Key Points.
Croup can be one of the many manifestations of COVID‐19 in children.
SARS‐CoV‐2 can lead to a more severe and acute presentation refractory to initial treatment.
More studies are needed to establish the current course and treatment of COVID‐19 croup.
The new coronavirus (SARS‐CoV‐2) responsible for the COVID‐19 disease can affect children of all ages. 1 The disease is usually milder in children compared with adults. 1
Most cases present asymptomatically or with symptoms such as cough or fever. 2 Croup (or laryngotracheitis) is a common childhood illness, mainly in pre‐school‐aged children, usually caused by viral infection. Parainfluenzaviruses (mainly types 1 and 3), respiratory syncytial virus, influenza, rhinovirus and enterovirus are among the main culprits. 3 Viral croup is usually a self‐limited illness with most cases occurring in the fall or winter. 3 , 4
We report a case of severe croup requiring intensive care in a healthy boy who was found to have SARS‐CoV‐2 infection. This case report joins the few case reports of severe upper airway disease COVID‐19 related cases, and adds to the growing literature of the SARS‐CoV‐2 clinical presentations. 4 , 5 , 6 , 7 , 8
Case Report
In May 2021, a previously healthy 11‐year‐old male presented to the emergency department with sudden dyspnoea, dysphonia and cough. He had not received Haemophilus influenzae type b (Hib) vaccination. There was no history of foreign body inhalation, drug intake or recent sick contacts, including COVID‐19 positive patients.
On admission, he was febrile (38.3°C), tachypnoeic (47 breaths per minute), hypoxaemic (83% oxygen saturation in room air) and had a blood pressure of 117/73 mmHg. His physical examination revealed inspiratory stridor at rest, barking cough, global chest retraction and no wheezing. He was able to talk only in short sentences.
Capillary blood gas revealed respiratory acidosis (pH 7.32, pCO2 47.4 mmHg).
Oral dexamethasone (0.18 mg/kg/dose), intravenous methylprednisolone (1 mg/kg/dose) and nebulised adrenaline (2 mL) were administered with slight improvement, but persistent hoarseness and stridor at rest.
Due to early concern for epiglottitis, in view of the patient's being unvaccinated against Hib, ceftriaxone was administered.
Later on, when the patient showed more stable clinical condition, neck and chest radiographs were obtained.
Anteroposterior cervical radiograph revealed severe glottis/subglottic narrowing with a ‘steeple sign’ image (Fig. 1). Lateral soft tissue neck radiograph revealed no signs of epiglottitis and chest radiograph was normal. Complete blood count was unremarkable with normal serum C‐reactive protein. Blood cultures taken before antibiotic therapy were negative. His respiratory viral panel tested by polymerase chain reaction (PCR) was negative for 20 viruses tested in the panel, including respiratory syncytial virus, influenza A/B and four types of parainfluenzavirus; the only positive was for SARS‐CoV‐2 infection.
Fig. 1.
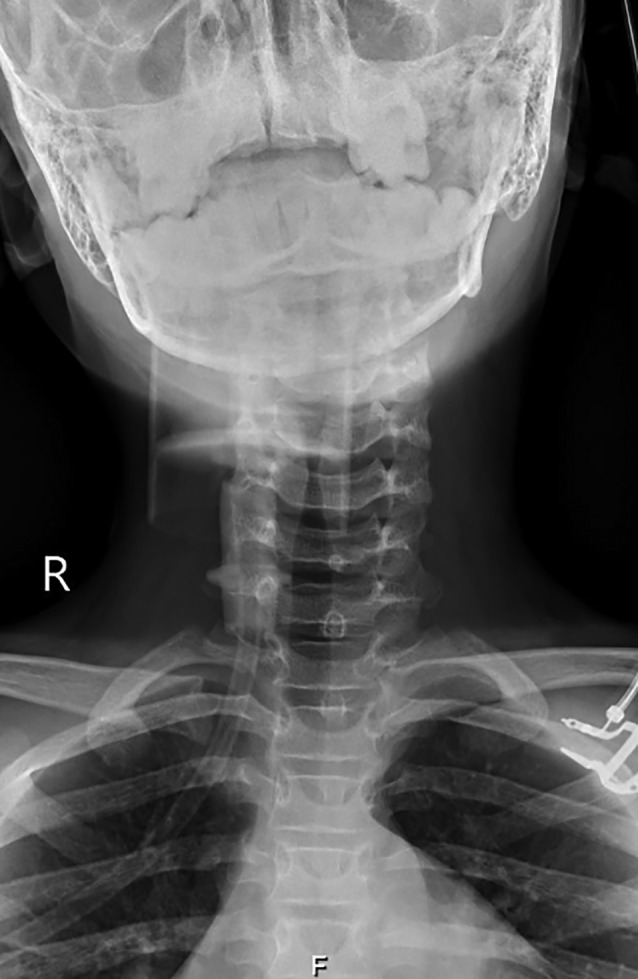
Anteroposterior cervical radiograph revealed severe glottis/subglottic narrowing with a ‘steeple sign’ image.
Otorhinolaryngology was consulted for persistent stridor; the patient was admitted to the paediatric intensive care unit (PICU) for close cardiopulmonary monitoring with supplementary oxygen at 6 L/min by face mask.
He was treated with nebulised adrenaline (2 mL) every 4 h, methylprednisolone (1 mg/kg/day) and ceftriaxone (100 mg/kg/day).
After 24 h PICU admission, he had a barking cough, hoarseness and stridor only at exertion, and was transferred to the paediatric ward. He was discharged after 3 days of hospitalisation.
Discussion
Upper airway obstruction causes can range from croup to epiglottitis, foreign body inhalation and anaphylaxis. In the post‐Hib vaccine era, there are few acute epiglottitis cases, which tend to occur in older children. 3 , 4 , 5 , 6
SARS‐CoV‐2 infection has been recently reported to be a rare cause of croup in children. Coronaviruses have been implicated before: Hoek et al. 9 described a strong association of human coronavirus NL63 (HCoV‐NL63) with croup in young children, being frequently found in young children with lower respiratory tract infections. The diagnosis of croup is clinical, dismissing the need for radiograph or laboratory tests. 3 However, identifying a specific viral aetiology may be helpful regarding cohort isolation and public health issues.
Our patient had stridor at rest which was relatively unresponsive to nebulised adrenaline, which works by decreasing airway oedema. 3 McGrath et al. 10 describe the potential of SARS‐CoV‐2 to cause subtle airway oedema resulting in intubation failure and stridor following tracheal extubation in some patients. As a result, they recommend treating the airway of COVID‐19 patients as a technical challenge.
To our knowledge, there are few reported paediatric cases of COVID‐19 related upper airway disease 4 , 5 , 6 , 7 , 8 ; of the seven reported cases, four needed PICU admission. 4 , 6 , 7 , 8 In one case series, 4 all three patients had more than three nebulised adrenaline cycles together with corticosteroid treatment and one received additional treatment with BiPAP and heliox. Nadiger et al. 6 describe the first case of supraglottitis with vocal cord hypomobility who needed high dose steroids and nebulised epinephrine for a week; on follow‐up, the patient presented with persistent hoarseness and dysphagia and is, currently, receiving speech therapy. Aghdam et al. 7 report the second case of bacterial superinfection pneumonia following COVID‐19 croup in a child who received mechanical ventilation for 17 days. In a recent report, Lim et al. 8 describe the evolution of croup secondary to SARS‐CoV‐2 in a young child who subsequently developed multi‐inflammatory syndrome of childhood. Two of the patients developed seizures, probably hypoxia‐induced by severe airway obstruction. 7 , 8
Fewer than 15% of children with croup require hospital admission, which is usually short and requires little intervention. 3 Only 1% require PICU admission. 3 In a retrospective descriptive study, paediatric patients with croup who received ≥3 nebulised adrenaline were more likely to need intensive care. 4 These findings suggest a more severe COVID‐19 croup versus the previously described group.
Unlike most cases of croup, which are more frequent in fall or winter, our patient developed croup during late spring, as in the Pitstick et al. 5 and Nadiger et al. 6 case reports. In Venn et al.'s 4 case series and Aghdam et al. 7 case report, the disease was also reported in the summer.
As the COVID‐19 pandemic continues to evolve, new clinical presentations are emerging. It is important for clinicians to be aware of unusual presentations. Children with SARS‐CoV‐2 infection can present with severe croup and might not improve as fast as in a typical case of croup. Given the current pandemic, screening for SARS‐CoV‐2 in paediatric croup may have prognostic significance and may direct appropriate infection control measures to limit disease transmission.
Conflict of interest: None declared.
References
- 1. Howard‐Jones A, Bowen AC, Danchin M et al. COVID‐19 in children: I. Epidemiology, prevention and indirect impacts. J. Paediatr. Child Health 2022; 58: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J. SARS‐CoV‐2 infection in children. N. Engl. J. Med. 2020; 382: 1663–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjornson CL, Johnson DW. Croup in children. Can. Med. Assoc. J. 2013; 185: 1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venn AMR, Schmidt JM, Mullan PC. Pediatric croup with COVID‐19. Am. J. Emerg. Med. 2021; 43: e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pitstick CE, Rodriguez KM, Smith AC, Herman HK, Hays JF, Nash CB. A curious case of croup: Laryngotracheitis caused by COVID‐19. Pediatrics 2021; 147: e2020012179. [DOI] [PubMed] [Google Scholar]
- 6. Nadiger M, Ness‐Cochinwala M, Sanchez‐Vegas C et al. Pediatric COVID‐19 presenting as supraglottitis with vocal cord hypomobility. SAGE Open Med. Case Rep. 2021; 9: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aghdam MK, Mirzaee HS, Eftekhari K. Croup is one of the clinical manifestations of novel coronavirus in children. Case Rep. Pulmonol. 2021; 2: 8877182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim CC, Saniasiaya J, Kulasegarah J. Croup and COVID‐19 in a child: A case report and literature review. BMJ Case Rep. 2021; 14: e244769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van der Hoek L, Sure K, Ihorst G et al. Croup is associated with the novel coronavirus NL63. PLoS Med. 2005; 2: e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McGrath BA, Wallace S, Goswamy J. Laryngeal oedema associated with COVID‐19 complicating airway management. Anaesthesia 2020; 75: 962–77. [DOI] [PubMed] [Google Scholar]


