Abstract
Background
Previous studies reported regional differences in end‐of‐life care (EoLC) for critically ill patients in Europe.
Objectives
The purpose of this post‐hoc analysis of the prospective multicentre COVIP study was to investigate variations in EoLC practices among older patients in intensive care units during the coronavirus disease 2019 pandemic.
Methods
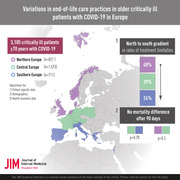
A total of 3105 critically ill patients aged 70 years and older were enrolled in this study (Central Europe: n = 1573; Northern Europe: n = 821; Southern Europe: n = 711). Generalised estimation equations were used to calculate adjusted odds ratios (aORs) to population averages. Data were adjusted for patient‐specific variables (demographic, disease‐specific) and health economic data (gross domestic product, health expenditure per capita). The primary outcome was any treatment limitation, and 90‐day mortality was a secondary outcome.
Results
The frequency of the primary endpoint (treatment limitation) was highest in Northern Europe (48%), intermediate in Central Europe (39%) and lowest in Southern Europe (24%). The likelihood for treatment limitations was lower in Southern than in Central Europe (aOR 0.39; 95% confidence interval [CI] 0.21–0.73; p = 0.004), even after multivariable adjustment, whereas no statistically significant differences were observed between Northern and Central Europe (aOR 0.57; 95%CI 0.27–1.22; p = 0.15). After multivariable adjustment, no statistically relevant mortality differences were found between Northern and Central Europe (aOR 1.29; 95%CI 0.80–2.09; p = 0.30) or between Southern and Central Europe (aOR 1.07; 95%CI 0.66–1.73; p = 0.78).
Conclusion
This study shows a north‐to‐south gradient in rates of treatment limitation in Europe, highlighting the heterogeneity of EoLC practices across countries. However, mortality rates were not affected by these results.
Keywords: COVID‐19, critical care, frail elderly, public health systems research, resuscitation orders
Introduction
Many individuals receive prolonged and invasive intensive care treatments at the end of their lives. The definition of treatment goals represents an important component in the treatment of critically ill patients, and therapy limitations need to be discussed when specific therapies become futile and/or death inevitable. These decisions can be difficult, and practices vary by physician, hospital and country. A general principle of medicine is to do no harm. However, there is a fine line between where modern intensive care encounters biological limits, especially in very old and severely ill patients. Finally and most importantly, the preservation of an individual's dignity and autonomy should always come first, and protracted suffering should be avoided. Avidan et al. recently confirmed in the Ethicus‐2 study that end‐of‐life practices are subject to regional variations [1]. These differences are observed worldwide, which is presumably due to social, religious, and/or legal reasons [1, 2]. However, a deeper understanding of these regional differences could contribute to a better mutual understanding and help decision makers adjust guidelines and organisational frameworks accordingly.
However, the data from Avidan et al. predate the COVID‐19 era [1]. The COVID‐19 pandemic put immense pressure on Europe's heterogenous but highly developed health‐care systems, and in particular on intensive care units (ICUs) [3]. Even before COVID‐19, older critically ill patients posed not only a medical but also an ethical challenge, as the added value of intensive care for older patients and especially octogenarians is the subject of ongoing debate [4, 5, 6, 7]. In addition to a clinical assessment and a structured evaluation of the severity of an acute illness, the evaluation of frailty—which reflects the functional capacity of patients prior to the acute illness—has also been proven to be helpful in increasingly ageing societies [8]. In particular, the Clinical Frailty Scale (CFS), among others, has been confirmed by our group as an independent predictor of mortality in critically ill older patients [2, 9, 10].
The aim of this study was to investigate regional variations within Europe with regard to the use of treatment limitations in older (≥70 years) critically ill patients with COVID‐19. Furthermore, baseline characteristics and mortality between three distinct European regions were compared.
Materials and methods
COVIP
COVIP (COVID‐19 in very old intensive care patients) is a multicentre investigation, which is part of the Very Old Intensive Care Patients (VIP) project (www.vipstudy.org). The study has been endorsed by the European Society of Intensive Care Medicine (ESICM). COVIP was registered at ClinicalTrials.gov (NCT04321265). The COVIP study adhered to the European Union General Data Privacy Regulation (GDPR) directive. As in the previous VIP studies, the national coordinators recruited the ICUs, coordinated national and local ethical permissions and supervised patient recruitment at the national level [2, 10]. The COVIP study protocol is available at https://vipstudy.org/covip‐study/.
Study participants and general information
In this post‐hoc analysis of the prospective COVIP study, all patients aged 70 years and older admitted to the ICU with confirmed COVID‐19 by means of polymerase chain reaction with complete data on the primary endpoint (any treatment limitation) were included. All patients admitted between 19 March 2020 and 4 February 2021 were included. Data collection started upon ICU admission. The admission day was defined as day one, and all consecutive days were numbered sequentially from the admission date. For each patient, baseline characteristics (including age, sex, main reason for admission and frailty) and management strategies (including use of renal replacement therapy [RRT], mechanical ventilation [MV], noninvasive ventilation [NIV] and use of vasoactive drugs) were documented. Also, any treatment limitations (treatment withheld or withdrawn) were documented. Patients were defined as belonging to Central Europe (Austria, Belgium, France, Germany, Poland, Switzerland), Northern Europe (Denmark, Ireland, Netherlands, Norway, UK) or Southern Europe (Greece, Israel, Italy, Portugal, Romania, Spain). In this respect, we have used a country classification similar to the Ethicus‐2 study [11]. Ninety‐day follow‐up was obtained by means of telephone interviews. The primary endpoint of this study was any treatment limitation, and the secondary endpoints were ICU‐, 30‐ and 90‐day‐mortality rates and withholding and withdrawing treatment. Frailty was assessed by the Clinical Frailty Scale (CFS), and the respective visual and simple descriptions were used with permission [8, 12]. The gross domestic product (GDP) per capita for 2019 in USD was retrieved from the International Money Fund (IMF) [13], the human development index (HDI) from the United Nations Development Program (UNDP) [14] and the total (compulsory, out‐of‐pocket, voluntary) amount of health spending per capita in USD in 2019 from the Organisation for Economic Co‐operation and Development (OECD) [15]. A general review of the literature prior to conducting the study was conducted, and the corresponding search terms can be found in Supplement 1.
Each study site obtained institutional research ethics board approval. Many countries could recruit patients without informed consent while others had to collect informed consent as ethical consent practices vary across Europe.
Statistical analysis
The primary exposure was belonging to one of the three European regions. Central Europe was chosen as the reference category and compared to either Northern or Southern Europe. Missing data (including loss to follow‐up) were addressed by listwise deletion. The data are likely to be clustered at an ICU level. To compensate for possible confounders, a multilevel regression analysis was used. As health economic data do not vary within a given cluster (as patients in one ICU belong to one country), generalised estimation equations with robust standard errors were used to produce population average odds ratios for the binary endpoints treatment limitation and mortality. Adjusted odds ratios (aORs) and respective 95% confidence intervals (95%CI) were obtained. We fitted a multilevel linear regression model to evaluate the association of the primary exposure with the length of ICU stay as dependent variable and obtained regression coefficients and respective 95%CIs. The regression analyses were conducted using only robust estimators of the standard errors and not in the sense of robustness against violations of normality assumptions as for the methods (e.g., Mann–Whitney tests) used for the univariate analyses. Model‐1 includes only the ICU as a panel. Model‐2 includes patient‐specific factors (sex, age per year, sequential organ failure assessment [SOFA] score per point, frailty scale per CFS point). Model‐3 adds the number of ICU beds per 100,000 inhabitants. Model‐4 includes the health economic data (HDI). Model‐5 adds the treatment limitations (treatment withdrawal or withholding) and calculates mortality and the ICU length of stay. Sensitivity analyses stratifying treatment limitation were done. Continuous data are given as median ± interquartile range and compared using the Mann–Whitney U test or given as mean ± standard deviation and compared using the Student's t‐test. Categorical data are given as numbers (percentages) and compared using the chi‐square test. All tests were two sided, and a p‐value of <0.05 was considered statistically significant. Stata/IC 17 (Stata Statistical Software: Release 17. StataCorp LLC, College Station, Texas, USA) was used for all statistical analyses.
Results
Baseline demographics in Central versus Northern and Southern Europe
In total, 3105 older (≥70 years) critically ill patients were included in this study—1573 in Central, 821 in Northern and 711 in Southern Europe. The baseline characteristics are given in Table 1. No differences in sex distribution within regions were found. Patients in Central Europe (23%) were more frequently older than 80 years old compared to Northern (14%) and Southern Europe (16%) (p < 0.001). Likewise, patients in Central Europe (19%) were more likely to be frail and suffer from cardiovascular comorbidities than those from Northern and Southern Europe (Table 1). The ICUs included in Central Europe had a higher median number of ICU beds (12) compared to Northern (7) and Southern Europe (10) (p < 0.001). The results of the univariate analysis on baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of the cohort
| Patient‐specific variables | Central Europe (n = 1573) | Northern Europe (n = 821) | Southern Europe (n = 711) | p‐Value |
|---|---|---|---|---|
| Sex | 0.43 | |||
| Male, n (%) | 1117 (71) | 594 (72) | 493 (69) | |
| Female, n (%) | 456 (29) | 227 (28) | 218 (31) | |
| Age at admission, years | 76 (5) | 75 (4) | 75 (5) | <0.001 |
| Age categories | <0.001 | |||
| Age <80 years, n (%) | 1211 (77) | 703 (86) | 595 (84) | |
| Age >79 years, n (%) | 362 (23) | 118 (14) | 115 (16) | |
| BMI, kg/m2 | 28 (5) | 28 (5) | 29 (5) | 0.007 |
| Comorbidities | ||||
| Arterial hypertension, n (%) | 1084 (69) | 467 (57) | 509 (72) | <0.001 |
| Diabetes (any type), n (%) | 558 (36) | 240 (29) | 244 (34) | 0.007 |
| Ischemic heart disease, n (%) | 379 (24) | 194 (24) | 121 (17) | <0.001 |
| Chronic heart failure, n (%) | 237 (15) | 122 (15) | 87 (12) | 0.19 |
| Pulmonary comorbidity, n (%) | 359 (23) | 206 (25) | 134 (19) | 0.011 |
| Renal insufficiency, n (%) | 285 (18) | 118 (14) | 99 (14) | 0.009 |
| Frailty | ||||
| Clinical Frailty Scale, pts | 3 (2) | 3 (1) | 3 (1) | 0.002 |
| SOFA score on admission, pts | 6 (3) | 5 (3) | 6 (3) | <0.001 |
| Frailty categories | <0.001 | |||
| Fit, n (%) | 941 (60) | 507 (62) | 496 (70) | |
| Vulnerable, n (%) | 253 (16) | 114 (14) | 69 (9) | |
| Frail, n (%) | 288 (18) | 125 (15) | 84 (12) | |
| Frailty category unavailable, n (%) | 91 (6) | 75 (9) | 62 (9) | |
| Region‐specific variables | ||||
| ICU beds per 100,000 population, no. | 12 (12–16) | 7 (6–7) | 10 (6–10) | <0.001 |
| ICU bed categories | <0.001 | |||
| <10 ICU beds per 100,000, n (%) | 134 (9) | 821 (100) | 695 (98) | |
| ≥10 ICU beds per 100,000, n (%) | 1439 (91) | 0 (0) | 16 (2) | |
| HDI | 0.92 (0.03) | 0.94 (0.01) | 0.89 (0.02) | <0.001 |
| GDP per capita in USD | 41,897 (41,897–46,473) | 52,646 (42,379–59,770) | 29,993 (23,132–29,993) | <0.001 |
| Health spending per capita in USD | 5376 (5376–6646) | 5568 (4653–5765) | 3616 (3379–3616) | <0.001 |
Abbreviations: BMI, body mass index; GDP, gross domestic product; HDI, human development index; ICU, intensive care unit; SOFA, sequential organ failure assessment.
Organ support and management
In the univariate analysis, ICU length of stay was significantly longer in Southern Europe (median 21 days) than in Central and Northern Europe (median 15 days each; p < 0.001). This finding remained after performing multilevel linear regression analysis across all five models (see table in Supplement 2). The figure in Supplement 3 shows the median length of stay in the groups with and without treatment limitations according to the three regions. Intubation and MV rates were highest in Southern Europe (85%), followed by Central (69%) and Northern Europe (67%) (p < 0.001). Likewise, tracheostomy rates were highest in Southern Europe (31%) and lowest in Central Europe (16%) (p < 0.001). By contrast, the use of RRT was most common in Central Europe (18%), and least common in Northern Europe (12%) (p < 0.001). The results on management strategies of the univariate analysis are shown in Table 2.
Table 2.
Management strategies and endpoints
| Central Europe (n = 1573) | Northern Europe (n = 821) | Southern Europe (n = 711) | p‐Value | |
|---|---|---|---|---|
| Management strategies | ||||
| Noninvasive ventilation, n (%) | 419 (27) | 220 (27) | 160 (23) | 0.077 |
| Intubation and mechanical ventilation, n (%) | 1080 (69) | 550 (67) | 602 (85) | <0.001 |
| Tracheostomy, n (%) | 254 (16) | 163 (20) | 222 (31) | <0.001 |
| Vasoactive drugs used, n (%) | 1083 (69) | 555 (68) | 541 (77) | <0.001 |
| Renal replacement therapy used, n (%) | 290 (18) | 96 (12) | 108 (15) | <0.001 |
| ICU length of stay, days | 15 (15) | 15 (14) | 21 (17) | <0.001 |
| Primary endpoint | ||||
| Any treatment limitation, n (%) | 616 (39) | 396 (48) | 172 (24) | <0.001 |
| Secondary endpoints | ||||
| Life sustaining care withheld, n (%) | 527 (34) | 315 (38) | 139 (20) | <0.001 |
| Life sustaining care withdrawn, n (%) | 283 (18) | 249 (30) | 93 (13) | <0.001 |
| ICU mortality, n (%) | 692 (44) | 349 (43) | 354 (51) | 0.003 |
| 30‐day mortality, n (%) | 744 of 1550 (48) | 391 of 798 (49) | 341 of 696 (49) | 0.93 |
| 3‐month mortality, n (%) | 804 of 1436 (56) | 421 of 752 (56) | 399 of 644 (62) | 0.019 |
Note: Due to missing values, 30‐day and 3‐month outcomes were calculated only for the set with an available outcome value.
Abbreviation: ICU, intensive care unit.
Treatment limitation analysis
The frequency of the primary endpoint (any treatment limitation) was highest in Northern Europe (48%), lowest in Southern (24%) and intermediate in Central Europe (39%; p < 0.001). A decreasing incidence of withholding and withdrawing treatment was observed from north to south (Table 2). Table 3 shows the multivariable regression analyses. The odds for any treatment limitation were lower in Southern Europe compared to Central Europe (aOR 0.39 95%CI 0.21–0.73; p = 0.004) even after adjustment for patient‐specific characteristics, ICU beds per 100,000 population and the HDI. No statistically significant differences were observed between Northern and Central Europe (reference category) (aOR 0.57 95%CI 0.27–1.22; p = 0.15). The rates of withholding and withdrawing treatment showed a similar pattern, with the highest rates in Northern Europe and the lowest rates in Southern Europe (Table 2).
Table 3.
Generalised estimation equations based analysis producing population average odds ratios. Model‐1 includes only the ICU as the panel. Model‐2 includes patient‐specific factors (sex, age per year, SOFA score per point and frailty score per CFS point). Model‐3 adds the amount of ICU beds per 100,000 population. Model‐4 includes the HDI. Model‐5 adds the rates of any treatment limitations and hence calculated only 90‐day mortality. Adjusted odds ratios and respective 95%CIs were obtained
| Primary endpoint (any treatment limitation) | Secondary endpoint (90‐day mortality) | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p‐Value | OR | 95%CI | p‐Value | |
| Model‐1: ICU as panel variable | ||||||
| Central Europe | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Northern Europe | 1.53 | 1.10–2.14 | 0.01 | 0.96 | 0.71–1.30 | 0.81 |
| Southern Europe | 0.56 | 0.38–0.83 | 0.004 | 1.31 | 0.92–1.87 | 0.14 |
| Model‐2: model‐1 + age, sex, SOFA and CFS | ||||||
| Central Europe | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Northern Europe | 1.63 | 1.06–2.52 | 0.03 | 1.24 | 0.85–1.82 | 0.27 |
| Southern Europe | 0.48 | 0.26–0.88 | 0.02 | 1.43 | 0.97–2.11 | 0.07 |
| Model‐3: model‐2 + ICU beds per 100,000 population | ||||||
| Central Europe | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Northern Europe | 0.87 | 0.52–1.46 | 0.60 | 0.84 | 0.53–1.35 | 0.48 |
| Southern Europe | 0.30 | 0.15–0.58 | <0.001 | 1.11 | 0.71–1.73 | 0.66 |
| Model‐4: model‐3 + HDI | ||||||
| Central Europe | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Northern Europe | 0.57 | 0.27–1.22 | 0.15 | 1.29 | 0.80–2.09 | 0.30 |
| Southern Europe | 0.39 | 0.21–0.73 | 0.004 | 1.07 | 0.66–1.73 | 0.78 |
| Model‐5: model‐4 + any treatment limitation | ||||||
| Central Europe | N/A | N/A | N/A | ‐ | ‐ | ‐ |
| Northern Europe | N/A | N/A | N/A | 1.99 | 0.98–4.03 | 0.06 |
| Southern Europe | N/A | N/A | N/A | 1.40 | 0.75–2.61 | 0.29 |
Abbreviations: CFS, Clinical Frailty Scale; CI, confidence interval; HDI, human development index; ICU, intensive care unit; OR, odds ratio; SOFA, sequential organ failure assessment.
Mortality analysis
ICU mortality was highest in Southern Europe (51%), and lower in Northern (43%) and Central Europe (44%) (p = 0.003). The 30‐day mortality rate was similar in the three regions (Table 2). In univariate analysis, 90‐day mortality was highest in Southern Europe (62%) and similar in Central (56%) and Northern Europe (56%) (p = 0.019). After adjustment for patient‐specific characteristics, ICU beds per 100,000 population and HDI, no mortality differences were found between Northern Europe compared to Central Europe (aOR 1.29 95%CI 0.80–2.09; p = 0.30), and Southern Europe compared to Central Europe (aOR 1.07; 95%CI 0.66–1.73; p = 0.78). Figure 1 shows a comparison of the survival probability of patients from the three regions.
Fig. 1.
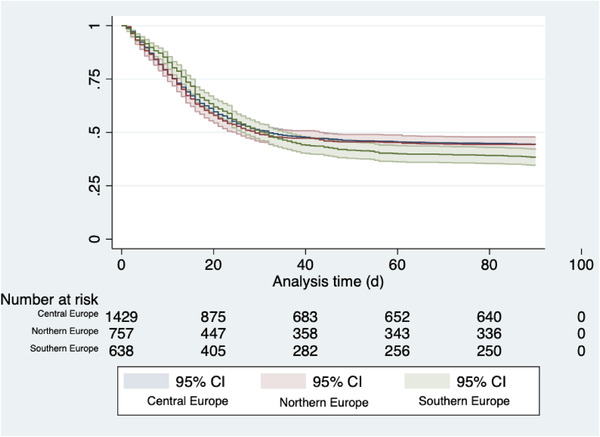
Kaplan–Meier curve depicting survival (with 95%CI) of patients in Northern Europe (red), Central Europe (blue) and Southern Europe (green). Abbreviation: CI, confidence interval.
Sensitivity analyses
In sensitivity analyses comparing treatment limitations between European regions, there was a consistent trend towards lower odds for any treatment limitation in Southern Europe versus Central Europe (see Forest plot in Fig. 2) and higher odds for any treatment limitation in Northern Europe versus Central Europe (see Forest plot in Fig. 3).
Fig. 2.
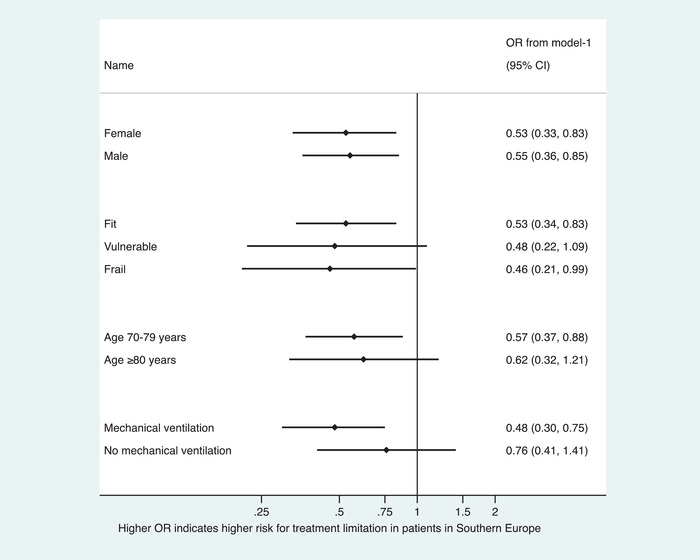
Sensitivity analyses stratifying any treatment limitations in subgroups for patient‐specific characteristics using generalised estimation equations producing population average odds ratios. The depicted adjusted ORs from model‐1 include only the intensive care unit as the panel. Abbreviations: CI, confidence interval; OR, odds ratio.
Fig. 3.
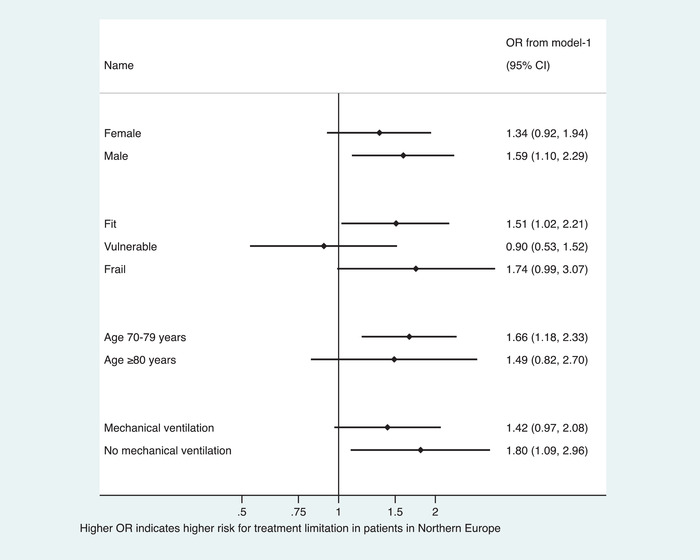
Sensitivity analyses stratifying any treatment limitations in subgroups for patient‐specific characteristics using generalised estimation equations producing population average odds ratios. The depicted adjusted ORs from model‐1 include only the intensive care unit as the panel. Abbreviations: CI, confidence interval; OR, odds ratio.
Discussion
In this study, differences in the incidence of treatment limitations among critically ill older (≥70 years) patients in three European regions were investigated. A north–south divide was observed—treatment limitations were more common in Northern Europe than in Southern Europe. However, the use of treatment limitations does not appear to translate into a mortality difference after correcting for various confounders.
In general, treatment goals should always be set for critically ill patients. If feasible, the benefits versus the risks of treatment should be considered for every patient. Ideally, a decision to withhold invasive intensive care measures should be made prior to ICU admission, yet in reality these expectations are not always met [16]. In addition to the declared will of an individual (if available and realistic) and the current state of the acute illness, the patient's past medical history (including previous illnesses, frailty, duration of inpatient stay and previous hospitalisations) should also be taken into consideration for any treatment decision [16]. Furthermore, functional and nutritional status are of paramount importance. Additionally, it is essential to distinguish between withholding or withdrawal of treatment, as well as to note that limitations of treatment (especially withholding of ICU care) are not necessarily associated with an increased short‐term mortality [17].
The fact that a north–south divide in end‐of‐life care practices exists in Europe is a well‐known phenomenon. Sprung et al. showed that the number of patients with treatment limitations put in place is gradually increasing. This could be for a number of reasons. It is certainly not only due to the ageing population of critically ill patients, often with significant comorbidities, but also due to increased public awareness, improved diagnostics and the growing body of guidelines and treatment recommendations, which allow for a more structured approach in the daily intensive care routine [18, 19].
Baseline risk distribution
This study observed discrete variations in patient‐specific differences between the three European regions studied, which may reflect regional variations in ICU admission policies [20, 21, 22]. In addition to the known increased number of critical care beds in Central Europe, a higher proportion of patients over 80 years of age and individuals with previously known frailty were found in this region [23]. Nevertheless, a significant—though not vast—difference was observed between the three regions with regard to median patient age and the severity of the acute illness. This also fits well with one of our preliminary studies, in which we showed that countries with different health‐care systems admitted patients with different characteristics to their local ICUs [24]. In addition to the expected difference in GDP between Northern and Southern Europe, there is also a higher level of health spending per capita in Central Europe. Whether the rate of admission of older and more frail patients is a ‘phenomenon of prosperity’ or due to cultural differences can only be speculated. There may be a higher rate of time‐limited ICU trials for older patients in Central Europe or a more liberal ICU admission policy as a result of the larger number of ICU beds available per capita; however, that is out of the scope of the present study.
Organ support and management
Furthermore, evidence of variations in intensive care management practices between different regions was found. For example, the rate of mechanically ventilated patients was significantly higher in Southern Europe, translating into a higher proportion of tracheostomies performed. The number of patients who required circulatory support with vasopressors was also higher in Southern Europe. This is particularly interesting given the relatively similar median SOFA score, and may reflect different regional treatment strategies. When treating COVID‐19, the early or late use of MV—which allows the application of lung‐protective ventilation—and the optimal timing for tracheostomy are the subject of ongoing scientific and clinical debate [25, 26]. As is well known, Italy, Spain, Portugal and Israel—all countries in Southern Europe—were hit hard relatively early in the COVID‐19 pandemic, and this may have affected how treatment was delivered. Especially in severely affected countries, conventional ICU measures such as NIV were often performed in normal wards during times of surge, whereas invasive MV generally remained within the intensive care domain [27]. This could have indirectly contributed to the higher numbers of intubated patients on MV in Southern Europe. Finally, given the similar disease severity described above, a different approach to time limits of NIV trials and variations in intubation criteria must be considered.
Treatment limitation analysis
The reasons for or against treatment limitations may be due to a patient's personal preference, general ethical/intensive care considerations and local ICU admission policies. Inherent factors in the health system—such as the training and education of clinicians, existing guidelines or the weight given to the wishes of the patient's family—may also influence the likelihood of a treatment [18]. In the Ethicus‐2 study, it was suggested that the similar observed differences within Europe could also be an expression of changing practices over time, and that changes are implemented more quickly in Northern Europe [28]. However, given the current data, this seems unlikely as the observed differences in 2020 and 2021 show a similar pattern to those seen in the Ethicus‐2 study conducted in 2015 and 2016. We therefore believe that the differences observed—now repeatedly and reproducibly—are more structural in nature, although the exact causes remain unclear.
Outcome analysis
Interestingly, the different rates of treatment limitations did not lead to a major difference in regional 90‐day mortality rates of critically ill older patients with COVID‐19. Ultimately, one can only speculate about the reasons for this finding. A slightly higher proportion of patients over 80 years of age and patients with cardiovascular comorbidities was observed in Central Europe compared with Northern and Southern Europe. Individual treatment strategies also varied—which may be due to different structures of care, but on the other hand could be due to the stress put on ICUs during surge. Another influencing factor could be the differences in ICU bed numbers, as mentioned previously. Systems with a larger pre‐existing ICU bed base are more likely to be able to compensate quickly in times of crisis compared with systems in which new capacity must be created. There may also be differences in terms of staffing–patient ratios. Also, different ICU capacities may imply different up‐ and downstream structures (intermediate care or monitoring areas in normal wards). Accordingly, patients with NIV may be admitted to an ICU in some settings and may be cared for in a monitored normal ward in other systems. Thus, in our collective, more patients in Central and Northern Europe received NIV, whereas more individuals in Southern Europe required invasive ventilation. Another interesting finding is that ICU length of stay varied significantly between regions, with the longest lengths of stay in Southern Europe. This is noteworthy, as the rate of treatment limitations was lower there, but there was no statistically significant difference in terms of mortality. It must be taken into account that infrastructure and management vary regionally, and not all details on local structures of care are available in our study. Nevertheless, considering these findings, the structured use of treatment limitations may be useful—especially in times of overcrowding in ICUs in the context of the pandemic—if a possibly longer invasive treatment does not result in a survival advantage for the affected patients. Because the secondary endpoints of 30‐ and 90‐day mortality are not available for all patients, a selection or reporting bias may also exist. This study regardless shows that the different systems had comparable results due to the large amount of time and work invested during the pandemic. In general, a multinational comparison is difficult due to the significantly different health‐care systems and ICU bed capacities (as well as subsequent care structures) in the various countries of the three European regions. Nevertheless, the multivariable analysis corrected for all of these factors, which was intended to achieve a balanced comparison across regions.
Conclusion
We conclude that rates of treatment limitations in older patients during the COVID‐19 pandemic period in Europe show a north to south gradient. When performing such evaluations, different health‐care systems, patient characteristics and local treatment strategies must be considered. Interestingly, this did not lead to significant differences in mortality rates in a multivariable analysis.
Limitations
One limitation is that we do not know the exact timing of when a treatment limitation was pronounced. We also do not have detailed information on how a ‘treatment withhold’ was defined in an individual case, and it would likely not be possible to evaluate this uniformly for such a large cohort. Unfortunately, we also do not have information on whether patients were transferred to intermediate care units or elsewhere after a treatment limitation was imposed. Further limitations of this study—besides its unblinded design—are the lack of knowledge about the functional outcome, and the problem of a potential self‐fulfilling prophecy, as always, with treatment limitations. Also, in addition to a comparison group with younger patients, long‐term outcome would be interesting because of the often‐protracted hospital stay in severe COVID‐19 infections. Although it is difficult to generalise qualitative data such as treatment limitations, we think that our study provides useful data for health‐care providers and decision makers due to the large number of participants and good patient characterisation.
Conflict of interests
J.C.S. (full departmental disclosure) reports grants from Orion Pharma, Abbott Nutrition International, B. Braun Medical AG, CSEM AG, Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, Nestle, Pierre Fabre Pharma AG, Pfizer, Bard Medica S.A., Abbott AG, Anandic Medical Systems, Pan Gas AG Healthcare, Bracco, Hamilton Medical AG, Fresenius Kabi, Getinge Group Maquet AG, Dräger AG, Teleflex Medical GmbH, Glaxo Smith Kline, Merck Sharp and Dohme AG, Eli Lilly and Company, Baxter, Astellas, Astra Zeneca, CSL Behring, Novartis, Covidien, Phagenesis and Nycomed outside of the submitted work. The money was paid into departmental funds. No personal financial gain applied. The other authors declare that they have no conflict of interests.
Author contributions
Conceptualisation: Bernhard Wernly, Hans Flaatten, Michael Joannidis, Dylan W. De Lange, Bertrand Guidet and Christian Jung. Methodology: Bernhard Wernly, Hans Flaatten and Raphael R. Bruno. Validation: Michael Beil, Sviri Sigal, Peter V. van Heerden, Rui Moreno, Ariane Boumendil and Bertrand Guidet. Formal analysis: Bernhard Wernly. Investigation: Jesper Fjølner, Antonio Artigas, Bernardo B. Pinto, Joerg C. Schefold, Sviri Sigal, Peter V. van Heerden, Wojciech Szczeklik, Muhammed Elhadi, Michael Joannidis, Sandra Oeyen, Georg Wolff, Brian Marsh, Finn H. Andersen, Rui Moreno, Susannah Leaver, Dylan W. De Lange, Bertrand Guidet and Christian Jung. Resources: Hans Flaatten, Michael Joannidis, Dylan W. De Lange, Bertrand Guidet and Christian Jung. Data curation: Michael Beil and Ariane Boumendil. Writing – original draft: Bernhard Wernly, Richard Rezar and Sarah Wernly. Writing – review and editing: Bernhard Wernly, Richard Rezar, Sarah Wernly, Susannah Leaver and Christian Jung. Visualisation: Richard Rezar. Supervision: Bertrand Guidet, Malte Kelm and Dylan W. De Lange. Project administration: Bernhard Wernly, Richard Rezar and Christian Jung.
Supporting information
Supplement 1: Evidence before this study.
Supplement Table 1: Multilevel linear regression model to evaluate the association of the primary exposure (belonging to one of the three regions) with the length of ICU stay as dependent variable. Model‐1 includes only the ICU as panel. Model‐2 includes patient‐specific factors (sex, age per year, SOFA score per point, and frailty score per CFS point). Model‐3 adds the amount of ICU beds per 100,000 population. Model‐4 includes the HDI. Model‐5 adds the rates of any treatment limitations and hence calculated only 90‐day mortality.
Figure legend: Median length of stay in patients with no treatment limitation versus individuals with any treatment limitation.
Acknowledgements
The map in the Graphical Abstract was generated using https://mapchart.net/ (licensed under a Creative Commons Attribution‐ShareAlike 4.0 International License). No (industry) sponsorship has been received for this investigator‐initiated study.
Open Access funding enabled and organized by Projekt DEAL.
Wernly B, Rezar R, Flaatten H, Beil M, Fjølner J, Bruno RR, et al. Variations in end‐of‐life care practices in older critically ill patients with COVID‐19 in Europe. J Intern Med. 2022;1–12.
Bernhard Wernly and Richard Rezar share first authorship.
Data availability statement
All data relevant for this study will be given by the authors upon specific request without restriction.
References
- 1. Avidan A, Sprung CL, Schefold JC, Ricou B, Hartog CS, Nates JL, et al. Variations in end‐of‐life practices in intensive care units worldwide (Ethicus‐2): a prospective observational study. Lancet Respir Medicine. 2021;9(10):1101–10. [DOI] [PubMed] [Google Scholar]
- 2. Guidet B, Flaatten H, Boumendil A, Morandi A, Andersen FH, Artigas A, et al. Withholding or withdrawing of life‐sustaining therapy in older adults (≥80 years) admitted to the intensive care unit. Intens Care Med. 2018;44(7):1027–38. [DOI] [PubMed] [Google Scholar]
- 3. Flaatten H, Van Heerden V, Jung C, Beil M, Leaver S, Rhodes A, et al. The good, the bad and the ugly: pandemic priority decisions and triage. J Med Ethics. 2020;0:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flaatten H, De Lange DW, Jung C, Beil M, Guidet B. The impact of end‐of‐life care on ICU outcome. Intens Care Med. 2021;47:624–5. [DOI] [PubMed] [Google Scholar]
- 5. Guidet B, De Lange DW, Flaatten H. Should this elderly patient be admitted to the ICU? Intens Care Med. 2018;44(11):1926–8. [DOI] [PubMed] [Google Scholar]
- 6. Beil M, Sviri S, Flaatten H, De Lange DW, Jung C, Szczeklik W, et al. On predictions in critical care: the individual prognostication fallacy in elderly patients. J Crit Care. 2021;61:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo‐Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173(5):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guidet B, De Lange DW, Boumendil A, Leaver S, Watson X, Boulanger C, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intens Care Med. 2020;46(1):57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flaatten H, De Lange DW, Morandi A, Andersen FH, Artigas A, Bertolini G, et al. The impact of frailty on ICU and 30‐day mortality and the level of care in very elderly patients (≥ 80 years). Intens Care Med. 2017;43(12):1820–8. [DOI] [PubMed] [Google Scholar]
- 11. Wagstaff A. Social health insurance vs. tax‐financed health systems—evidence from the OECD. The World Bank, Policy Research Working Paper Series. 2009.
- 12. Katz S. Assessing self‐maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721–7. [DOI] [PubMed] [Google Scholar]
- 13. International Monetary Fund . World economic outlook reports 2020. [Internet] https://www.imf.org/en/Publications/WEO/Issues/2020/09/30/world‐economic‐outlook‐october‐2020 (2020). Accessed 13 May 2021.
- 14. United Nations Development Program . Human development report 2020 [Internet]. http://hdr.undp.org/sites/default/files/hdr2020.pdf (2020). Accessed 13 May 2021.
- 15. OECD . Health at a glance [Internet]. https://data.oecd.org/healthres/health‐spending.htm (2020). Accessed 13 May 2021.
- 16. Friesenecker B, Fruhwald S, Hasibeder W, Hörmann C, Hoffmann ML, Krenn CG, et al. Therapiezieländerungen auf der intensivstation: definitionen, entscheidungsfindung und dokumentation. Anästhesiol Intensivmed Notfallmed Schmerzther. 2013;48(4):216–23. [DOI] [PubMed] [Google Scholar]
- 17. Rubio O, Arnau A, Cano S, Subirà C, Balerdi B, Perea ME, et al. Limitation of life support techniques at admission to the intensive care unit: a multicenter prospective cohort study. J Intensive Care. 2018;6(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sprung CL, Ricou B, Hartog CS, Maia P, Mentzelopoulos SD, Weiss M, et al. Changes in end‐of‐life practices in European intensive care units from 1999 to 2016. JAMA. 2019;322(17):1692–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ehni H, Wiesing U, Ranisch R. Saving the most lives—a comparison of European triage guidelines in the context of the COVID‐19 pandemic. Bioethics. 2021;35(2):125–34. [DOI] [PubMed] [Google Scholar]
- 20. Wilkinson D, Zohny H, Kappes A, Sinnott‐Armstrong W, Savulescu J. Which factors should be included in triage? An online survey of the attitudes of the UK general public to pandemic triage dilemmas. BMJ Open. 2020;10(12):e045593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Netters S, Dekker N, van de Wetering K, Hasker A, Paasman D, de Groot JW, et al. Pandemic ICU triage challenge and medical ethics. BMJ Supportive Palliat Care. 2021;11(2):133–7. [DOI] [PubMed] [Google Scholar]
- 22. Darvall JN, Bellomo R, Bailey M, Anstey J, Pilcher D. Long‐term survival of critically ill patients stratified by pandemic triage categories: a retrospective cohort study. Chest. 2021;160(2):538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rhodes A, Ferdinande P, Flaatten H, Guidet B, Metnitz PG, Moreno RP. The variability of critical care bed numbers in Europe. Intens Care Med. 2012;38(10):1647–53. [DOI] [PubMed] [Google Scholar]
- 24. Wernly B, Beil M, Bruno RR, Binnebössel S, Kelm M, Sigal S, et al. Provision of critical care for the elderly in Europe: a retrospective comparison of national healthcare frameworks in intensive care units. BMJ Open. 2021;11(6):e046909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bavishi AA, Mylvaganam RJ, Agarwal R, Avery RJ, Cuttica MJ. Timing of intubation in coronavirus disease 2019: a study of ventilator mechanics, imaging, findings, and outcomes. Critical Care Explor. 2021;3(5):e0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schultz MJ, Teng MS, Brenner MJ. Timing of tracheostomy for patients with COVID‐19 in the ICU—setting precedent in unprecedented times. JAMA Otolaryngology Head Neck Surg. 2020;146(10):887–8. [DOI] [PubMed] [Google Scholar]
- 27. Bellani G, Grasselli G, Cecconi M, Antolini L, Borelli M, De Giacomi F, et al. Noninvasive ventilatory support of patients with COVID‐19 outside the intensive care units (WARd‐COVID). Ann Am Thorac Soc. 2021;18(6):1020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Avidan A, Sprung CL, Schefold JC, Ricou B, Hartog CS, Nates JL, et al. Variations in end‐of‐life practices in intensive care units worldwide (Ethicus‐2): a prospective observational study. Lancet Respir Med. 2021;9(10):1101–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1: Evidence before this study.
Supplement Table 1: Multilevel linear regression model to evaluate the association of the primary exposure (belonging to one of the three regions) with the length of ICU stay as dependent variable. Model‐1 includes only the ICU as panel. Model‐2 includes patient‐specific factors (sex, age per year, SOFA score per point, and frailty score per CFS point). Model‐3 adds the amount of ICU beds per 100,000 population. Model‐4 includes the HDI. Model‐5 adds the rates of any treatment limitations and hence calculated only 90‐day mortality.
Figure legend: Median length of stay in patients with no treatment limitation versus individuals with any treatment limitation.
Data Availability Statement
All data relevant for this study will be given by the authors upon specific request without restriction.


