6%) and weekly DEBs ranged from the lowest in Germany (1.1%) to the highest in the USA (10.1%). The meta‐analysis found a high value of I 2 for both lifetime (I 2 = 96.2%) and weekly DEBs (I 2 = 94.6%), suggesting a high degree of heterogeneity for the prevalence of DEBs across different countries (Figure 1).
FIGURE 1.
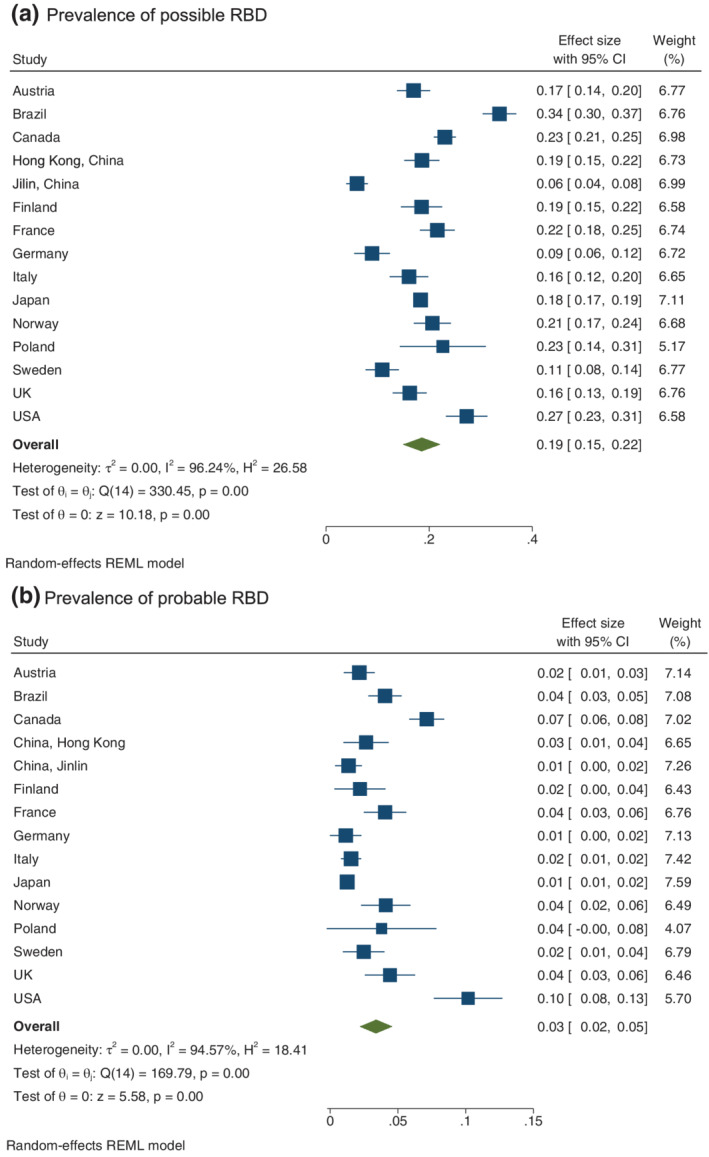
Weighted prevalence of possible and probable rapid eye movment sleep behaviour disorder (RBD) in different countries/regions
Weekly smoking and alcohol drinking were associated with higher rate of both frequency of DEBs after adjusted for age and sex. The ORs ranged from 1.28 to 2.66. Intriguingly, daily caffeine consumption was found to be associated with higher rate of lifetime DEBs (OR 1.14, 95% CI 1.04–1.24) but lower rate of weekly DEBs (OR 0.60, 95% CI 0.50–0.71). Physical activity level was not associated with lifetime DEBs; however, it was found to be positively associated with weekly DEBs (OR 1.37, 95% CI 1.28–1.47).
In the overall sample of this study, 650 participants (3%) reported having a diagnosis of COVID‐19. COVID‐19‐positive cases were younger than those without COVID‐19 (mean [SD] age 42.6 [15.4] versus 47.5 [18.0] years, p < 0.001). The prevalence of COVID‐19 diagnosis decreased with age in the sample (3.4% among participants aged 18–49 years; 2.5% among participants aged 50–59 years; and 1.7% among participants aged ≥60 years). There was no difference in the sex distribution between participants with and without COVID‐19. Participants with a COVID‐19 diagnosis had higher rate of lifetime DEBs (29.2% versus 19.0%; OR 1.66, 95% CI 1.39–1.99) and weekly DEBs (8.1% versus 2.9%; OR 2.79, 95% CI 2.06–3.76) when compared to those without a COVID‐19 diagnosis. Among these COVID‐19 participants, their disease severity was as follows: 41.0% were mild, 44.6% were moderate, and 14.3% were severe. There was an increasing trend of weekly DEBs across the severity of COVID‐19 from 6.3% in mild cases, 13.6% in moderate, and 16.7% in severe cases (p for linear‐by‐linear association: 0.003). There was no significant association between lifetime DEBs and severity of COVID‐19. Participants who were hospitalised had a higher rate of lifetime DEBs (49.5% versus 27.4%, p < 0.001) and weekly DEBs (23.8% versus 7.8%, p < 0.001) than participants who were not hospitalised (Table 2).
TABLE 2.
Correlates of lifetime and weekly dream‐enactment behaviours
| Correlate | Lifetime DEBs | Weekly DEBs | ||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) a | Unadjusted OR (95% CI) | Adjusted OR (95% CI) a | |
| Among overall sample (n = 21,870) | ||||
| Smoking weekly, yes versus no | 1.50 (1.38–1.63)*** | 1.41 (1.30–1.53)*** | 2.66 (2.26–3.13)*** | 2.49 (2.11–2.94)*** |
| Alcohol drinking weekly, yes versus no | 1.25 (1.17–1.34)*** | 1.28 (1.19–1.37)*** | 1.55 (1.32–1.81)*** | 1.54 (1.31–1.82)*** |
| Caffeine taking daily, yes versus no | 1.04 (0.95–1.13) | 1.14 (1.04–1.24)** | 0.56 (0.47–0.67)*** | 0.60 (0.50–0.71)*** |
| Physical activity level | 1.07 (1.04–1.11)*** | 1.03 (1.00–1.07) | 1.42 (1.33–1.52)*** | 1.37 (1.28–1.47)*** |
| Among restricted participants | 1.08 (1.03–1.14)** | 1.05 (1.01–1.11)* | 1.43 (1.32–1.56)*** | 1.42 (1.30–1.55)*** |
| Among no restricted participants | 1.03 (0.99–1.08) | 1.00 (0.95–1.05) | 1.27 (1.13–1.42)*** | 1.20 (1.07–1.34)*** |
| Olfactory impairment, yes versus no | 2.39 (1.86–3.07)*** | 2.09 (1.62–2.69)*** | 4.96 (3.42–7.19)*** | 4.32 (2.97–6.28)*** |
| PHQ‐4 score | 1.16 (1.15–1.17)*** | 1.15 (1.14–1.16)*** | 1.29 (1.27–1.32)*** | 1.29 (1.27–1.32)*** |
| STOP score | 1.15 (1.13–1.16)*** | 1.16 (1.15–1.18)*** | 1.28 (1.25–1.31)*** | 1.30 (1.27–1.33)*** |
| PTSD score | 1.26 (1.25–1.28)*** | 1.25 (1.23–1.27)*** | 1.47 (1.43–1.52)*** | 1.47 (1.43–1.52)*** |
| COVID‐19 diagnosis, yes versus no | 1.74 (1.46–2.08)*** | 1.66 (1.39–1.99)*** | 2.92 (2.16–3.94)*** | 2.79 (2.06–3.76)*** |
| Among participants who reported having COVID‐19 (n = 549) | ||||
| COVID‐19 severity | ||||
| Mild | Reference | Reference | Reference | Reference |
| Moderate | 1.01 (0.69–1.48) | 1.13 (0.76–1.67) | 2.34 (1.23–4.43)** | 3.04 (1.54–6.00)** |
| Severe | 1.17 (0.68–2.00) | 1.28 (0.74–2.24) | 2.96 (1.34–6.54)** | 3.73 (1.61–8.64)** |
| Hospitalisation, yes versus no | 1.72 (1.08–2.73)* | 1.77 (1.10–2.84)* | 3.00 (1.56–5.78)** | 3.10 (1.59–6.03)** |
Abbreviations: CI, confidence interval; COVID‐19, coronavirus disease 2019; DEBs, dream‐enactment behaviours; OR, odds ratio; PHQ, Patient Health Questionnaire; PTSD, post‐traumatic stress disorder; STOP, questionnaire for screening Snoring, Tired, Observed stop breathing, and high blood Pressure.
Binary logistic model adjusted for age and sex.
p < 0.05.
p < 0.01.
p < 0.001.
Lifetime DEBs were significantly associated with almost all symptoms of COVID‐19, even after performing correction for multiple comparisons in the adjusted model (Table 3). However, only chills, gastrointestinal symptoms, running rose, respiratory symptoms, and sore throat were found to be significantly associated with weekly DEBs after performing correction for multiple comparisons in the adjusted model.
TABLE 3.
Association of coronavirus disease 2019 (COVID‐19) symptoms with lifetime and weekly dream‐enactment behaviours
| Lifetime DEBs (n = 428) | Weekly DEBs (n = 428) | |||
|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) a | Unadjusted OR (95% CI) | Adjusted OR (95% CI) a | |
| Chills | 2.71 (1.62–4.53)** | 3.31 (1.94–5.64)** | 8.54 (1.95–37.41)** | 10.24 (2.31–45.33)** |
| Cough | 1.43 (0.87–2.36) | 1.56 (0.93–2.59) | 3.32 (1.06–10.45) | 3.59 (1.13–11.36) |
| Excessive sleepiness | 1.85 (1.07–3.20) | 2.20 (1.25–3.86)* | 1.69 (0.65–4.44) | 2.00 (0.75–5.33) |
| Fatigue | 2.58 (1.21–5.51) | 3.00 (1.38–6.50)** | 6.73 (0.74–61.46) | 7.86 (0.85–72.42) |
| Fever | 1.83 (1.04–3.23) | 2.26 (1.26–4.06)* | 2.92 (0.87–9.77) | 3.56 (1.04–12.11) |
| Gastrointestinal symptoms (e.g., diarrhoea, stomach pain) | 2.38 (1.43–3.96)** | 2.55 (1.52–4.28)** | 8.88 (1.98–39.81)** | 9.60 (2.13–43.22)** |
| Headache | 2.78 (1.56–4.98)** | 2.56 (1.42–4.61)** | 3.19 (1.00–10.14) | 3.02 (0.94–9.70) |
| Loss of taste or smell | 2.47 (1.43–4.25)** | 2.73 (1.57–4.74)** | 3.23 (1.02–8.99) | 3.27 (1.09–9.77) |
| Muscle pain | 1.66 (0.97–2.86) | 1.85 (1.06–3.21) | 2.09 (0.73–5.98) | 2.23 (0.77–6.45) |
| Running nose | 1.97 (1.24–3.13)* | 2.09 (1.30–3.35)** | 5.60 (1.80–17.42)** | 5.62 (1.79–17.63)** |
| Shortness of breath or difficulty breathing | 2.25 (1.39–3.66)** | 2.36 (1.44–3.87)** | 12.66 (2.35–68.25)** | 13.54 (2.50–73.26)** |
| Sore throat | 2.17 (1.35–3.50)** | 2.24 (1.38–3.63)** | 9.20 (2.20–38.40)** | 9.78 (2.33–41.02)** |
Abbreviations: CI, confidence interval; DEBs, dream‐enactment behaviours; OR, odds ratio.
Binary logistic model adjusted for age and sex.
Note: ***p < 0.001, **p < 0.01, and *p < 0.05 after adjustment by the Holm's Sequential Bonferroni Procedure for multiple comparisons.
Among COVID‐19‐positive cases, 39.9% reported olfactory impairment including 9.7% who felt worse than before, 7.8% who reported loss of smell, and 22.4% reported transient worsening of smell (Figure 2). However, among the general population participants who were free of COVID‐19, only 9.3% reported olfactory impairment (p < 0.001). When compared with participants with normal olfaction, those with persistent olfaction impairments (subjects felt worse than before or had loss of smell) had higher rate of lifetime DEBs (35.7% versus 19.1%; OR 2.09, 95% CI 1.62–2.69) and weekly DEBs (14.8% versus 2.9%; OR 4.32, 95% CI 2.97–6.28; Table 2).
FIGURE 2.
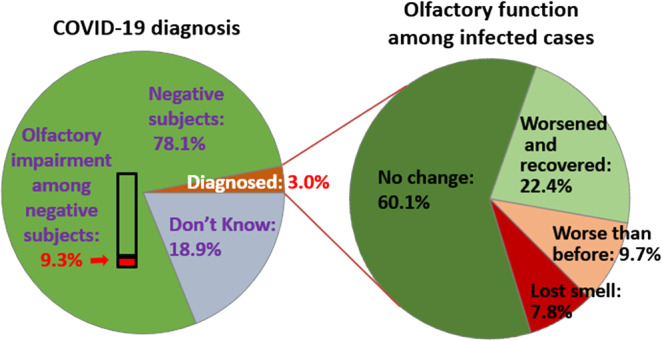
Olfactory impairment among participants stratified by coronavirus disease 2019 (COVID‐19) status
3.1. Sensitivity analyses
We performed sensitivity analysis after excluding data from Brazil for lifetime DEBs and the USA for weekly DEBs, as they had the highest prevalence of lifetime DEBs and weekly DEBs, respectively. Similar to the results in the full sample, meta‐analysis still found high heterogeneity of prevalence of lifetime (I 2 = 94.5%) and weekly DEBs (I 2 = 90.7%) among the remaining countries/regions. Binary logistic regression revealed a significant association between either lifetime or weekly DEBs and life‐style factors, COVID‐19 diagnosis, olfactory impairment, and higher scores on the PHQ‐4, STOP, and PTSD questionnaires (Table S1). However, COVID‐19 severity was not associated with weekly DEBs.
4. DISCUSSION
This large‐scale international online survey found that DEBs were quite prevalent in the general population during the COVID‐19 pandemic and were associated with factors including male sex, life‐style factors, olfactory impairment, depression and anxiety symptoms, OSA symptoms, and PTSD symptoms. In particular, we found that COVID‐19 diagnosis was associated with increased prevalence of DEBs. Moreover, COVID‐19‐related symptoms including chills, gastrointestinal symptoms, loss of taste and smell, running rose, respiratory symptoms and sore throat were significantly associated with DEBs. As one of the core symptoms of RBD, recurrent DEBs in a large‐scale epidemiological study may signify the potential occurrence of idiopathic/isolated RBD. In this regard, the finding of co‐occurrence of olfactory impairment and gastrointestinal symptoms with DEBs in our study replicated some previously known neurodegenerative and epidemiological correlates of idiopathic/isolated RBD in general population studies (Haba‐Rubio et al., 2018; Ma et al., 2017; Yao et al., 2018). Although our findings of the association of DEBs and COVID‐19 were novel, there is a need to investigate the association further, especially with the need for confirmatory polysomnographic studies in future.
When compared to previous studies, the prevalence of DEBs in the general population was strikingly high during the COVID‐19 pandemic. With various assessment tools, several previous epidemiological studies have revealed that the prevalence of DEBs in the general population ranged from 2.7% to 18.4% (Haba‐Rubio et al., 2018; Ma et al., 2017). Compared to studies that have employed the identical screening questionnaire as in our survey, the prevalence of DEBs in the present ICOSS survey was almost 2–4‐times higher than that of previous studies (ranged from 4.9% (Yao et al., 2018) to 9.9% (Shprecher et al., 2020) in previous research).
Several explanations may address the high prevalence of DEBs among the general population during the COVID‐19 pandemic. Firstly, a high proportion of these screened‐positive cases may likely turn out to be false‐positive cases for RBD (pseudo‐RBD), as the apparent DEB features might be mimicked by OSA (Iranzo & Santamaria, 2005), and the risk of OSA was found to be increased during the COVID‐19 pandemic (Chung et al., 2021). Additionally, other parasomnias and sleep‐related movement disorders, such as nightmare disorder and periodic limb movement disorder, may be mis‐recognised as RBD symptoms, as these sleep disorders are difficult to differentiate from RBD by the screening question (Bjorvatn, Gronli, & Pallesen, 2010).
We noted that the global population was suffering from a relatively high level of anxiety, depression, and PTSD symptoms during the COVID‐19 pandemic (Morin et al., 2021; Salari et al., 2020), which might have been associated with an abundance of distress that may culminate into the development of parasomnia (Barone, 2020). Previous studies suggested that parasomnia including RBD is common among the psychiatric population (Lam, Fong, Ho, Yu, & Wing, 2008; Wang et al., 2021). Traumatic events and PTSD have been reported to increase the odds of RBD (Elliott et al., 2020) and a global pandemic could be considered as a such. Additionally, several epidemiological studies found that PTSD was associated with DEBs in the general population (Ma et al., 2017; Yao et al., 2018). In fact, a transient form of RBD has been proposed to be related to a series of acute conditions including central nervous system diseases (e.g., encephalitis), drug consumption or alcohol withdrawal, and also possibly PTSD (Manni, Ratti, & Terzaghi, 2011).
A rather unexpected finding of the association of DEB with younger age is in contrast to the finding that increasing age is a risk factor for RBD (Zhang et al., 2022). In other words, there will be a distinct possibility that this young age group may suffer from DEB‐like episodes related to non‐REM (NREM) parasomnia during the COVID‐19 pandemic. In a series of 43 patients with sleepwalking and sleep terrors at a mean age of 26 years, short unpleasant dream‐like mentations could occur during NREM parasomnia (Oudiette et al., 2009). In addition, almost two‐thirds (62.8%) of patients reported at least one incidence of recalling mental content during the episode. Thus, in our present study, dream‐like mentations during NREM episodes may have been misclassified as DEBs as based on a single‐question screen assessment. Uguccioni et al. (2013) also postulated that different threat simulations may contribute to different types of parasomnia (i.e., natural disaster for NREM parasomnia versus human or animal aggression for REM parasomnia). It is possible that the catastrophic nature of the COVID‐19 pandemic might enhance the distress and sleep disruption that will contribute to the dream‐like mentation in the NREM parasomnia of young subjects. (Uguccioni et al., 2013; Merikanto et al., 2021; Morin et al., 2021).
Interestingly, DEBs were associated with a higher level of physical activity in our study. The lockdown or restriction during pandemic may potentially alter the physical activity pattern of participants and the possibility of reverse causality could not be excluded (Bu, Bone, Mitchell, Steptoe, & Fancourt, 2021). Nonetheless, most of the factors associated with DEBs of the present ICOSS study were consistent with previous general population studies, including male sex (Yao et al., 2018), smoking (Shrestha et al., 2018; Yao et al., 2018), alcohol consumption (Ma et al., 2017; Shprecher et al., 2020; Yao et al., 2018), olfactory impairment (Ma et al., 2017; Shprecher, Zhang, Halverson, & Savica, 2019; Shrestha et al., 2018), and mood and PTSD symptoms (Ma et al., 2017; Mahlknecht et al., 2015; Yao et al., 2018; Wang et al., 2021).
It is also worth noting that DEBs can be associated with narcolepsy, a sleep disorder characterised by excessive daytime sleepiness and which increased following the 2009 H1N1 flu pandemic (Han et al., 2011). Whether COVID‐19 and related vaccines will increase the incidence of narcolepsy remains unknown and deserves further investigation.
4.1. Limitations
Like other online surveys, a response bias is worth noting. There was a large proportion of younger and female responders. Thus, we applied a weighting method in the statistical analyses. Nevertheless, the older adult subjects or the patients with COVID‐19 with more severe symptoms may have less accessibility to the internet or be less capable of completing an online survey. Although this study included >20,000 participants, it was still underpowered to analyse the association between DEBs and COVID‐19 symptoms, as the number of cases with both conditions was limited. Additionally, we did not explore the chronological relationship between DEBs and COVID‐19. Thus, whether COVID‐19 led to the increase of DEBs or vice versa will need further investigation. In addition, the diagnosis and severity of COVID‐19 was based on self‐report, which might lead to potential reporting bias. Finally, we only employed one screening question to assess lifetime DEB (RBD‐like) symptoms without further information about age‐onset of the symptom. DEB could also be related to other conditions that were not addressed in our study, i.e., other parasomnias, synucleinopathies, certain medications, and withdrawal of alcohol or drugs.
4.2. Future research directions
Firstly, there is a need for further larger scale studies of COVID‐19 cases, especially among those with long COVID to determine whether DEBs are transient or may have a tardive onset and most importantly, their future neurodegenerative predisposition. Secondly, emerging evidence suggests the occurrence of a complex neuropsychiatric syndrome after SARS‐Cov‐2 infection (Li et al., 2020). Multidisciplinary collaboration is needed to longitudinally follow‐up these COVID‐19‐confirmed cases to have deeper insight into the pathophysiology of the long‐term impact on the nervous system.
5. CONCLUSIONS
Dream‐enactment behaviours are quite common during the COVID‐19 pandemic in the general population, and are associated with olfactory impairment, mood, PTSD, and OSA symptoms. COVID‐19 survivors reported a 2–3‐times higher risk of experiencing DEBs, especially among those with olfactory dysfunction and gastrointestinal symptoms. Further studies with a larger sample size of COVID‐19‐positive cases are warranted to investigate the potential neurodegenerative effect of long COVID.
CONFLICT OF INTEREST
Yaping Liu: nothing to report. Eemil Partinen: reports other from Jazz Pharmaceuticals, grants from UCB‐Pharma, personal fees from Janssen‐Cilag, other from Idorsia, outside the submitted work. Ngan Yin Chan: nothing to report. Yves Dauvilliers: advisory board member for Jazz, Bioprojet, Takeda, Avadel, UCB, Idorsia, which is outside of the submitted work. Yuichi Inoue: nothing to report. Luigi De Gennaro: nothing to report. Giuseppe Plazzi: advisory board member for Jazz, Bioprojet, Takeda, Idorsia, which is outside of the submitted work. Courtney J. Bolstad: nothing to report. Michael R. Nadorff: nothing to report. Ilona Merikanto: nothing to report. Bjørn Bjorvatn: received personal fees from Sanofi for lecture, and Cura of Sweden for consultant work, both outside of the submitted work. Fang Han: nothing to report. Bin Zhang: nothing to report. Ana Suely Cunha: nothing to report. Sérgio Mota‐Rolim: nothing to report. Damien Léger: advisory board member for Jazz, Sanofi, Bioserenity, Idorsia, which is outside of the submitted work. Kentaro Matsui: received speaker fees from Eisai, Meiji Seika Pharma, MSD, Otsuka, Yoshitomi, and Takeda, outside of the submitted work. Colin A Espie: nothing to report. Frances Chung: reports research support from the University Health Network Foundation, Up‐to‐date royalties, consultant to Takeda Pharma and Masimo, STOP‐Bang proprietary to University Health Network. Charles M. Morin: nothing to report. Mariusz Sieminski: nothing to report. Thomas Penzel: received travel support and speaker fees from Jazz Pharma, Löwenstein Medical, Neuwirth Medical Instruments. Received consultancy fees from Bayer, Cerebra, Jazz, National Sleep Foundation, Received grants from Löwenstein medical, Cidelec. Owns shares of Advanced Sleep Research, The Sieatagroup, and Nukute, all outside of the submitted work. Brigitte Holzinger: nothing to report. Markku Partinen: reports grants from Finnish Parkinson foundation, during the conduct of the study; other from Bioprojet, other from Jazz Pharmaceuticals, personal fees from UCB‐Pharma, personal fees from GSK, personal fees from Takeda, other from MSD, personal fees from Orion, other from Flamel, outside the submitted work. Yun Kwok Wing: received personal fees from Eisai Co., Ltd for lecture, travel support from Lundbeck HK Limited, which are outside the submitted work.
AUTHOR CONTRIBUTIONS
Yaping Liu and Eemil Partinen conducted data analysis and drafted the manuscript. All authors conceptualised and conducted the study, collected the data, and critically revised the manuscript.
Supporting information
Table S1
Liu, Y. , Partinen, E. , Chan, N. Y. , Dauvilliers, Y. , Inoue, Y. , De Gennaro, L. , Plazzi, G. , Bolstad, C. J. , Nadorff, M. R. , Merikanto, I. , Bjorvatn, B. , Han, F. , Zhang, B. , Cunha, A. S. , Mota‐Rolim, S. , Léger, D. , Matsui, K. , Espie, C. A. , Chung, F. , Morin, C. M. , Sieminski, M. , Thomas, P. , Holzinger, B. , Partinen, M. , & Wing, Y. K. (2022). Dream‐enactment behaviours during the COVID‐19 pandemic: an international COVID‐19 sleep study. Journal of Sleep Research, 1–11. 10.1111/jsr.13613
Contributor Information
Markku Partinen, Email: markku.partinen@helsinki.fi.
Yun Kwok Wing, Email: ykwing@cuhk.edu.hk.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this international survey may be requested from the ICOSS group, via the corresponding authors.
REFERENCES
- Barone, D. A. (2020). Dream enactment behavior‐a real nightmare: A review of post‐traumatic stress disorder, REM sleep behavior disorder, and trauma‐associated sleep disorder. Journal of Clinical Sleep Medicine, 16(11), 1943–1948. 10.5664/jcsm.8758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorvatn, B. , Gronli, J. , & Pallesen, S. (2010). Prevalence of different parasomnias in the general population. Sleep Medicine, 11(10), 1031–1034. 10.1016/j.sleep.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Boldrini, M. , Canoll, P. D. , & Klein, R. S. (2021). How COVID‐19 affects the brain. JAMA Psychiatry, 78(6), 682–683. 10.1001/jamapsychiatry.2021.0500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin, P. , Nath, A. , & Beckham, J. D. (2020). Is COVID‐19 a perfect storm for Parkinson's Disease? Trends in Neurosciences, 43(12), 931–933. 10.1016/j.tins.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu, F. , Bone, J. K. , Mitchell, J. J. , Steptoe, A. , & Fancourt, D. (2021). Longitudinal changes in physical activity during and after the first national lockdown due to the COVID‐19 pandemic in England. Scientific Reports, 11(1), 17723. 10.1038/s41598-021-97065-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, F. , Waseem, R. , Pham, C. , Penzel, T. , Han, F. , Bjorvatn, B. , … International COVID Sleep Study (ICOSS) group . (2021). The association between high risk of sleep apnea, comorbidities, and risk of COVID‐19: A population‐based international harmonized study. Sleep & Breathing, 25(2), 849–860. doi: 10.1007/s11325-021-02373-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, F. , Yegneswaran, B. , Liao, P. , Chung, S. A. , Vairavanathan, S. , Islam, S. , … Shapiro, C. M. (2008). STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology, 108(5), 812–821. doi: 10.1097/ALN.0b013e31816d83e4 [DOI] [PubMed] [Google Scholar]
- Cohen, M. E. , Eichel, R. , Steiner‐Birmanns, B. , Janah, A. , Ioshpa, M. , Bar‐Shalom, R. , … Yahalom, G. (2020). A case of probable Parkinson's disease after SARS‐CoV‐2 infection. Lancet Neurology, 19(10), 804–805. 10.1016/S1474-4422(20)30305-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, J. E. , Opel, R. A. , Pleshakov, D. , Rachakonda, T. , Chau, A. Q. , Weymann, K. B. , & Lim, M. M. (2020). Posttraumatic stress disorder increases the odds of REM sleep behavior disorder and other parasomnias in veterans with and without comorbid traumatic brain injury. Sleep, 43(3), zsz237. 10.1093/sleep/zsz237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber, I. , Brandao, P. R. P. , Menegatti, F. , de Carvalho Bispo, D. D. , Maluf, F. B. , & Cardoso, F. (2020). Coronavirus Disease 2019 and parkinsonism: A non‐post‐encephalitic case. Movement Disorders, 35(10), 1721–1722. 10.1002/mds.28277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fränkl, E. , Scarpelli, S. , Nadorff, M. R. , Bjorvatn, B. , Bolstad, C. J. , Chan, N. Y. , … Holzinger, B. (2021). How our dreams changed during the COVID‐19 pandemic: Effects and correlates of dream recall frequency ‐ a multinational study on 19,355 adults. Nat Sci Sleep, 13, 1573–1591. 10.2147/nss.S324142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati, A. , Verga, L. , Giora, E. , Zucconi, M. , & Ferini‐Strambi, L. (2019). The risk of neurodegeneration in REM sleep behavior disorder: A systematic review and meta‐analysis of longitudinal studies. Sleep Medicine Reviews, 43, 37–46. 10.1016/j.smrv.2018.09.008 [DOI] [PubMed] [Google Scholar]
- Haba‐Rubio, J. , Frauscher, B. , Marques‐Vidal, P. , Toriel, J. , Tobback, N. , Andries, D. , … Heinzer, R. (2018). Prevalence and determinants of rapid eye movement sleep behavior disorder in the general population. Sleep, 41(2), zsx197. 10.1093/sleep/zsx197 [DOI] [PubMed] [Google Scholar]
- Han, F. , Lin, L. , Warby, S. C. , Faraco, J. , Li, J. , Dong, S. X. , … Mignot, E. (2011). Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Annals of Neurology, 70(3), 410–417. 10.1002/ana.22587 [DOI] [PubMed] [Google Scholar]
- Heidbreder, A. , Sonnweber, T. , Stefani, A. , Ibrahim, A. , Cesari, M. , Bergmann, M. , … Hogl, B. (2021). Video‐polysomnographic findings after acute COVID‐19: REM sleep without atonia as sign of CNS pathology? Sleep Medicine, 80, 92–95. 10.1016/j.sleep.2021.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel, S. , Berg, D. , Gasser, T. , Chen, H. , Yao, C. , Postuma, R. B. , & MDS Task Force on the Definition of Parkinson's Disease . (2019). Update of the MDS research criteria for prodromal Parkinson's disease. Movement Disorders, 34(10), 1464–1470. 10.1002/mds.27802 [DOI] [PubMed] [Google Scholar]
- Iranzo, A. , & Santamaria, J. (2005). Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder. Sleep, 28(2), 203–206. 10.1093/sleep/28.2.203 [DOI] [PubMed] [Google Scholar]
- Kroenke, K. , Spitzer, R. L. , Williams, J. B. , & Lowe, B. (2009). An ultra‐brief screening scale for anxiety and depression: The PHQ‐4. Psychosomatics, 50(6), 613–621. 10.1176/appi.psy.50.6.613 [DOI] [PubMed] [Google Scholar]
- Lam, M. H. , Wing, Y. K. , Yu, M. W. , Leung, C. M. , Ma, R. C. , Kong, A. P. , … Lam, S. P. (2009). Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long‐term follow‐up. Archives of Internal Medicine, 169(22), 2142–2147. 10.1001/archinternmed.2009.384 [DOI] [PubMed] [Google Scholar]
- Lam, S. P. , Fong, S. Y. , Ho, C. K. , Yu, M. W. , & Wing, Y. K. (2008). Parasomnia among psychiatric outpatients: A clinical, epidemiologic, cross‐sectional study. The Journal of Clinical Psychiatry, 69(9), 1374–1382. 10.4088/jcp.v69n0904 [DOI] [PubMed] [Google Scholar]
- Lang, A. J. , Wilkins, K. , Roy‐Byrne, P. P. , Golinelli, D. , Chavira, D. , Sherbourne, C. , … Stein, M. B. (2012). Abbreviated PTSD checklist (PCL) as a guide to clinical response. General Hospital Psychiatry, 34(4), 332–338. 10.1016/j.genhosppsych.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. C. , Bai, W. Z. , & Hashikawa, T. (2020). The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. Journal of Medical Virology, 92(6), 552–555. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, J. F. , Qiao, Y. , Gao, X. , Liang, L. , Liu, X. L. , Li, D. H. , … Chen, S. D. (2017). A community‐based study of risk factors for probable rapid eye movement sleep behavior disorder. Sleep Medicine, 30, 71–76. 10.1016/j.sleep.2016.06.027 [DOI] [PubMed] [Google Scholar]
- Mahlknecht, P. , Seppi, K. , Frauscher, B. , Kiechl, S. , Willeit, J. , Stockner, H. , … Hogl, B. (2015). Probable RBD and association with neurodegenerative disease markers: A population‐based study. Movement Disorders, 30(10), 1417–1421. doi: 10.1002/mds.26350 [DOI] [PubMed] [Google Scholar]
- Manni, R. , Ratti, P. L. , & Terzaghi, M. (2011). Secondary “incidental” REM sleep behavior disorder: Do we ever think of it? Sleep Medicine, 12(Suppl 2), S50–S53. doi: 10.1016/j.sleep.2011.10.011 [DOI] [PubMed] [Google Scholar]
- Mendez‐Guerrero, A. , Laespada‐Garcia, M. I. , Gomez‐Grande, A. , Ruiz‐Ortiz, M. , Blanco‐Palmero, V. A. , Azcarate‐Diaz, F. J. , … Gonzalez de la Aleja, J. (2020). Acute hypokinetic‐rigid syndrome following SARS‐CoV‐2 infection. Neurology, 95(15), e2109–e2118. doi: 10.1212/WNL.0000000000010282 [DOI] [PubMed] [Google Scholar]
- Merikanto, I. , Kortesoja, L. , Benedict, C. , Chung, F. , Cedernaes, J. , Espie, C. A. , … Bjorvatn, B. (2021). Evening‐types show highest increase of sleep and mental health problems during the COVID‐19 pandemic ‐ multinational study on 19,267 adults. Sleep, 45, zsab216. doi: 10.1093/sleep/zsab216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, C. M. , Bjorvatn, B. , Chung, F. , Holzinger, B. , Partinen, M. , Penzel, T. , … Espie, C. A. (2021). Insomnia, anxiety, and depression during the COVID‐19 pandemic: An international collaborative study. Sleep Medicine, 87, 38–45. doi: 10.1016/j.sleep.2021.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette, D. , Leu, S. , Pottier, M. , Buzare, M. A. , Brion, A. , & Arnulf, I. (2009). Dreamlike mentations during sleepwalking and sleep terrors in adults. Sleep, 32(12), 1621–1627. doi: 10.1093/sleep/32.12.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partinen, M. , Bjorvatn, B. , Holzinger, B. , Chung, F. , Penzel, T. , Espie, C. A. , … ICOSS‐collaboration group . (2021). Sleep and circadian problems during the coronavirus disease 2019 (COVID‐19) pandemic: The international COVID‐19 sleep study (ICOSS). Journal of Sleep Research, 30(1), e13206. doi: 10.1111/jsr.13206 [DOI] [PubMed] [Google Scholar]
- Postuma, R. B. , Arnulf, I. , Hogl, B. , Iranzo, A. , Miyamoto, T. , Dauvilliers, Y. , … Montplaisir, J. Y. (2012). A single‐question screen for rapid eye movement sleep behavior disorder: A multicenter validation study. Movement Disorders, 27(7), 913–916. 10.1002/mds.25037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma, R. B. , Iranzo, A. , Hu, M. , Hogl, B. , Boeve, B. F. , Manni, R. , … Pelletier, A. (2019). Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain, 142(3), 744–759. 10.1093/brain/awz030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salari, N. , Hosseinian‐Far, A. , Jalali, R. , Vaisi‐Raygani, A. , Rasoulpoor, S. , Mohammadi, M. , … Khaledi‐Paveh, B. (2020). Prevalence of stress, anxiety, depression among the general population during the COVID‐19 pandemic: A systematic review and meta‐analysis. Globalization and Health, 16(1), 57. 10.1186/s12992-020-00589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprecher, D. , Zhang, N. , Halverson, M. , & Savica, R. (2019). Parkinsonism risk factors in Salt Lake City, Utah: A community‐based study. Brain Sciences, 9(3), 71. 10.3390/brainsci9030071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprecher, D. R. , Serrano, G. E. , Zhang, N. , Intorcia, A. , Davis, K. J. , Glass, M. , … Beach, T. G. (2020). Prevalence of REM sleep behavior disorder in Sun City, Arizona. Heliyon, 6(1), e03140. 10.1016/j.heliyon.2019.e03140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha, S. , Kamel, F. , Umbach, D. M. , Fan, Z. , Beane Freeman, L. E. , Koutros, S. , … Chen, H. (2018). Factors associated with dream enacting behaviors among US farmers. Parkinsonism & Related Disorders, 57, 9–15. 10.1016/j.parkreldis.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uguccioni, G. , Golmard, J. L. , de Fontreaux, A. N. , Leu‐Semenescu, S. , Brion, A. , & Arnulf, I. (2013). Fight or flight? Dream content during sleepwalking/sleep terrors vs. rapid eye movement sleep behavior disorder. Sleep Medicine, 14(5), 391–398. 10.1016/j.sleep.2013.01.014 [DOI] [PubMed] [Google Scholar]
- von Bartheld, C. S. , Hagen, M. M. , & Butowt, R. (2020). Prevalence of chemosensory dysfunction in COVID‐19 patients: A systematic review and meta‐analysis reveals significant ethnic differences. ACS Chemical Neuroscience, 11(19), 2944–2961. 10.1021/acschemneuro.0c00460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Chau, S. W. H. , Lam, S. P. , Liu, Y. , Zhang, J. , Chan, N. Y. , … Wing, Y. K. (2021). Prevalence and correlates of REM sleep behaviour disorder in patients with major depressive disorder: A two‐phase study. Journal of Neurology, Neurosurgery, and Psychiatry. doi: 10.1136/jnnp-2021-327460 [DOI] [PubMed] [Google Scholar]
- WHO Coronavirus Disease (COVID‐19) Dashboard . (2020). Retrieved from https://covid19.who.int/
- World Population Prospects . (2021). United Nations. Retrieved from https://population.un.org/wpp/Download/Standard/Population/
- Yao, C. , Fereshtehnejad, S. M. , Keezer, M. R. , Wolfson, C. , Pelletier, A. , & Postuma, R. B. (2018). Risk factors for possible REM sleep behavior disorder: A CLSA population‐based cohort study. Neurology, 92(5), e475–e485. 10.1212/WNL.0000000000006849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Iranzo, A. , Hogl, B. , Arnulf, I. , Ferini‐Strambi, L. , Manni, R. , … Postuma, R. B. (2022). Risk factors for Phenoconversion in rapid eye movement sleep behavior disorder. Annals of Neurology, 91(3), 404–416. 10.1002/ana.26298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data supporting the findings of this international survey may be requested from the ICOSS group, via the corresponding authors.


