Abstract
Aim
Multisystem inflammatory syndrome in children (MIS‐C) may cause shock and even death in children. The aim of this study is to describe the clinical features, laboratory characteristics and outcome of children diagnosed with MIS‐C in 25 different hospitals in Turkey.
Methods
The retrospective study was conducted between 8 April and 28 October 2020 in 25 different hospitals from 17 cities. Data were collected from patients' medical records using a standardised form. Clinical and laboratory characteristics and outcomes according to different age groups, gender and body mass index percentiles were compared using multivariate logistic regression analysis.
Results
The study comprised 101 patients, median age 7 years (interquartile range (IQR) 4.6–9.3); 51 (50.5%) were boys. Reverse‐transcriptase polymerase chain reaction (PCR) assay was positive in 21/100 (21%) patients; 62/83 (74.6%) patients had positive serology for SARS‐CoV‐2. The predominant complaints were fever (100%), fatigue (n = 90, 89.1%), and gastrointestinal symptoms (n = 81, 80.2%). Serum C‐reactive protein (in 101 patients, median 165 mg/L; range 112–228), erythrocyte sedimentation rate (73/84, median 53 mm/s; IQR 30–84) and procalcitonin levels (86/89, median 5 μg/L; IQR 0.58–20.2) were elevated. Thirty‐eight patients (37.6%) required admission to intensive care. Kawasaki disease (KD) was diagnosed in 70 (69.3%) patients, 40 of whom had classical KD. Most patients were treated with intravenous immunoglobulin (n = 92, 91%) and glucocorticoids (n = 59, 58.4%). Seven patients (6.9%) died.
Conclusion
The clinical spectrum of MIS‐C is broad, but clinicians should consider MIS‐C in the differential diagnosis when persistent fever, fatigue and gastrointestinal symptoms are prominent. Most patients diagnosed with MIS‐C were previously healthy. Immunomodulatory treatment and supportive intensive care are important in the management of cases with MIS‐C. Glucocorticoids and intravenous immunoglobulins are the most common immunomodulatory treatment options for MIS‐C. Prompt diagnosis and prompt treatment are essential for optimal management.
Keywords: child, COVID‐19, Kawasaki disease, MIS‐C, shock
What is already known on this topic
Multisystem inflammatory syndrome in children (MIS‐C) occurs in association with SARS‐CoV‐2 infection and presents with fever, involvement of multiple organ systems and evidence of inflammation.
Most patients with MIS‐C are diagnosed with serological tests.
Unlike classical Kawasaki disease, most patients are over 5 years.
What this paper adds
Fever lasted at least 3 days in all patients.
Kawasaki‐like features were primarily detected in children aged 5 to 12 years.
Lymphopenia was predominant over 12 years.
Some patients had bradycardia after starting treatment.
After the global severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic was announced, China's first reports showed that children with ‘Coronavirus Disease 2019’ (COVID‐19) were mostly asymptomatic or had very mild clinical symptoms, unlike the severe disease reported in adults. 1 Nevertheless, in April 2020, a small group of children were identified with shock and a multisystem inflammation in the United Kingdom. 2 Some had coronary artery aneurysms while some had less severe symptoms. 2 This syndrome has clinical similarities with Kawasaki disease (KD), toxic shock syndrome and macrophage activation syndrome. 3 Most of these cases had a history of SARS‐CoV‐2 infection, close contact with COVID‐19 patients or positive laboratory test results for SARS‐CoV‐2. 4 , 5 Later, many cases from the United States and Europe were reported. 6 , 7 In the United Kingdom and Australia this condition was initially called Paediatric Inflammatory Multisystem Syndrome temporally associated with SARS‐CoV‐2 (PIMS‐TS), 8 , 9 but the World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) uses the term multisystem inflammatory syndrome in children (MIS‐C). 10 , 11 Prompt identification of MIS‐C is important as untreated it can lead to severe organ dysfunction and death. 5 , 7 , 12
The aim of this study is to describe the clinical features, laboratory characteristics and outcome of children diagnosed with MIS‐C in 25 different hospitals in Turkey.
Methods
Study design
Our study was a multicentre, retrospective case series in children younger than 18 years of age. Children with MIS‐C hospitalised in one of 25 centres from 17 cities in Turkey between 8 April and 28 October 2020 were enrolled. Data collected from patients included demographic, clinical features and laboratory parameters. Laboratory and clinical parameters (lymphocyte counts, neutrophil counts, blood pressure, respiratory rate and heart rate) were compared to age‐specific normal ranges. We also recorded echocardiographic findings, the need for intensive care, the presence of shock with inotrope support or fluid resuscitation, and the need for invasive/non‐invasive mechanical ventilation (MV) or extracorporeal membrane oxygenation (ECMO). The American Heart Association criteria were used to define incomplete and complete KD. 13 As described by the CDC and the WHO, criteria were used for the case definition of MIS‐C. 10 , 11
Inclusion criteria included a proof of SARS‐CoV‐2 infection: positive reverse‐transcriptase polymerase chain reaction (RT‐PCR) or positive immunoglobulin M (IgM), or immunoglobulin G (IgG) in a rapid test, or positive enzyme‐linked immunosorbent assay, or antigen assay or known contact with a confirmed COVID‐19 patient. We compared clinical features, laboratory features and MIS‐C outcomes by age group, gender, and body mass index (BMI) percentiles. Ethics Committee approval was obtained from the Ethics Committee of Tepecik Training and Research Hospital dated 12.10.2020, reference number was 2020/12–17.
Statistical analysis
The median, first quartile and third quartile were used to express continuous variables. The differences between the two groups were analysed using the Mann–Whitney U test and categorical variables were compared using the χ2 or Fisher's exact tests. For normally distributed levels, we used t‐tests and ANOVA to compare the mean of the groups. The Kruskal‐Wallis test was also used to compare the groups of distributed with not normally, and the Dunn‐Bonferroni post hoc method was used for the data. P < 0.05 was considered significant. Binary logistic regression analysis using the Enter method was performed separately to determine the effects of various symptoms and laboratory findings on the probability of patient gender, percentile of BMI (normal/overweight and obese), need for ICU or not and survivor/non‐survivor. Clinical and laboratory characteristics and outcomes by different age groups were compared using multinomial logistic regression analysis. Statistical analyses were performed using the SPSS software version 25 for Windows (IBM, Armonk, NY, USA) and the MedCalc program (MedCalc Software, Mariakerke, Belgium).
Results
Between 8 April and 28 October 2020, we enrolled 113 patients. Nine patients were excluded as they did not fit MIS‐C definition. Three patients who hadwith negative laboratory results offor SARS‐CoV‐2 and had no history of contact towith COVİD‐19 patients were also excluded. The study comprised 101 patients (Fig. 1). The distribution of the 101 patients with MIS‐C by months is presented in Figure 2.
Fig. 1.
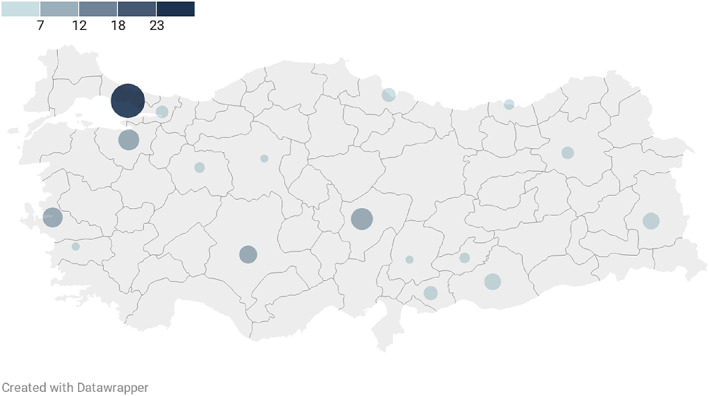
The distribution of patients with MIS‐C by cities in Turkey
Fig. 2.
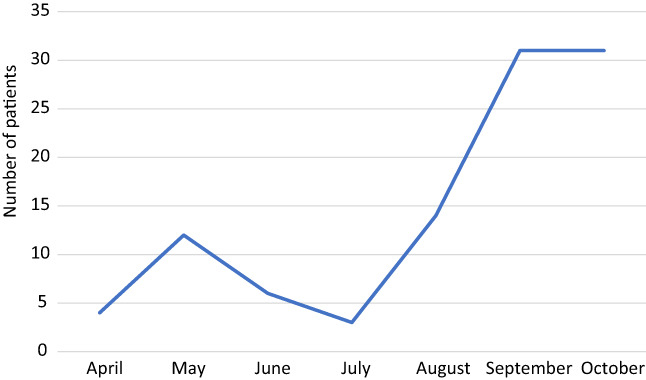
Number of patients with MIS‐C by months
Clinical characteristics of patients
The median age was 7 years (interquartile range (IQR) 4.6–9.3; range 2–208 months) and 51 (50.5%) were boys (Table 1). Nineteen patients (17.8%) had comorbid diseases, including five with neurodevelopmental disabilities, three with Familial Mediterranean Fever, two with asthma, two with chronic renal failure, two with autoimmune disease, one with acute lymphoblastic leukaemia (ALL), one with acute myeloblastic leukaemia, one with common variable immunodeficiency (CVID), one with a kidney transplant and one with neuroendocrine tumour. Two of these children were receiving chemotherapy for acute myeloblastic leukaemia and ALL, respectively.
Table 1.
Demographic and clinical characteristics of the patients with multisystem inflammatory syndrome in children (MIS‐C)
| Characteristic | All patients (n = 101) | Laboratory confirmation of SARS‐CoV‐2 infection (n = 83) | Epidemiologic link to person with COVID‐19 (n = 18) | |
|---|---|---|---|---|
| RT‐PCR positive (n = 25) | Antibody test positive. RT‐PCR negative (n = 58) | |||
| Age, median (IQR), year | 7.08 (4.6–9.3) | 6.08 (2.1–12.3) | 7.25 (5.5–9.87) | 4.91 (1.1–8.41) |
| Sex, no. (%) | ||||
| Boy | 51 (50.5) | 10 (40) | 31 (53.4) | 10 (55.6) |
| Girl | 50 (49.5) | 15 (60) | 27 (46.6) | 8 (44.4) |
| Race/ethnicity (%) | ||||
| Turkish | 97 (96) | 25 (100) | 55 (94.8) | 17 (94.4) |
| Others | 4 (4) | 0 | 3 (5.2) | 1 (5.6) |
| Age group, no (%) | ||||
| 0–5 y | 29 (28.7) | 10 (40) | 9 (15.5) | 10 (55.5) |
| 5–12 y | 56 (55.4) | 8 (32) | 42 (72.4) | 6 (33.4) |
| >12 y | 16 (15.9) | 7 (28) | 7 (12.1) | 2 (11.1) |
| BMI percentile median/patient no. (IQR) | 39.7/97 (14.5–83) | 44.4/25 (18.5–83.8) | 41.9/54 (11.52–83.2) | 31.5/18 (14.7–84.7) |
| Overweight, patient no./total no. (%) | 13/97 (13.4) | 3/25 (12) | 7/54 (12.9) | 3/18 (16.6) |
| Obese. patient no./total no. (%) | 9/97 (9.2) | 3/25 (12) | 5/54 (9.2) | 1/18 (5.5) |
| Clinical features at presentation | ||||
| Fever, n (%) | 101 (100) | 25 (100) | 58 (100) | 18 (100) |
| Degree of fever (°C), median (IQR) | 39 (38.6–39.5) | 39 (38.5–39) | 39 (38.7–39.5) | 39 (38.9–39.6) |
| Duration of fever (day), median (IQR) | 7 (5–7.5) | 7 (6–7.5) | 6.5 (5–8) | 6.5 (5–7) |
| Fatigue, n (%) | 90 (89.1) | 19 (76) | 55 (94.8) | 16 (88.8) |
| Gastrointestinal symptoms, n (%) | 81 (80.2) | 19 (76) | 48 (82.8) | 14 (77.8) |
| Abdominal pain, patient no./total no. (%) | 68/85 (80) | 15/17 (88.2) | 41/55 (74.5) | 12/13 (92.3) |
| Vomiting, n (%) | 55 (54.5) | 14 (56) | 30 (51.7) | 11 (61.1) |
| Nausea, patient no./total no. (%) | 57/95 (60) | 13/19 (68.4) | 33/58 (56.8) | 11/18 (61.1) |
| Diarrhoea, n (%) | 43 (42.5) | 9 (36) | 26 (44.8) | 8 (44.4) |
| Conjunctival injection, n (%) | 65 (64.4) | 10 (40) | 45 (77.6) | 10 (55.6) |
| Rash, n (%) | 65 (64.4) | 14 (56) | 37 (63.8) | 14 (77.8) |
| Maculopapular | 33 (32.6) | 6 (24) | 18 (31) | 9 (50) |
| Macule | 29 (28.7) | 7 (28) | 17 (29.3) | 5 (27.7) |
| Petechiae/ecchymosis | 3 (2.9) | 1 (4) | 2 (3.4) | 0 |
| Muscle ache, patient no./total no. (%) | 60/85 (70.5) | 12/18 (66.6) | 37/56 (66) | 11/11 (100) |
| Mucous membrane changes, n (%) | 59 (58.4) | 8 (32) | 37 (63.8) | 14 (77.8) |
| Peripheral cutaneous inflammation signs, n (%) | 46 (45.5) | 9 (36) | 27 (46.6) | 10 (55.6) |
| Any respiratory symptoms, n (%) | 44 (38.6) | 9 (36) | 28 (48.3) | 7 (38.9) |
| Arthralgia, patient no./total no. (%) | 40/85 (47) | 10/17 (58.8) | 21/55 (38.1) | 9/13 (69.2) |
| Shock, n (%) | 39 (38.6) | 7 (28) | 24 (41.3) | 8 (44.4) |
| Headache, patient no./total no. (%) | 36/79 (45.5) | 9/17 (52.9) | 23/52 (44.2) | 4/10 (40) |
| Sore throat, patient no./total no. (%) | 24/82 (29.2) | 6/18 (33.3) | 14/54 (25.9) | 4/10 (40) |
| Dry cough, n (%) | 23 (22.8) | 5 (20) | 16 (27.6) | 2 (11.1) |
| Lethargy, n (%) | 23 (22.8) | 6 (24) | 10 (17.2) | 7 (38.8) |
| Desquamation, n (%) | 20 (19.8) | 4 (16) | 10 (17.2) | 6 (33.3) |
| Joint swelling, n (%) | 11 (10.9) | 3 (12) | 6 (10.3) | 2 (11.1) |
| Ileus, n (%) | 9 (8.9) | 2 (8) | 3 (5.2) | 4 (22.2) |
| Odynophagia, n (%) | 8/82 (9.7) | 2/17 (11.7) | 3/55 (5.4) | 3/10 (30) |
| Lymphadenopathy, n (%) | 7 (6.9) | 1 (4) | 4 (6.8) | 2 (11.1) |
| Taste loss, patient no./total no. (%) | 5/83 (6) | 0/18 | 2/55 (3.6) | 3/10 (30) |
| Runny nose, n (%) | 4 (3.9) | 1 (4) | 2 (3.4) | 1 (5.6) |
| Loss of smell, patient no./total no. (%) | 3/83 (3.6) | 1/18 (5.5) | 1/55 (1.81) | 1/10 (10) |
BMI, body mass index; IQR, interquartile range; RT‐PCR, reverse‐transcriptase polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome virus 2.
The median time from onset of symptoms to hospitalisation was 5 days (IQR 3–7; range 1–24 days). All patients except one had fever for at least 3 days. The median height of fever was 39°C (IQR 38.6–39.5°C; range 37–40.5°C), and the median duration of fever was 7 days (IQR 5–7.5; range 1–20 days). Joint swelling was noted in 11 patients, 10 of whom were male (odds ratio 10.75; 95% confidence interval 0.011–0.811; P = 0.032).
SARS‐CoV‐2 test results
A total of 100 patients with nasopharyngeal and oropharyngeal swab sampling were tested for SARS‐CoV‐2 nucleic acid detection using RT‐PCR assay (Table 1). The median number of RT‐PCR assays was two (IQR 1–2 times). Twenty‐one patients (21%) had only positive RT‐PCR assay results, and four patients (4%) were positive for both PCR and serological tests for SARS‐CoV‐2 infection. One patient only had contact with a proven COVID‐19 patient. IgG or IgM antibody against SARS‐CoV‐2 was positive in 62 of 83 patients (74.6%). Among these patients, 30 (36.1%) had positive results for IgM and IgG antibodies, 17 (20.4%) had confirmed total antibody, 13 (15.6%) patients had only IgG antibody, and 2 (2.4%) had only IgM antibody. Eighteen patients had negative test results for antibody and PCR assay with SARS‐CoV‐2, but had close contact with a person with proven COVID‐19. While 63 patients had a history of contact with COVID‐19 patients in the last 4 weeks, 4 of these patients had a history of contact within the last 6 weeks.
Laboratory investigations
Demographic characteristics and laboratory test results of the patients by age groups are presented in Table 2 and Figure 3. Lymphopenia was detected in 68.3% (median 1080 cell/μL (IQR 715–1845)). At admission, the majority of patients had markedly elevated inflammatory markers. Elevated C‐reactive protein (CRP) levels were found in all patients (CRP > 100 mg/L in 78.2%), elevated erythrocyte sedimentation rate (ESR) in 86% (ESR > 40 mm/s in 64.2%), elevated procalcitonin levels in 96% (procalcitonin >0.2 μg/L in 91%), ferritin in 69% (ferritin >400 μg/L in 65.9%) and interleukin 6 in 70% (interleukin 6 > 16.4 pg./mL in 70%). In univariate analysis of laboratory results, no statistical difference was found between sex, age groups and normal weight/obese or overweight groups. There was no association between laboratory results and sex, age groups and normal weight/overweight‐obese patients in logistic regression analyses.
Table 2.
Laboratory findings of the patients with multisystem inflammatory syndrome in children (MIS‐C) by age range
| Characteristic | All patients (n = 101) | 0–5 years (n = 29) | 5–12 years (n = 56) | >12 years (n = 16) | P |
|---|---|---|---|---|---|
| Haematology | |||||
| Total white blood cell count cells × 103/μL, median (IQR) | 8.3 (5.6–13.1) | 11.9 (7–18.1) | 7.9 (5.6–11.7) | 6.9 (3.9–9.9) | 0.018 |
| Neutrophil count cells × 103/μL, median (IQR) | 6.4 (3.8–10.4) | 8.39 (4.3–14) | 6.31 (4.2–10) | 5.77 (2.7–8.7) | 0.308 |
| Lymphocyte count cells/μL, median (IQR) | 1080 (715–1845) | 2000 (1380–5105) | 930 (707–1495) | 635 (510–995) | <0.001 |
| Platelet count cells × 103/μL, median (IQR) | 152 (103–257) | 267 (131–399) | 137 (100–180) | 162 (102–208) | 0.011 |
| Haemoglobin, g/dL, mean ± SD | 10.8 ± 1.5 | 10.6 ± 1.5 | 10.6 ± 1.3 | 11.9 ± 1.9 | 0.009 |
| Inflammatory markers | |||||
| CRP, mg/L, median (IQR) | 165 (112–228) | 129 (78.5–193) | 161 (128–228) | 203 (119–299) | 0.088 |
| ESR, mm/h, median (IQR) | 53 (30–84) [n = 84] | 51 (21–85) [n = 23] | 52 (27–87) [n = 49] | 55 (35–82) [n = 12] | 0.894 |
| Procalcitonin, μg/L, median (IQR) | 5 (0.58–20.2) [n = 89] | 3.72 (0.3–10.1) [n = 25] | 7 (0.9–24.5) [n = 49] | 2.39 (0.41–9.2) [n = 15] | 0.187 |
| Ferritin, μg/L, median (IQR) | 553 (272–1022) [n = 97] | 262 (141–646) [n = 27] | 630 (406–1084) [n = 54] | 545 (385–1032) [n = 16] | 0.054 |
| Biochemistry | |||||
| LDH, U/L, median (IQR) | 323 (264–404) | 325 (260–431) | 326 (268–394) | 310 (248–579) | 0.944 |
| Albumin, g/dL, median (IQR) | 3 (2.5–3.4) | 2.9 (2.4–3.6) | 3 (2.6–3.2) | 3.4 (2.5–3.9) | 0.158 |
| Sodium, mmol/L, median (IQR) | 133 (130–136) [n = 95] | 133 (131–136) [n = 27] | 132 (129–135) [n = 53] | 134 (131–139) [n = 15] | 0.308 |
| Urea, mg/dL, median (IQR) | 18.8 (12.7–28.5) | 15 (9–19.9) | 20 (14.7–27.7) | 29 (16.1–58.3) | 0.001 |
| Creatinine, mg/dL, median (IQR) | 0.48 (0.34–0.73) [n = 96] | 0.30 (0.25–0.47) [n = 26] | 0.49 (0.38–0.66) [n = 55] | 0.83 (0.7–1.24) [n = 15] | <0.001 |
| Triglyceride, mg/dL, median (IQR) | 230 (176–324) [n = 68] | 182 (113–320) [n = 18] | 269 (204–333) [n = 40] | 191 (103–239) [n = 10] | 0.006 |
| Cardiac markers | |||||
| Troponin, ng/L, median (IQR) | 13.3 (2.4–63) [n = 93] | 5.3 (1.8–16.2) [n = 26] | 19.8 (0.7–86.6) [n = 53] | 36.2 (4.37–519) [n = 14] | 0.035 |
| NT‐pro‐BNP, pg/mL, median (IQR) | 5656 (1577–12 503) [n = 44] | 1307 (400–8547) [n = 11] | 6773 (3180–14 151) [n = 25] | 4473 (206–8047) [n = 8] | 0.087 |
| Coagulation | |||||
| Fibrinogen, mg/dL, median (IQR) | 451 (350–628) [n = 92] | 423 (305–570) [n = 24] | 410 (341–612) [n = 52] | 577 (460–770) [n = 16] | 0.054 |
| D‐dimer, μg/L, median (IQR) | 2787 (1405–4500) [n = 99] | 2850 (1245–4727) [n = 28] | 2400 (1700–4850) [n = 55] | 2975 (1101–4245) [n = 16] | 1.000 |
CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; IQR, interquartile range; LDH, lactate dehydrogenase; NT‐pro‐BNP, N‐terminal pro‐B type natriuretic peptide; SD, standard deviation. Significant p values are indicated in bold.
Fig. 3.
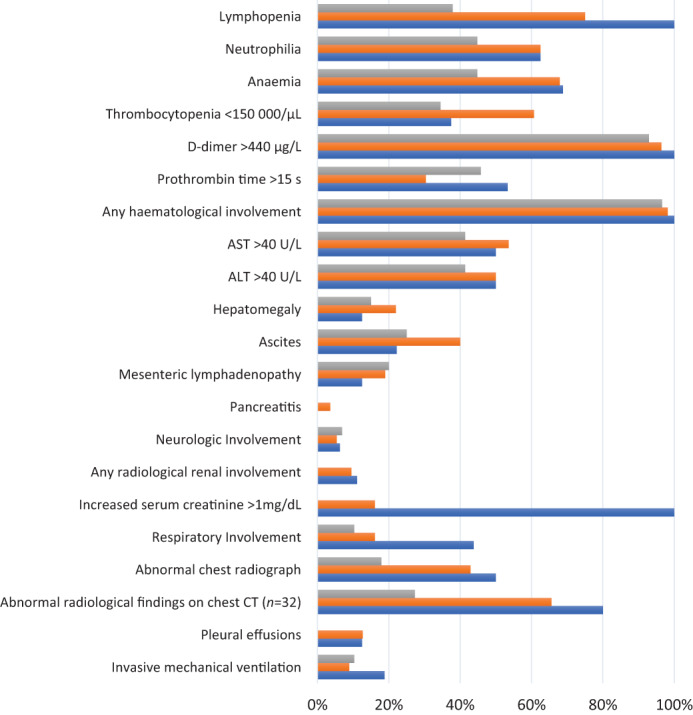
Laboratory markers, radiological findings and system involvement in patients with MIS‐C ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, computed tomography.  , 0‐5 yr;
, 0‐5 yr;  , 5‐12;
, 5‐12;  , 12yr
, 12yr
Clinical outcomes and treatment
The median time of hospitalisation was 10 days (IQR 7–14 days) (Table 3). Thirty‐eight patients (37.6%) required intensive care unit (ICU) admission, and invasive MV was used for respiratory support in 11 patients (10.8%). Children who required an ICU stay had numerically higher procalcitonin, CRP, international normalised ratio, D‐dimer, indirect bilirubin and creatinine levels, as well as prolonged prothrombin time and lower lymphocyte and platelet counts, and lower total protein levels compared to children who did not require intensive care treatment (Table 4). There were no significant differences between gender, age groups and overweight‐obese/normal‐weight groups regarding the need for staying in an ICU. Seven patients died; four had underlying diseases (ALL, CVID, pustular psoriasis, vesicoureteral reflux), while no comorbid conditions were found in three cases. One patient had severe myocarditis, one patient died from ventricular fibrillation, and two patients died after ECMO. Laboratory findings were compared betwen those who died and survived; higher procalcitonin levels, gamma‐glutamyl transferase, creatinine kinase levels, and lower platelet counts were detected in patients who died (P = 0.017, P = 0.025, P = 0.017, P = 0.01, respectively).
Table 3.
Clinical outcomes and management of the patients with multisystem inflammatory syndrome in children (MIS‐C) by age groups
| All patients (n = 101) | 0–5 years (n = 29) | 5–12 years (n = 56) | >12 years (n = 16) | P | |
|---|---|---|---|---|---|
| Characteristic | |||||
| ICU admission, n (%) | 38 (37.6) | 9 (31) | 20 (35.7) | 9 (56.2) | 0.235 |
| Duration of ICU median (IQR) | 6 (3.0–9.0) | 7 (3.5–11.0) | 4 (3.0–8.5) | 7 (4.5–8.0) | 0.245 |
| Duration of hospitalisation median (IQR) | 10 (7–14) | 9.5 (7–13.7) | 10 (7.2–13.5) | 10 (7–15) | 0.873 |
| Shock†, n (%) | 39 (38.6) | 6 (20.6) | 25 (44.6) | 8 (50) | 0.059 |
| Cardiac dysfunction, n (%) | 18 (17.8) | 2 (6.8) | 12 (21.4) | 4 (25) | 0.159 |
| Respiratory failure, n (%) | 19 (18.8) | 3 (10.3) | 9 (16) | 7 (43.7) | 0.018 |
| Acute kidney injury‡, n (%) | 18 (17.8) | 3 (10.3) | 8 (14.2) | 7 (43.7) | 0.012 |
| Hematologic failure§, n (%) | 96 (95) | 27 (93.1) | 53 (94.6) | 16 (100) | 0.328 |
| Meets KD or incomplete KD criteria¶, n (%) | 70 (69.3) | 20 (68.9) | 41 (73.2) | 9 (56.2) | 0.430 |
| Pharmacotherapy | |||||
| Intravenous immunoglobulin (IVIG), n (%) | 92 (91%) | 27 (93.1) | 50 (89.2) | 15 (93.7) | 0.775 |
| Corticosteroids, n (%) | 59 (58.4) | 10 (34.4) | 41 (73.2) | 8 (50) | 0.020 |
| Acetylsalicylic acid, n (%) | 51 (50.4) | 18 (62) | 27 (48.2) | 6 (37.5) | 0.253 |
| Anticoagulants, n (%) | 48 (47.5) | 10 (34.4) | 27 (48.2) | 11 (68.7) | 0.087 |
| Oxygen replacement, n (%) | 44 (43.5) | 7 (24.1) | 27 (48.2) | 10 (62.5) | 0.026 |
| Inotropes, n (%) | 28 (27.7) | 5 (17.2) | 17 (30.3) | 6 (37.5) | 0.280 |
| Anakinra, n (%) | 11 (10.8) | 3 (10.3) | 7 (12.5) | 1 (6.2) | ‐ |
| Plasma exchange, n (%) | 7 (6.9) | 1 (3.4) | 3 (5.3) | 3 (18.7) | ‐ |
| Tocilizumab, n (%) | 3 (2.9) | ‐ | 1 (1.7) | 2 (12.5) | ‐ |
| Infliximab, n (%) | 1 (0.9) | 1 (3.4) | ‐ | ‐ | ‐ |
| Outcomes | |||||
| NIMV, n (%) | 16 (15.8) | 3 (10.3) | 8 (14.2) | 5 (31.2) | 0.165 |
| Intubation, n (%) | 11 (10.8) | 3 (10.3) | 5 (8.9) | 3 (18.7) | 0.535 |
| Prone position, n (%) | 5 (4.9) | 1 (3.4) | 2 (3.5) | 2 (12.5) | 0.098 |
| ECMO, n (%) | 2 (1.9) | 1 (3.4) | 1 (1.7) | ‐ | ‐ |
| Coronary artery dilatation patient no./total no. (%) | 19/85 (22.3) | 6/25 (24) | 10/47 (21.2) | 3/13 (23) | 0.963 |
| Coronary artery aneurysm patient no./total no. (%) | 5/85 (8%) | 1/25 (4%) | 4/47 (8.5%) | −/13 | 0.459 |
| Death, n (%) | 7 (6.9%) | 2 (6.8%) | 4 (7.1%) | 1 (6.2%) | ‐ |
Shock was defined as needing inotrope support or fluid resuscitation >20 mL/kg.
Acute kidney injury defined by creatinine level greater than the upper limit for age.
American Heart Association criteria for the definition of KD is to have persistent fever and four of the following five mucocutaneous features: erythema and cracking of lips/strawberry tongue and/or erythema of oral and pharyngeal mucosa; bilateral bulbar conjunctival injection without exudate; rash; erythema and oedema of the hands and feet in acute phase and/or periungual desquamation in subacute phase and cervical lymphadenopathy.
Hematologic failure was defined as D‐dimer elevation and/or neutrophilia and/or lymphopenia. Neutrophilia was defined as ANC ≥7700/μg/L. Lymphopenia was defined as below 4500 μL in children under 8 months of age and below 1500 μL above 8 months of age.
ANC, absolute neutrophil count; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range; KD, Kawasaki disease; NIMV, non‐invasive mechanical ventilation. Significant p values are indicated in bold.
Table 4.
The optimal cut‐off values, sensitivities, specificities, positive predictive value, and negative predictive value of laboratory findings were presented as indices predictive of the need for intensive care unit admission of patients with multisystem inflammatory syndrome in children (MIS‐C)†
| n | AUC | P | Cut‐Off | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| Procalcitonin levels, μg/L | 89 | 0.686 | 0.001 | >6 | 67.65 | 69.09 | 57.5 | 77.6 |
| INR | 99 | 0.681 | 0.003 | >1.25 | 55.26 | 85.25 | 70.0 | 75.4 |
| CRP, mg/L | 101 | 0.670 | 0.002 | >177 | 63.16 | 68.25 | 54.5 | 75.4 |
| Total protein, mg/dL | 82 | 0.659 | 0.009 | ≤5.9 | 77.78 | 54.35 | 57.1 | 75.8 |
| Creatinine levels, mg/dL | 96 | 0.654 | 0.011 | >0.5 | 65.79 | 72.41 | 61.0 | 76.4 |
| D‐dimer, μg/L | 99 | 0.651 | 0.007 | >2787 | 65.79 | 60.66 | 51.0 | 74.0 |
| Prothrombin time, s | 85 | 0.637 | 0.035 | >15.1 | 48.65 | 83.33 | 69.2 | 67.8 |
| Indirect bilirubin, mg/dL | 92 | 0.635 | 0.023 | >0.23 | 63.89 | 69.64 | 57.5 | 75.0 |
| Lymphocyte cells/μL | 101 | 0.633 | 0.021 | ≤930 | 63.16 | 66.67 | 53.3 | 75.0 |
| Platelet counts cells/μL | 101 | 0.628 | 0.026 | ≤173 000 | 78.95 | 49.21 | 48.4 | 79.5 |
Receiver operating characteristic analysis was used to determine the optimal cut‐off values of laboratory findings to predict the need for an intensive care unit.
AUC, area under an ROC curve; CRP, C‐reactive protein; INR,international normalized ratio; NPV, negative predictive value; PPV, positive predictive value.
Most patients were treated with intravenous immunoglobulin (n = 92, 91%); the dosage was 2 g/kg in 82% (76/92) and 4 g/kg in 10% (10/92) of patients. Fifty‐nine patients received methylprednisolone treatment; 37 patients received a dose of 2 mg/kg, and 10 patients received pulse methylprednisolone.
Due to the risk of thrombosis associated with SARS‐CoV‐2 infection, almost half of the patients received anticoagulants and aspirin. High‐dose aspirin (60–100 mg/kg/day) was used in 37 patients, moderate dose in 4 patients, and low dose (3–5 mg/kg/day) in 10 patients from the beginning of treatment. Also, 11 patients received anakinra, 3 patients received tocilizumab, and one patient received infliximab. Two of the patients who died were treated with anakinra, while one was treated with tocilizumab.
System involvement and imaging findings
System involvements of the patients with MIS‐C between the different age groups is shown in Figure 3. Haematological involvement was most common in the patients (96/101, 95%). Echocardiography was performed in 85 (84.1%) patients and abnormal findings were noted in 45 (52.9%) patients. Kawasaki‐like features were found in 70 (69.3%) patients, of whom 40 had classic KD. Laboratory findings in children with and without Kawasaki‐like features were not statistically different (Fig. 4). Coronary artery aneurysm was reported in 5.8% (5/85) and coronary artery dilatation in 22% (19/85). Interestingly, sinus bradycardia developed in four patients with MIS‐C after treatment. This transient sinus bradycardia resolved within a week without the need for treatment.
Fig. 4.
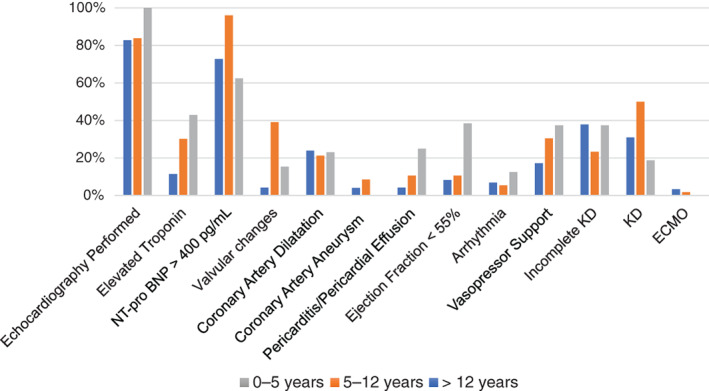
Cardiovascular involvement in patients with MIS‐C ECMO, extracorporeal membrane oxygenation; KD, Kawasaki Disease; NT‐pro‐BNP, N‐terminal pro‐B‐type natriuretic peptide.
Discussion
To the best of our knowledge, this is the most extensive retrospective study of MIS‐C from Turkey. In this report, laboratory‐confirmed SARS‐CoV‐2 infection was present in 82% of the patients by detection mainly with serological assays. In most studies, serological evidence of SARS‐CoV‐2 was detected in a higher proportion than RT‐PCR positivity. In studies, SARS‐CoV‐2 serology was positive in 87%, 91% and 78%, respectively. 4 , 5 , 14 Since antibodies are produced some time after the onset of infection, it is thought that MIS‐C may be an immune response that develops after SARS‐CoV‐2 infection. 15 A higher level of confirmation of infection in severe cases may result in higher sensitivity and fewer false‐negative results, while mild disease, especially in children, may result in detectable levels not always being reached in antibody‐based tests. 1 , 15 , 16 , 17 , 18
The temporal distribution of the MIS‐C cases showed a rapid upward trend in the Republic of Turkey in the late stages of the pandemic. However, on 10 March 2020, the first case with COVID‐19 has announced after detection from many European countries; thanks to the country's early isolation decisions, there were a small number of cases in the early stage of the pandemic. Then, there was a noteworthy increase in the number of patients with MIS‐C in September and October. MIS‐C cases' temporal distribution suggests an increased awareness of health‐care workers in the later stages of the outbreak and the detection of previously undiagnosed patients. It is thought that people's migration to many regions in the country after the country's national holiday at the end of August resulted in the rapid spread of SARS‐CoV‐2 and increased the number of patients with COVID‐19.
Unlike other studies, we found that boy and girl proportions were close to each other in patients with MIS‐C. 4 , 7 , 19 We found that the median age of the children was 7 years, in contrast to KD, which usually occurs before the age of 5. 13 Other studies came to the same conclusions: the median age was 9, 7, 8 and 7 years, respectively. 4 , 5 , 7 , 14
In our report, fever lasted at least 3 days in almost all cases. The presence and duration of fever may be important because body temperature may rise in humans due to many different infections and other diseases. However, the presence of fever for 7 days is a long time, as in our study. In this context, even if clinicians cannot detect SARS‐CoV‐2 infection, they may also consider MIS‐C in children who do not respond to antibiotics if the fever lasts longer and there is a pronounced acute phase reaction. Interestingly, our data showed that after fever, fatigue was the most commonly cited complaint. Like previous reports, we also showed that gastrointestinal symptoms were the predominant complaint in hospital admissions. 5 , 6 , 7 , 14 , 20 Muscle pain, conjunctival injection and rash were the other main complaints, which is consistent with reports by Whittaker et al. 4 in the United Kingdom, Pouletty et al. 20 in France and Lee et al. 19 in the United States.
In our study, lymphopenia was found in 68% of children with MİS‐C; other studies showed this in 54%, 64%, over 75%, and 81% of patients, respectively. 6 , 7 , 14 , 19 Lymphopenia tended to be more pronounced in older children, especially those more than 12 years of age, similar to Feldstein et al. 7 In our study, elevated inflammatory markers such as CRP (CRP > 100 mg/L was in 78.2%), ESR (ESR > 40 mm/s was in 64.2%), ferritin (ferritin >400 μg/L was in 65.9%), procalcitonin (procalcitonin >0.2 μg/L was in 91%) and interleukin 6 (interleukin>16.4 pg/mL was in 70%) were detected. It may be related to cytokine‐storm in human immune dysregulation. Raised serum D‐dimer and fibrinogen may cause a hypercoagulative situation such as pulmonary emboli or deep vein thrombosis. In our study, 91.9% of patients had D‐dimer levels higher than two times the upper limit of normal.
An aspect of cardiac involvement was markedly high N‐terminal pro‐B‐type natriuretic peptide (NT‐pro‐BNP) levels which were high in 84% of the patients. In our study, markedly elevated NT‐pro‐BNP might have been due to cardiomyocyte damage and oedema. At the same time, almost half of the echocardiography resulted normally. Interestingly, our data showed that coronary artery abnormalities were detected in one‐third of the patients like KD. 21 Unlike KD, which occurs predominantly less than 5 years old, we found that the patients with complete or incomplete Kawasaki‐like features were more between 5 and 12 years. 13 , 22
In the treatment of MISC, immunomodulatory agents are usually chosen as first‐line therapy after fluid support. Intravenous immunoglobulin (IVIG) was administered to nearly all of the patients as suggested in the study of Toubiana et al. 6 More than half of the patients received glucocorticoid treatment, like some studies. 4 , 7 Immunomodulators such as infliximab, anakinra and tocilizumab were used less frequently. Two of the patients who died were treated with anakinra, while one was treated with tocilizumab.
Previous studies have shown that about 60%–80% of children with MIS‐C needed ICU admission; however, in our study the rate was 37%. 5 , 6 One reason for the low rate of ICU admission was the high number of children with mild and moderate MIS‐C. Also, the need of MV was in 10.8% of patients furthermore, non‐invasive MV rate was in 15%. Among the age groups, respiratory failure was significantly increased over 12 years of age. As it is known, the SARS‐CoV‐2 uses the angiotensin‐converting enzyme 2 (ACE2) receptor to gain entrance to cells. 23 , 24 In their study, Xie et al. 25 showed a decrease number of ACE2 receptors with age in mouse models. Perhaps this may explain the cause of respiratory failure in patients over 12 years old. While there were no 6 , 20 or few deaths 4 , 7 , 14 reported in publications about MIS‐C, in our cohort, seven patients died due to MIS‐C. Four of these had underlying diseases (ALL, CVID, pustular psoriasis, vesicoureteral reflux), while no comorbid conditions were found in three of the patients. One patient had severe myocarditis, and one patient died from ventricular fibrillation. Two patients died after ECMO, and one of them had CVID.
Interestingly, in our findings, significant bradycardia developed in some patients towards the end of their treatment. These patients were monitored closely and followed up with repeated echocardiography and Holter electrocardiogram. The bradycardia resolved spontaneously within a week and no treatment was required. Interestingly, a low dose of intravenous steroids was used for treatment in all these four patients. The heart‐related complications might be related to ACE2 receptors expressed in endothelial and cardiomyocytes in the heart. Increasing evidence suggests that ACE2 enzyme activity has a protective role in cardiovascular damage caused by SARS‐CoV‐2 infection. The impairment of ACE2 activity results in impaired heart perfusion, vascular permeability, vasospasm and myocardial injury. 26 This effect may have shown itself with rhythm problems in the heart. Besides, bradycardia might be caused by treatments such as steroids. Further evidence is urgently needed to assess the mechanism of cardiac damage in MIS‐C patients.
This is the first nationwide study focusing on the epidemiological characteristics, laboratory findings, system involvement and outcomes of patients with MIS‐C in Turkey. This study has several limitations. First, we excluded the patients who were not proved SARS‐CoV‐2 by laboratory tests or contact with a person with proven COVID‐19. We did not show the evidence of SARS‐CoV‐2 infection in these patients. However, since serological tests' reliability in publications is around 70%, it is thought that excluded patients may be overlooked. 15 Second, the patients were treated in different hospitals, so their treatment protocols were not applied in consensus. Although long‐term follow‐up of the patients after discharge was not the subject of this study but studies are needed in terms of long‐term results of MIS‐C. Finally, due to the increased rates of seroconversion in the population with the spread of SARS‐CoV‐2 infection, symptoms of MIS‐C, KD or acute COVID‐19 might be intertwined. Therefore, revealing the pathogenesis of MIS‐C may guide in diagnosis.
Conclusion
The clinical spectrum of MIS‐C is broad, but clinicians should consider MIS‐C in the differential diagnosis when persistent fever, fatigue and gastrointestinal symptoms are prominent. Most patients diagnosed with MIS‐C appear to have been previously healthy. Immunomodulatory treatments and supportive intensive care are important in the management of cases with MIS‐C. Glucocorticoids and intravenous immunoglobulins are the most common immunomodulatory treatment options for MIS‐C. Prompt diagnosis and prompt treatment are essential for the management of these patients.
Conflict of interest: None declared.
Author's contribution: Yilmaz‐Ciftdogan and Ekemen‐Keles conceptualized and designed the study, collected data, drafted the initial manuscript and reviewed and revised the manuscript. Karbuz, Cetin, Elmas Bozdemir, Kepenekli Kadayifci, Ozer, Erat, Sutcu, Buyukcam, Belet, Oncel, Orbak, Turel, Gayretli Aydin, O Kilic, Yahsi, Kara Aksay, Ergenc, Petmezci, Oflaz, Sarikaya, Otar Yener, Ozen, Gul, Arslan, SS Kara, Demirkol, Yazici Ozkaya, Yozgat, Varan, M Kara, Arga, Yakut, AO Kilic, Cakici, Kucuk, Kaba, Karaoglu Asrak, Bursal Duramaz, Dalkiran, Berna Anil, Turgut, Karapinar, Somer, Elmali, Dinleyici and Ciftci designed the data collection instruments, collected data and reviewed and revised the manuscript. Kara Aksay, Cetin, Elmas Bozdemir, Kepenekli Kadayifci, Metin Akcan, Erdeniz, Dalgic Karabulut, Hancerli Torun and A Kara conceptualized and designed the study, drafted the initial manuscript, reviewed, coordinated and supervised data collection and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1. Dong Y, Mo X, Hu Y et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics 2020; 145: e20200702 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 2. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet 2020; 23: 1607–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verdoni L, Mazza A, Gervasoni A et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: An observational cohort study. Lancet 2020; 395: 1771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whittaker E, Bamford A, Kenny J et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA 2020; 324: 259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moraleda C, Serna‐Pascual M, Soriano‐Arandes Soriano‐Arandes A, Simó S, Epalza C, Santos M, Grasa C, Rodríguez M, Soto B, Gallego N, Ruiz Y, Urretavizcaya‐Martínez M, Pareja M, Sanz‐Santaeufemia FJ, Fumadó V, Lanaspa M, Jordan I, Prieto L, Belda S, Toral‐Vázquez B, Rincón E, Gil‐Villanueva N, Méndez‐Echevarría A, Castillo‐Serrano A, Rivière JG, Soler‐Palacín P, Rojo P, Tagarro A, EPICO‐AEP Working Group . Multi‐inflammatory syndrome in children related to SARS‐CoV‐2 in Spain. Clin. Infect. Dis. 2021;72:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toubiana J, Poirault C, Corsia A et al. Kawasaki‐like multisystem inflammatory syndrome in children during the covid‐19 pandemic in Paris, France: Prospective observational study. BMJ 2020; 369: m2094 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feldstein LR, Rose EB, Horwitz SM et al. Multisystem inflammatory syndrome in US children and adolescents. N. Engl. J. Med. 2020; 383: 334–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Royal College of Paediatrics and Child Health (2020), Health C. Guidance‐Paediatric multisystem inflammatory syndrome temporally associated with COVID‐19 Available from: . https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance. [DOI] [PubMed]
- 9. Singh‐Grewal D, Lucas R, McCarthy K et al. Update on the COVID‐19‐associated inflammatory syndrome in children and adolescents; paediatric inflammatory multisystem syndrome‐temporally associated with SARS‐CoV‐2. J. Paediatr. Child Health 2020; 56: 1173–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention (2020). Multisystem Inflammatory Syndrome in Children (MIS‐C) Associated with Coronavirus Disease 2019 (COVID‐19). Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6932e2.htm [accessed August 2020].
- 11. World Health Organization (2020). Multisystem inflammatory syndrome in children and adolescents with COVID‐19 Available from:. https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 [].
- 12. Dufort EM, Koumans EH, Chow EJ et al. Multisystem inflammatory syndrome in children in New York state. N. Engl. J. Med. 2020; 383: 347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCrindle BW, Rowley AH, Newburger JW et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation 2017; 135: e927–99. [DOI] [PubMed] [Google Scholar]
- 14. Mamishi S, Movahedi Z, Mohammadi M et al. Multisystem inflammatory syndrome associated with SARS‐CoV‐2 infection in 45 children: A first report from Iran. Epidemiol. Infect. 2020; 28: e148–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu R, Liu X, Han H et al. The comparative superiority of IgM‐IgG antibody test to real‐time reverse transcriptase PCR detection for SARS‐CoV‐2 infection diagnosis. medRxiv 2020; 1–17. 10.1101/2020.03.28.20045765 (forthcoming). [DOI] [Google Scholar]
- 16. Dong Y, Mo X, Hu Y et al. Epidemiology of COVID‐19 among children in China. Pediatrics 2020; 145: e20200702 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 17. Wölfel R, Corman VM, Guggemos W et al. Virological assessment of hospitalized patients with COVID‐2019. Nature 2020; 581: 465–9. [DOI] [PubMed] [Google Scholar]
- 18. Traugott M, Aberle SW, Aberle JH et al. Performance of severe acute respiratory syndrome coronavirus 2 antibody assays in different stages of infection: Comparison of commercial enzyme‐linked immunosorbent assays and rapid tests. J Infect Dis 2020; 222: 362–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee PY, Day‐Lewis M, Henderson LA et al. Distinct clinical and immunological features of SARS‐COV‐2‐induced multisystem inflammatory syndrome in children. J. Clin. Invest. 2020; 130: 5942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pouletty M, Borocco C, Ouldali N et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS‐CoV‐2 mimicking Kawasaki disease (Kawa‐COVID‐19): A multicentre cohort. Ann. Rheum. Dis. 2020; 79: 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dominguez SR, Anderson MS, El‐Adawy M, Glodé MP. Preventing coronary artery abnormalities: A need for earlier diagnosis and treatment of Kawasaki disease. Pediatr. Infect. Dis. J. 2012; 31: 1217–20. [DOI] [PubMed] [Google Scholar]
- 22. Rowley AH, Shulman ST. Kawasaki syndrome. Pediatr. Clin. 1999; 46: 313–29. [DOI] [PubMed] [Google Scholar]
- 23. Yan R, Zhang Y, Li Y, Xia L., Guo Y., Zhou Q. Structural basis for the recognition of the SARS‐CoV‐2 by full‐length human ACE2. Science 2020;367:1444–48. doi:10.1126/science.abb2762. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li W, Moore MJ, Vasilieva N et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426: 450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xie X, Chen J, Wang X et al. Age‐ and gender‐related difference of ACE2 expression in rat lung. Life Sci. 2006; 78: 2166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuba K, Imai Y, Rao S, Jiang C, Penninger JM. Lessons from SARS: Control of acute lung failure by the SARS receptor ACE2. J. Mol. Med. 2006; 84: 814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


