At a time when hepatitis C virus clearance can be obtained by DAAs in almost all infected patients, global infection burden control is an objective within reach, even if achieving the WHO HCV elimination targets by 2030 may not be attainable. 1 The lowest cost intervention is an awareness campaign to bring in those who are recently diagnosed and those who were previously diagnosed but not treated. Only 30% of all HCV‐diagnosed patients are linked‐to‐care. 2 The next level of intervention is case‐finding for disease control and screening. Screening invites people who do not have symptoms to undergo testing, whereas health professionals are focused on detecting conditions as early as possible among people with symptoms to avoid late clinical presentation. 3 , 4 With continuous efforts for disease control as a priority, early diagnosis in those with liver disease, but unknown HCV status, is the key intervention to avoid further disease progression and costs (Figure 1).
FIGURE 1.
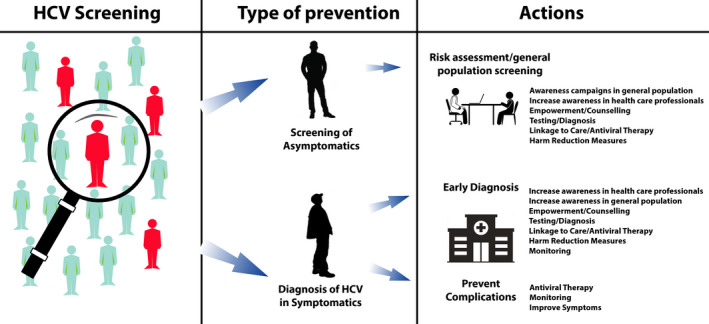
HCV screening for disease control and infection burden reduction
To achieve HCV elimination, screening programs in the general population, who are unaware of the infection status, are needed, focusing on the social and age groups most at risk for viral transmission. 5 Universal screening, while theoretically feasible and conceivably highly effective in identifying most HCV‐infected persons, would put major organizational and financial hurdles in countries with low‐to‐moderate prevalence. 6 , 7 , 8 Hence, if appropriately conceived, focused screenings would identify a high rate of infected subjects, because of the higher infection rate related to risk factors, and simultaneously reduce onward viral transmission by allowing to clear HCV from those at higher risk of spreading the infection. 9 , 10 , 11 , 12 , 13
An important step in deciding on a mass screening program is to model the number of people with infection and their outcomes over time. The challenges in defining the HCV prevalence values have been partly covered by modelling exercises which, despite not providing the precise values, are useful to understand whether the screening is cost‐effective and affordable. 8 , 13 , 14 , 15 In Italy, a cost‐effectiveness analysis, based on modelling chronic infection burden in untreated individuals, focused on persons born in the period 1948–1987, graduating the intervention in the first 2 years in key populations and younger cohort (1968–1987), to detect those persons most exposed to risk factors, specifically, prior or current drug use, tattoos and other at‐risk cosmetics or nosocomial interventions. 8 These individuals are asymptomatic, because of the short time of chronic infection and have a low awareness of their exposure risk and disease progression. In light of these considerations and based on HCV elimination goals, screening firstly this cohort would be the most cost‐effective strategy to reduce both the infection prevalence and incidence. 8 , 16
One of the models proposed to contain the medical and non‐medical infrastructure costs and to reach the targeted population is HCV screening to be held simultaneously with SARS‐CoV‐2 testing or COVID‐19 vaccination. 17 Two papers published in this issue of Liver International address HCV screening during COVID‐19 vaccination in Milan (Italy) and during SARS‐CoV‐2 testing and COVID‐19 vaccination in Salerno (Italy). 8 , 9 The approaches are reported as an HCV testing opportunity; however, several concerns on the expected HCV prevalence have been raised. The active infection prevalence was found to range from 0.07% to 0.1%, less than the estimated prevalence. 14 , 18 , 19 These pilot studies pose a matter of debate if the screening program extension to subjects born before 1969 could lead to improved HCV screening effectiveness.
Pilot testing can be an important preparatory step to scaling up the screening, but it should be representative of the average conditions of the target population in which the large‐scale screening program will function. 2 , 3 The success of any screening program will be determined by its coverage, uptake, which is the proportion of people who were invited and actually get screened, and linkage to care. Both studies did not report sufficient data to evaluate the sample representativeness versus the target population within a context of a refusal rate among different settings ranging from 15 to 70%, possibly owing to poor information, disease awareness and several potential selection biases acknowledged by the authors. Data from the Naples study show the presence of anti‐HCV in seven individuals, four of them in age 30–50 years. 19 These opportunistic anti‐HCV testing, a marker of exposure to the virus not necessarily related to active infection, yielded a significantly higher anti‐HCV positivity prevalence among individuals screened for SARS‐Cov2 (5.9%) than in those undergoing COVID‐19 vaccination (0.2%), strongly suggesting different populations in terms of cultural background, a key factor for evaluating the presence of risk factors for acquiring HCV infection.
People with high socioeconomic status and a low risk of having severe conditions tend to participate more in vaccination and screening programs than socioeconomically deprived people, who may have a higher risk of infection/disease. Social and cultural factors can influence screening participation, with it being lower among disadvantaged and underprivileged populations. This can lead to increasing health inequalities which should be addressed very carefully in a screening program. Moreover, it should be considered that, though the adherence of a population tested or vaccinated for COVID‐19 would be presumably greater (and with greater awareness of the value of prevention), for the same reasons the expected number of persons infected with HCV in the selected population could be lower than the general estimates.
The low prevalence found raises questions on the screening value in younger cohorts, as a prioritized screening intervention, to reach HCV elimination targets. Could the estimated prevalence of HCV active infection in the young population be related only to infection in active drug users and inmates, addressed by the free of charge screening in Italy 16 ? If so, a public health intervention, limiting the action only to micro‐elimination programs and harm‐reduction interventions, is obviously the better choice versus the mass screening. 5 The HCV infection risk factors are well defined in key populations such as drug users; however, previous drug users, inapparent nosocomial or aesthetic procedures, and other transmission routes have been reported and estimated in young asymptomatic people. 9 , 10 , 11 , 12 , 14 , 15 Active screening in the 1969–1989 cohort, with an estimated overall prevalence of 0.3%–0.6%, means focusing on better containing the risk of new infections, responding to the unexpressed health needs of an age group that is at risk of infection and disease progression, focusing also on a domino effect of screening for the entire family groups of those subjects (30–50 years old) with more frequent sexual relations and females of childbearing age. 8 , 13 Moreover, focusing on this age group entails strengthening and replicating the effect of micro‐elimination efforts on persons who use drugs and inmates (who on average are in the same age range), because it provides the opportunity to repeat screening also outside prisons and drug dependency centres and to perform it on their closest family and social contacts.
Although free‐of‐charge screening in Italy, firstly prioritized specific key populations and the 1969–1989 cohort as the best cost‐effectiveness strategy, additional educational and organizational planning and dedicated funds are necessary to give access to screening of the 1948–1968 cohort and other vulnerable at‐risk populations. 8 , 16 These efforts are indispensable to successfully perform the HCV screening program in Italy and to guarantee equity in diagnosis and treatment access.
Implementing and sustaining a screening program requires extensive human resources and health system capacity. As a rule of thumb, real‐life cost evaluations are useful in adapting a screening program. In both Italian studies, owing to the low prevalence of active infection found, the cost per detection of an active infection is shown to be high. 18 , 19 The data of both studies could be of help in determining the best resources allocation in the different options of screening settings. However, these estimations are not sufficient to query the cost‐effectiveness of a mass screening which requires Health Technology Assessment tools that evaluate the cost versus the long‐term efficacy of a public health intervention. With specific regard to HCV screening, it is evaluated as a cost‐effective intervention, including all screening and disease costs over time, in different countries and with particular regards in Italy, the best cost‐effectiveness profile was given by a graduated strategy. 6 , 7 , 8 Each strategy was weighted for the uncertainties on the prevalence values of active infection, evaluating the short‐mid and long‐term costs and benefits to the health system in the perspective of achieving the elimination by 2030. 8 Because a screening program is not just a single test but rather a pathway that starts by identifying the eligible people and stops when the outcomes are reported, the further treatment costs of patients diagnosed have shown to be economically balanced by the possible expenditure on medical care in Italy. 20 A POCT for COVID‐19 screening or vaccination should increase the HCV screening uptake and reduce the cost of HCV screening by using the same resources for both interventions, otherwise, as it has been shown in both Italian studies, the increasing costs cannot be justified. 18 , 19
In conclusion, despite the feasibility of this opportunistic approach, we strongly believe that HCV screening with COVID‐19‐related services is a chance too good to be missed, but should not be a potential generator of chance findings that could distract from the main aim of HCV elimination.
CONFLICT OF INTEREST
All authors declare no conflict of interest related to the subject matter of this paper.
Funding information
No funds were used for this Editorial.
References
- 1. WHO . Global Health Sector Strategy on Viral Hepatitis, 2016–2021 Towards Ending Viral Hepatitis. World Health Organization; 2016. https://www.who.int/hepatitis/strategy2016‐2021/ghss‐hep/en/ [Google Scholar]
- 2. Safreed‐Harmon K, Blach S, Aleman S, et al. The consensus hepatitis C cascade of care: standardized reporting to monitor progress toward elimination. Clin Infect Dis. 2019;69(12):2218‐2227. [DOI] [PubMed] [Google Scholar]
- 3. Screening Program a Short Guide Increased Effectiveness Maximize Benefits and Minimize Harm. World Health Organization; 2020. ISBN 978 92 890 5478 2 [Google Scholar]
- 4. Sagan A, McDaid D, Rajan S, Farrington J, McKee M. Screening. When is it appropriate and how can we get it right? Health systems and policy analysis. Policy Brief 35 Print ISSN 1997–8065 Web ISSN 1997–8073 [PubMed]
- 5. Cox AL, El‐Sayed MH, Kao J‐H, et al. Progress towards elimination goals for viral hepatitis. Nat Rev Gastroenterol Hepatol. 2020;17:533‐542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deuffic‐Burban S, Huneau A, Verleene A. Assessing the cost‐effectiveness of hepatitis C screening strategies in France. J Hepatol. 2018;69:785‐792. [DOI] [PubMed] [Google Scholar]
- 7. Cortesi PA, Barca R, Giudicatti G, et al. Systematic review: economic evaluations of HCV screening in the direct‐acting antivirals era. Aliment Pharmacol Ther. 2019;49:1126‐1133. [DOI] [PubMed] [Google Scholar]
- 8. Kondili LA, Gamkrelidze I, Blach S, et al. Optimization of hepatitis C virus screening strategies by birth cohort in Italy. Liver Int. 2020;40:1545‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andreoni M, Giacometti A, Maida I, Meraviglia P, Ripamonti D, Sarmati L. HIV‐HCV co‐infection: epidemiology, pathogenesis and therapeutic implications. Eur Rev Med Pharmacol Sci. 2012;16:1473‐1483. [PubMed] [Google Scholar]
- 10. Mariano A, Mele A, Tosti ME, et al. Role of beauty treatment in the spread of parenterally transmitted hepatitis viruses in Italy. J Med Virol. 2004;74:216‐220. [DOI] [PubMed] [Google Scholar]
- 11. Spada E, Mele A, Mariano A, Zuccaro O, Tosti ME; SEIEVA collaborating group . Risk factors for and incidence of acute hepatitis C after the achievement of blood supply safety in Italy: results from the national surveillance system. J Med Virol. 2013;85:433‐440. [DOI] [PubMed] [Google Scholar]
- 12. Spada E, Rezza G, Garbuglia AR, et al. Incidence and risk factors for hepatitis C virus infection among illicit drug users in Italy. J Urban Health. 2018;95:99‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kondili LA, Robbins S, Blach S, et al. Forecasting hepatitis C liver disease burden on real‐life data. Does the “hidden iceberg” matter to reach the elimination goals? Liver Int. 2018;38:2190‐2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kondili LA, Andreoni M, Alberti A, et al. Estimated prevalence of undiagnosed HCV infected individuals in Italy: a mathematical model by route of transmission and fibrosis progression. Epidemics. 2021;34:100442. [DOI] [PubMed] [Google Scholar]
- 15. Kondili LA, Andreoni M, Alberti A, et al. A mathematical model by route of transmission and fibrosis progression to estimate undiagnosed individuals with HCV in different Italian regions. BMC Infect Dis. 2022;22:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kondili LA, Aghemo A, Andreoni A, et al. Milestones to reach hepatitis C virus (HCV) elimination in Italy: from free‐of‐charge screening to regional roadmaps for an HCV‐free nation. Dig Liver Dis. 2022;54:237‐242. [DOI] [PubMed] [Google Scholar]
- 17. Kondili LA, Craxi A, Aghemo A. Absolute targets for HCV elimination and national health policy paradigms: foreseeing future requirements. Liver Int. 2021;41:649‐655. [DOI] [PubMed] [Google Scholar]
- 18. D’Ambrosio R, Rizzardini G, Puoti M, et al. Implementation of HCV screening in the 1969–1989 birth cohort undergoing COVID‐19 vaccination: a pivotal study in Italy. Liver Int. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Torre P, Annunziata M & Sciorio R et al. Hepatitis C screening during SARS‐CoV‐2 testing or vaccination. Experience in an area of Southern Italy in the province of Salerno [DOI] [PMC free article] [PubMed]
- 20. Marcellusi A, Simonelli C, Mennini FS, Kondili LA; PITER collaborating group . Economic consequences of anti‐HCV treatment of patients diagnosed through screening in Italy: a prospective modelling analysis. Appl Health Econ Health Policy. 2022;20:133‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]


