Abstract
Background and Aims
The coronavirus disease of 2019 (COVID‐19) causes considerable mortality worldwide. We aimed to investigate the frequency and predictive role of abnormal liver chemistries in different age groups.
Methods
Patients with positive severe acute respiratory distress syndrome‐coronavirus‐2 (SARS‐CoV‐2) polymerase chain reaction (PCR) test between 03/2020‐07/2021 at the Vienna General Hospital were included. Patients were stratified for age: 18–39 vs. 40–69 vs. ≥70 years (y). Aspartate aminotransferase (AST), alanine‐aminotransferase (ALT), alkaline phosphatase (ALP), gamma‐glutamyl transferase (GGT) and total bilirubin (BIL) were recorded.
Results
900 patients (18–39 years: 32.2%, 40–69 years: 39.7%, ≥70 years: 28.1%) were included. Number of comorbidities, median D‐dimer and C‐reactive protein increased with age. During COVID‐19, AST/ALT and ALP/GGT levels significantly increased. Elevated hepatocellular transaminases (AST/ALT) and cholestasis parameters (ALP/GGT/BIL) were observed in 40.3% (n = 262/650) and 45.0% (n = 287/638) of patients respectively. Liver‐related mortality was highest among patients with pre‐existing decompensated liver disease (28.6%, p < .001). 1.7% of patients without pre‐existing liver disease died of liver‐related causes, that is consequences of hepatic dysfunction or acute liver failure. Importantly, COVID‐19‐associated liver injury (16.0%, p < .001), abnormal liver chemistries and liver‐related mortality (6.5%, p < .001) were most frequent among 40–69 years old patients. Elevated AST and BIL after the first positive SARS‐CoV‐2 PCR independently predicted mortality in the overall cohort and in 40–69 years old patients.
Conclusions
Almost half of the COVID‐19 patients exhibit abnormal hepatocellular and cholestasis‐related liver chemistries with 40–69 years old patients being at particularly high risk for COVID‐19‐related liver injury and liver‐related mortality. Elevated AST and BIL after SARS‐CoV‐2 infection are independent predictors of mortality, especially in patients aged 40–69 years.
Keywords: acute respiratory distress syndrome, COVID‐19, liver chemistries, liver injury, SARS‐CoV‐2
Abbreviations
- 95% CI
95% confidence interval
- aHR
adjusted hazard ratio
- ALP
alkaline phosphatase
- ALT
alanine‐aminotransferase
- ARDS
acute respiratory distress syndrome
- AST
aspartate aminotransferase
- BIL
total bilirubin
- BMI
body mass index
- cACLD
compensated advanced chronic liver disease
- COVID‐19
coronavirus disease of 2019
- CRP
C‐reactive protein
- dACLD
decompensated advanced chronic liver disease
- EC
Ethics committee
- GGT
gamma‐glutamyl transferase
- HR
hazard ratio
- ICU
intensive care unit
- INR
international normalized ratio
- IQR
interquartile range
- n
number
- non‐ACLD
non‐advanced chronic liver disease
- PCR
polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory distress syndrome‐coronavirus‐2
- ULN
upper limit of normal
- WBC
white blood cell count
Lay summary.
Investigating liver chemistries in a large cohort of SARS‐CoV‐2 infected patients, we observed abnormal hepatocellular and cholestasis‐related liver chemistries in 40.3% and 45.0% of patients with COVID‐19. Patients aged 40‐69 years are at particularly high risk for COVID‐19‐related liver injury and liver‐related mortality. Elevated AST and BIL after the first positive SARS‐CoV‐2 PCR test are independent predictors for mortality, especially in 40‐69 years old patients.
1. INTRODUCTION
The coronavirus disease of 2019 (COVID‐19) pandemic, caused by severe acute respiratory distress syndrome‐coronavirus‐2 (SARS‐CoV‐2), is associated with substantial morbidity and mortality worldwide. 1 COVID‐19 affects lungs, liver, intestinal and neuronal systems, causing acute respiratory distress syndrome (ARDS) and multiorgan failure. 2 , 3 Risk factors for mortality due to COVID‐19 include old age, obesity, male sex and pre‐existing comorbidities including liver disease. 4 , 5 , 6
Previous studies have demonstrated liver enzyme abnormalities in a substantial number of patients with COVID‐19. 7 , 8 , 9 , 10 , 11 Meta‐analyses found liver transaminases to be elevated in approximately 20% of patients, and also reported increased parameters of cholestatic liver injury, that is alkaline phosphatase (ALP) in 6.1% and gamma‐glutamyl transferase (GGT) in 21.1% of COVID‐19 patients, respectively. 12 , 13
Elevations of liver enzymes were more frequently observed in patients with severe courses of COVID‐19 and in critically ill patients. 14 , 15 , 16 , 17 In a large retrospective Chinese study, elevated levels of aspartate aminotransferase (AST) and direct bilirubin at hospital admission were identified as independent predictors of COVID‐19‐associated mortality. 18 However, this study has some limitations, including the lack of detailed data on pre‐existing liver disease and the severity of systemic inflammation. 19 Another study reported progressively increasing levels of hepatic transaminases in COVID‐19 patients with severe courses of the disease. 20
Examining post‐mortem liver biopsies of patients with SARS‐CoV‐2 infection, viral particles were detected in the cytoplasm of hepatocytes, directly linking hepatocellular infection with COVID‐19‐associated liver injury. 21 Next to direct SARS‐CoV‐2‐mediated cytotoxicity, other pathomechanisms such as an excessive proinflammatory state, hypoxemia, drug‐induced liver injury, coagulopathy‐associated vascular dysfunction, cardiac congestion and sepsis are likely all contributing to liver injury in COVID‐19. 3 , 16
The aim of this study was to investigate (i) the rate of abnormal liver chemistries at the first blood withdrawal after the first positive SARS‐CoV‐2 polymerase chain reaction (PCR) test and (ii) the trajectory of hepatic transaminases in a large Austrian cohort of patients with COVID‐19. Moreover, we set out to determine (iii) the impact of liver abnormalities on clinical outcome of patients with COVID‐19 in different age strata.
2. PATIENTS AND METHODS
2.1. Study population
Adult patients with positive SARS‐CoV‐2 PCR test at the Vienna General Hospital between 03/2020 and 07/2021 were included in this retrospective study. Clinical and laboratory parameters, including age, body mass index (BMI), comorbidities (i.e. pre‐existing arterial hypertension, diabetes mellitus, hyperlipidemia, chronic liver disease, cardiovascular, lung, chronic kidney and malignant disease), liver chemistries (alkaline phosphatase [ALP], gamma‐glutamyl transferase (GGT), AST, alanine‐aminotransferase [ALT] and total bilirubin [BIL]), haemoglobin, platelet and white blood cell count (WBC), international normalized ratio (INR), D‐dimer, serum sodium, creatinine, albumin and C‐reactive protein (CRP), hospital admission, intensive care unit (ICU) admission, intubation, death, liver‐related death and COVID‐19‐related death were assessed via chart review. Patients were stratified for age (18–39 years, 40–69 years, and ≥70 years). Liver‐related death was defined as death directly associated with liver‐related complications. Pre‐existing liver disease was subdivided into non‐advanced chronic liver disease (non‐ACLD), compensated ACLD (cACLD) and decompensated ACLD (dACLD). Notably, due to the retrospective design of the study, not all parameters were available for every patient.
2.2. Laboratory parameters
All parameters were assessed by standard laboratory assays. For every parameter, the last available value prior to the positive SARS‐CoV‐2 PCR test (t0), as well as the first three available values after the positive SARS‐CoV‐2 PCR test (t1, t2 and t3 respectively) and the last available value (last) were recorded.
For parameters of hepatocellular (AST, ALT) and cholestatic liver injury (ALP, GGT, BIL), standard laboratory thresholds for men and women were used as upper limit of normal (ULN). Liver injury was defined as increased AST or ALT >3xULN or increased AP or BIL >2xULN, analogous to previous studies 18 , 20 and to the American College of Gastroenterology Clinical Guideline definition. 22
2.3. Statistical analysis
Categorical variables were reported as number (n) and proportion (%) of patients showing the parameter of interest. Where appropriate, the total number of available values (n total) was added. Continuous data were depicted as median and interquartile range (IQR). D'Agostino & Pearson and Shapiro‐Wilk normality tests were implemented to test for normal distribution. Mann‐Whitney U test was used for comparing non‐normally distributed continuous variables between two groups. For comparison of non‐normally distributed continuous variables in three or more groups, the Kruskal–Wallis test was computed. Dunn's multiple comparisons test was implemented as post hoc test. For group comparisons of categorical variables, Pearson's chi‐squared or Fisher's exact test was used. Kaplan‐Meier curves depicted differences in survival between groups of elevated versus non‐elevated levels of the parameters of interest. Differences in survival between these groups were assessed by a log‐rank test. Cox proportional hazard models were used to determine the impact of the parameters of interest on mortality. Multivariate analysis considered age, sex, creatinine, albumin, obesity, liver disease, diabetes mellitus, cardiovascular disease, lung disease and malignancy. Patients entered these models at the time of the first positive SARS‐CoV‐2 PCR test. IBM SPSS 22.0 statistic software (IBM) and GraphPad Prism 8 (Graphpad Software) were used for statistical analysis. A two‐sided p‐value of <.05 was considered as statistically significant.
2.4. Ethics
The study was approved by the ethics committee (EC) of the Medical University of Vienna (EK1461/2020). It was performed in accordance with the current version of the Helsinki Declaration. Due to the retrospective design of the study, the EC waived the need for informed consent.
3. RESULTS
3.1. Patient characteristics in different age strata
In total, 900 patients with positive SARS‐CoV‐2 PCR test were included in this study. 52.4% of patients were male. The median age was 52.9 years with 290 (32.2%) patients between 18 and 39 years, 357 (39.7%) patients between 40 and 69 years and 253 (28.1%) patients ≥70 years old. Overall, 60 patients had pre‐existing liver disease (non‐ACLD: 8.3% [n = 41], cACLD: 20.0% [n = 12], dACLD: 11.7% [n = 7]). The main aetiologies included non‐alcoholic fatty liver disease/non‐alcoholic steatohepatitis (NAFLD/NASH) in 58.3% (n = 35), alcohol‐related liver disease (ALD) in 16.7% (n = 10) and viral hepatitis in 8.3% (n = 5) of patients with pre‐existing liver disease. With progressive age, the prevalence of comorbidities, including pre‐existing liver disease, arterial hypertension, diabetes mellitus, dyslipidemia, cardiovascular disease, chronic renal deficiency, lung disease and malignancy increased. The rate of obesity was highest in 40–69 years old patients (Table 1).
TABLE 1.
Patient characteristics and comparison between patients stratified for age (18–39 years, 40–69 years, ≥70 years)
| Patient characteristics | All patients(n = 900) | Age | p‐value | ||
|---|---|---|---|---|---|
| 18–39 years(n = 290) | 40–69 years(n = 357) | ≥70 years(n = 253) | |||
| Sex, male/female (% male) | 472/428 (52.4%) | 151/139 (52.1%) | 204/153 (57.1%) | 117/136 (46.2%) | .029 |
| Age, years (IQR) | 52.9 (37.3) | 29.6 (9.4) | 55.4 (13.3) | 79.8 (9.7) | <.001 |
| Obesity, n/n total (%) | 143/390 (36.7%) | 24/69 (34.8%) | 110/198 (44.4%) | 31/123 (25.2%) | .002 |
| Liver disease, n/n total (%) | 60/741 (8.1%) | 2/198 (1.0%) | 27/298 (9.1%) | 31/245 (12.7%) | <.001 |
| Arterial Hypertension, n/n total (%) | 323/738 (44.4%) | 9/198 (13.3%) | 129/295 (43.7%) | 185/245 (75.5%) | <.001 |
| Diabetes mellitus, n/n total (%) | 141/740 (19.1%) | 5/198 (2.5%) | 68/296 (23.0%) | 68/246 (27.6%) | <.001 |
| Dyslipidemia, n/n total (%) | 158/741 (21.3%) | 7/198 (3.5%) | 61/297 (20.5%) | 90/246 (36.6%) | <.001 |
| Cardiovascular disease, n/n total (%) | 215/737 (29.2%) | 6/198 (3.0%) | 57/293 (19.5%) | 152/246 (61.8%) | <0.001 |
| Chronic renal insufficiency, n/n total (%) | 91/751 (12.1%) | 1/201 (0.5%) | 23/303 (7.6%) | 67/247 (27.1%) | <.001 |
| Lung disease, n/n total (%) | 126/743 (17.0%) | 11/198 (5.6%) | 53/298 (17.8%) | 62/247 (25.1%) | <.001 |
| Malignancy, n/n total (%) | 111/741 (15.0%) | 4/200 (2.0%) | 48/296 (16.2%) | 59/245 (24.1%) | <.001 |
| Liver injury, n/n total (%) a | 66/652 (10.3%) | 11/130 (8.5%) | 45/282 (16.0%) | 11/240 (4.6%) | <.001 |
| Alkaline phosphatase, U × L−1 (IQR) a | 72.5 (46.0) | 65.0 (34.0) | 73.0 (51.0) | 75.0 (53.0) | .080 |
| Alkaline phosphatase > 2xULN, n/n total (%) | 27/558 (4.8%) | 2/117 (1.7%) | 21/261 (8.0%) | 4/180 (2.2%) | .004 |
| Aspartate transaminase, U × L−1 (IQR) a | 32.0 (30.0) | 27.0 (20.0) | 34.0 (32.0) | 33.0 (29.0) | .044 |
| Aspartate transaminase > 3xULN, n/n total (%) a | 23/618 (3.7%) | 5/121 (4.1%) | 13/275 (4.7%) | 5/222 (2.3%) | .338 |
| Alanine aminotransferase, U × L−1 (IQR) a | 27.0 (25.0) | 28.0 (28.0) | 30.0 (32.8) | 23.0 (18.0) | .001 |
| Alanine aminotransferase > 3xULN, n/n total (%) a | 28/648 (4.3%) | 8/130 (6.2%) | 15/280 (5.4%) | 5/238 (2.1%) | .099 |
| Gamma‐glutamyl transferase, U × L−1 (IQR) a | 42.0 (84.5) | 28.0 (57.5) | 53.0 (143.0) | 42.0 (62.0) | <.001 |
| Gamma‐glutamyl transferase > 2xULN, n/Total n (%) a | 145/565 (25.7%) | 18/117 (15.4%) | 89/261 (34.1%) | 38/187 (20.3%) | <.001 |
| Bilirubin, mg × dl−1 (IQR) a | 0.5 (0.4) | 0.4 (0.3) | 0.5 (0.5) | 0.5 (0.3) | .137 |
| Bilirubin > 2xULN, n/n total (%) a | 23/638 (3.6%) | 3/124 (2.4%) | 17/275 (6.2%) | 3/236 (1.3%) | .009 |
| Thrombocytes, G × L−1 (IQR) a | 213.5 (107.0) | 229.5 (79.8) | 218.0 (114.0) | 203.0 (112.0) | .005 |
| D‐dimer, mg × dl−1 (IQR) a | 1.5 (2.6) | 0.5 (0.8) | 1.3 (2.8) | 1.3 (2.5) | <.001 |
| Albumin, g × L−1 (IQR) a | 33.7 (11.6) | 42.7 (13.6) | 32.7 (12.0) | 32.6 (7.9) | <.001 |
| Creatinine, mg × dl−1 (IQR) a | 0.9 (0.5) | 0.8 (0.3) | 0.8 (0.4) | 1.0 (0.7) | <.001 |
| C‐reactive pr/otein, mg × dl−1 (IQR) a | 2.6 (8.1) | 1.0 (3.3) | 3.3 (9.6) | 3.2 (7.3) | <.001 |
| Follow‐up and clinical outcomes | All patients (n = 697) | Age | p‐value | ||
|---|---|---|---|---|---|
| 18–39 years (n = 175) | 40–69 years (n = 293) | ≥70 years (n = 229) | |||
| Median follow‐up, days (IQR) | 63.0 (139.0) | ||||
| Hospital admission, n (%) | 458 (65.7%) | 52 (29.7%) | 200 (68.3%) | 206 (90.0%) | <.001 |
| Median hospital stay, days (IQR) | 22.0 (33.0) | 12.0 (25.0) | 25.5 (39.0) | 21.0 (28.0) | .011 |
| ICU admission, n (%) | 200 (28.7%) | 23 (13.1%) | 126 (43.0%) | 51 (22.3%) | <.001 |
| Median ICU stay, days (IQR) | 22.0 (30.0) | 18.0 (20.0) | 29.0 (32.0) | 11.0 (19.0) | .002 |
| Intubation, n (%) | 164 (23.5%) | 17 (9.7%) | 108 (36.9%) | 39 (17.0%) | <.001 |
| Median duration of intubation, days (IQR) | 21.5 (29.0) | 19.0 (14.0) | 26.5 (31.0) | 13.0 (19.0) | .001 |
| Death, n (%) | 154 (22.1%) | 0 (0.0%) | 61 (20.8%) | 93 (40.6%) | <.001 |
| COVID‐19‐related death, n (%) | 128 (18.4%) | 0 (0.0%) | 45 (15.4%) | 83 (36.2%) | <.001 |
| Liver‐related death, n (%) | 24 (2.7%) | 0 (0.0%) | 19 (6.5%) | 5 (2.2%) | <.001 |
At the first blood withdrawal after the first positive SARS‐CoV‐2 PCR test.
p‐values depicting statistically significant differences are presented as bold values.
At the blood withdrawal after the first positive SARS‐CoV‐2 PCR test, 10.3% (n = 66/652) of patients showed liver injury by biochemical definition. Parameters of hepatocellular liver injury (AST/ALT) were elevated in 40.3% (n = 262/650) of patients and parameters of cholestatic liver injury (ALP/GGT) in 45.0% (n = 287/638) of patients. Platelet count and albumin levels decreased throughout the age strata. Creatinine levels were significantly increased in patients ≥70 years. D‐dimer and CRP were particularly elevated in patients ≥40 years old.
3.2. Trajectory of liver values after SARS‐CoV‐2 infection
Over the course of the SARS‐CoV‐2 infection, plasma levels of parameters of hepatocellular injury (AST: p < .001; ALT: p = .002) and parameters of cholestatic liver injury (ALP: p = .012; GGT: p < .001) progressively increased (at t1/t2/t3 vs. t0), while there were no significant changes in BIL (p = .347; Figure‐1, Table‐S1).
FIGURE 1.
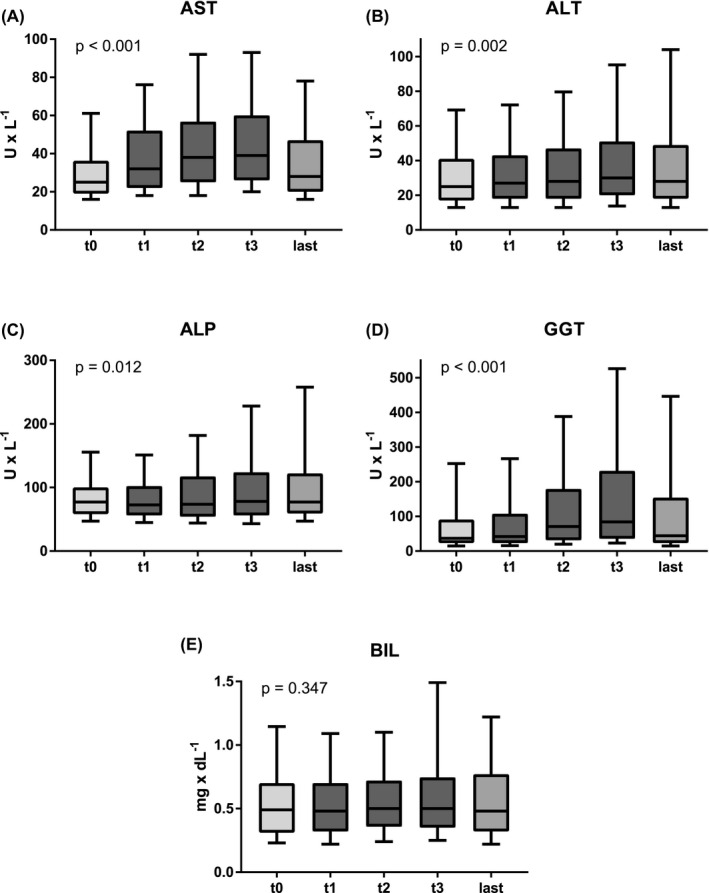
Trajectory of blood levels of (A) aspartate transaminase (AST), (B) alanine aminotransferase (ALT), (C) alkaline phosphatase (ALP), (D) gamma‐glutamyl transferase (GGT) and (E) bilirubin. The borders of the whiskers are the 10th and the 90th percentile. t0 = last available value before SARS‐CoV‐2 infection; t1/t2/t3 = first/second/third available value after SARS‐CoV‐2 infection; last = last available value
Median levels of AST were already increased at the first blood withdrawal after the first positive SARS‐CoV‐2 PCR test as compared to the last previous value (t1: 32.0 U/L vs. t0: 25.0 U/L, p < .001). For ALT (t0: 25.0 U/L vs. t1: 27.0 U/L, p = .700) and parameters of cholestatic liver injury (ALP: t0: 77.0 U/L vs. t1: 72.5 U/L, p = .999; GGT: t0: 37.0 U/L vs. t1: 42.0 U/L, p = .952), the median values at the first positive SARS‐CoV‐2‐PCR (t1) were similar to baseline values (t0).
During the COVID‐19 course, parameters of hepatocellular liver injury (AST: t1: 32.0 U/L vs. t3: 39.0 U/L, p < .001; ALT: t1: 27.0 U/L vs. t3: 30.0 U/L, p = .183) and parameters of cholestatic liver injury (ALP: t1: 72.5 U/L vs. t3: 78.0 U/L, p = .184; GGT: t1: 42.0 U/L vs. t3: 84 U/L, p < .001) increased, while median BIL levels remained unchanged (BIL: t1: 0.5 mg/dl vs. t3: 0.5 mg/dl, p = .611).
Finally, for the last available laboratory values, median ALP (t3: 78.0 U/L vs. last: 77.0 U/L, p = .999) and ALT levels (t3: 30.0 U/L vs. last: 28.0 U/L, p = .999) did not decrease, while levels of AST (t3: 39.0 U/L vs. last: 28.0 U/L, p < .001) and GGT (t3: 84.0 U/L vs. last: 44.0 U/L, p < .001) declined significantly.
3.3. COVID‐19‐related liver injury in different age strata after the first positive SARS‐CoV‐2 PCRtest
Interestingly, the proportion of patients with COVID‐19‐related liver injury at the first blood withdrawal after the first positive SARS‐CoV‐2 PCR test was particularly high in 40–69 years old patients (8.5% vs. 16.0% vs. 4.6%; p < .001). Consistently, median levels of parameters of hepatocellular injury (AST: 18–39 years: 27.0 U/L vs. 40–69 years: 34.0 U/L vs. ≥70 years: 33.0 U/L, p = .044; ALT: 18–39 years: 28.0 U/L vs. 40–69 years: 30.0 U/L vs. ≥70 years: 23.0 U/L, p = .001) were highest among patients between 40 and 69 years old. Parameters of cholestatic liver injury (ALP: 18–39 years: 65.0 U/L vs. 40–69 years: 73.0 U/L vs. ≥70 years: 75.0 U/L, p = .004; GGT: 18–39 years: 28.0 U/L vs. 40–69 years: 53.0 U/L vs. ≥70 years: 42.0 U/L, p < .001) showed similar results with increased values especially in patients ≥40 years old. Finally, BIL levels >2xULN were observed primarily in patients of 40–69 years of age (6.2% vs. 18–39 years: 2.4% vs. ≥70 years: 1.3%, p = .009; Table 1, Figure 2).
FIGURE 2.
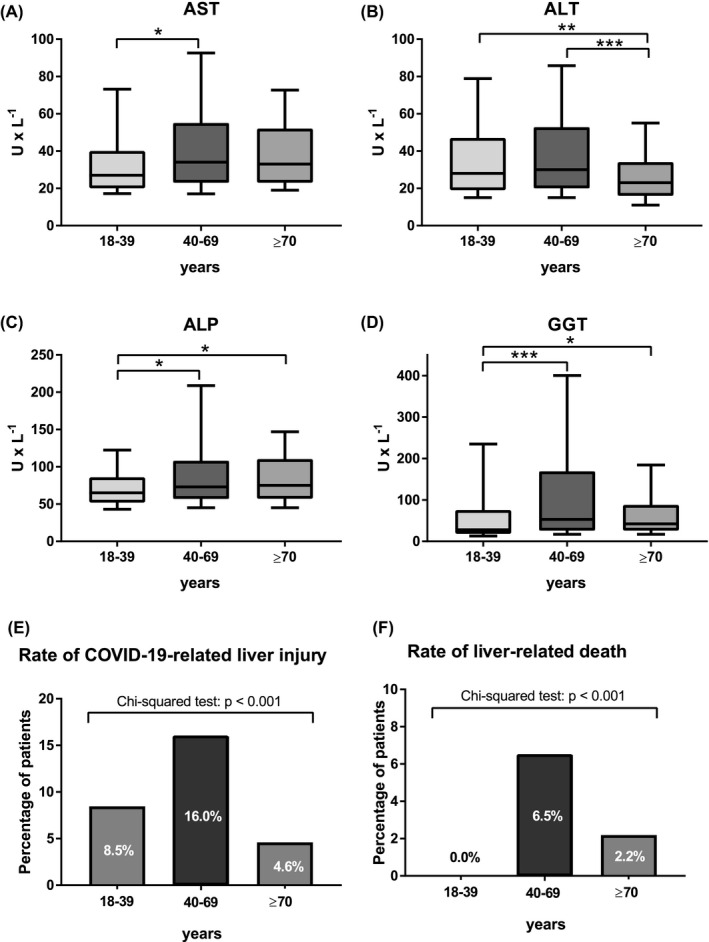
Comparison of plasma levels of (A) aspartate transaminase (AST), (B) alanine aminotransferase (ALT), (C) alkaline phosphatase (ALP) and (D) gamma‐glutamyl transferase (GGT) between different age strata (i.e. patients aged 18–39 years, 40–69 years and ≥70 years) at blood withdrawal after the first positive SARS‐CoV‐2 PCR test. The borders of the whiskers are the 10th and the 90th percentile. Comparison of (E) proportion of patients with COVID‐19‐related liver injury between different age strata and (F) proportion of liver‐related death among 40–69 years old and ≥70 years old patients
3.4. Clinical outcomes of patients in regard to the presence or absence of elevated liver chemistries
Follow‐up data were available for 697 patients with a median follow‐up duration of 63.0 [IQR 139.0] days. In total, 164 patients (23.5%) were intubated and 154 patients (22.1%) died, with 128 deaths (18.4%) being COVID‐19‐related and 24 deaths (3.4%) being liver‐related. While the rate of hospital admissions, death and COVID‐19 related death increased throughout the age strata, the rate of ICU admissions, intubations and median hospital stay was highest in 40–69 years old patients. There were no deaths among patients aged 18–39 years (Tables 1, 2, Figure 2).
TABLE 2.
Clinical outcomes of COVID‐19 patients with and without elevated AST at blood withdrawal after the first positive SARS‐CoV‐2 PCR
| Follow‐up and clinical outcomes | AST ≤ULN(n = 349) | AST > ULN(n = 194) | p‐value |
|---|---|---|---|
| Hospital admission, n (%) | 258 (73.9%) | 176 (90.7%) | <.001 |
| Median hospital stay, days (IQR) | 22.0 (41.0) | 32.0 (36.0) | .261 |
| ICU admission, n (%) | 97 (27.8%) | 99 (51.0%) | <.001 |
| Median ICU stay, days (IQR) | 24.5 (38.0) | 32.0 (29.0) | .775 |
| Intubation, n (%) | 76 (21.8%) | 84 (43.3%) | <.001 |
| Median duration of intubation, days (IQR) | 26.0 (34.0) | 27.0 (28.0) | .981 |
| Death, n (%) | 74 (21.2%) | 62 (32.0%) | .006 |
| COVID‐19‐related death, n (%) | 57 (16.3%) | 56 (28.9%) | .001 |
| Liver‐related death, n (%) | 8 (2.3%) | 16 (8.2%) | .001 |
p‐values depicting statistically significant differences are presented as bold values.
Of all patients with liver‐related death, 14 patients had no pre‐existing liver disease (1.7% of patients without preexisting liver disease), 6 had non‐ACLD (14.6% of non‐ACLD patients), 1 had cACLD (8.3% of cACLD patients) and 2 had dACLD (28.6% of dACLD patients, p < .001). Liver disease aetiology did not impact on liver‐related death (17.1% of NAFLD/NASH patients vs. 20.0% of ALD patients vs. 20.0% of viral hepatitis patients; p = .701). Importantly, liver‐related death occurred significantly more often in 40–69 years old patients (18–39 years: 0.0% vs. 40–69 years: 6.5% vs. ≥70 years: 2.2%; p < .001).
Patients with elevated AST at the first blood withdrawal after the first positive SARS‐CoV‐2 PCR test were more frequently admitted to the hospital (73.9% vs. 90.7%; p < .001) and to the ICU (27.8% vs. 51.0%; p < .001), they were intubated more often (21.8% vs. 43.3%; p < .001) and showed higher mortality (21.2% vs. 32.0%; p = .006), more COVID‐19‐related deaths (16.3% vs. 28.9%; p = .001) and more liver‐related deaths (2.3% vs. 8.2%; p = .001).
Elevated AST was associated with more frequent hospital admission, ICU admission and intubation in all age strata. Moreover, in patients aged 18–39 years, elevated AST (n = 27/93; 29.0%) was linked to longer median hospital stay (9.5 days vs. 16.5 days; p = .046, Table‐S2). COVID‐19‐related death occurred more often in 40–69 years (n = 93/247; 37.7%, Table‐S3) and ≥70 years old patients with elevated AST (n = 74/203; 36.5%, Table‐S4). However, overall death (19.5% vs. 31.2%; p = .037) and liver‐related death (3.2% vs. 15.1%; p = .027) was associated with elevated AST levels only in patients aged 40–69 years.
3.5. Association of survival and elevated liver enzymes after the first positive SARS‐CoV‐2 PCRtest
Assessed by log‐rank test, elevated levels of AST (n = 194/543; p < .001), ALP (n = 108/489; p = .004) and GGT (n = 244/493; p = .005) were associated with shorter survival, while ALT (n = 156/565; p = .253) and BIL (n = 44/557; p = .217) were not. In univariate Cox regression analysis, elevated AST (HR: 2.10; 95% CI: 1.25–3.51; p = .005) and ALP (HR: 1.70; 95% CI: 1.17–2.46; p = .005) were associated with increased mortality (Table‐S6). After adjustment for potentially confounding factors (pre‐existing liver disease, age, albumin, diabetes mellitus, cardiovascular disease, lung disease and malignancy), AST (aHR: 1.47; 95% CI: 1.01–2.14; p = .043) and BIL (aHR: 2.20; 95% CI: 1.22–3.98; p = .009) independently predicted survival after SARS‐CoV‐2 infection. In contrast, ALT (aHR: 0.95; 95% CI: 0.60–1.50; p = .842), ALP (aHR: 0.93; 95% CI: 0.60–1.44: p = .651) and GGT (aHR: 0.95; 95% CI: 0.61–1.50; p = .834) were not independently linked to mortality in multivariate analysis (Table‐3, Figure‐3).
TABLE 3.
Independent risk factors for mortality in COVID‐19 patients between 40 and 69 years old. Next to the univariate analysis (i), multivariate models including (ii) aspartate transaminase (AST), (iii) gamma‐glutamyl‐transferase (GGT) and (iv) bilirubin at blood withdrawal after the first positive SARS‐CoV‐2 PCR are shown
| Parameter of interest | HR | 95% CI | p‐value |
|---|---|---|---|
| (i) univariate (unadjusted) analysis | |||
| Alkaline phosphatase, >ULN | 1.54 | 0.89–2.67 | .121 |
| Aspartate transaminase, >ULN | 2.10 | 1.25–3.51 | .005 |
| Alanine aminotransferase, >ULN | 0.89 | 0.51–1.55 | .671 |
| Gamma‐glutamyl transferase, >ULN | 2.31 | 1.31–4.07 | .004 |
| Bilirubin, >ULN | 2.35 | 1.27–4.35 | .007 |
| Liver disease (present vs. absent) | 2.56 | 1.41–4.65 | .002 |
| Age, 10 years | 2.04 | 1.40–3.00 | <.001 |
| Sex (male) | 1.94 | 1.12–3.36 | .019 |
| Creatinine, mg × dl−1 | 1.16 | 1.01–1.34 | .040 |
| Albumin, g × L−1 | 0.91 | 0.88–0.95 | <.001 |
| Obesity (yes) | 1.17 | 0.68–2.01 | .572 |
| Diabetes mellitus (yes) | 1.13 | 0.64–2.00 | .682 |
| Cardiovascular disease (yes) | 1.18 | 0.65–2.14 | .596 |
| Lung disease (yes) | 1.68 | 0.96–2.94 | .070 |
| Malignancy (yes) | 1.56 | 0.89–2.73 | .120 |
| (ii) multivariate (adjusted) model including AST | |||
| Aspartate transaminase, >ULN | 1.78 | 1.04–3.06 | .037 |
| Liver disease (present vs. absent) | 1.73 | 0.90–3.31 | .100 |
| Age, 10 years | 1.37 | 0.93–2.01 | .107 |
| Sex (male) | 1.52 | 0.83–2.79 | .173 |
| Creatinine, mg × dl−1 | 1.06 | 0.91–1.25 | .445 |
| Albumin, g × L−1 | 0.92 | 0.88–0.96 | <.001 |
| Lung disease (yes) | 0.79 | 0.41–1.54 | .484 |
| (iii) multivariate (adjusted) model including GGT | |||
| Gamma‐glutamyl‐transferase, >ULN | 0.88 | 0.45–1.74 | .719 |
| Liver disease (present vs. absent) | 1.93 | 1.03–3.63 | .040 |
| Age, 10 years | 1.33 | 0.91–1.95 | .139 |
| Sex (male) | 1.66 | 0.91–3.04 | .099 |
| Creatinine, mg × dl−1 | 1.04 | 0.89–1.22 | .617 |
| Albumin, g × L−1 | 0.93 | 0.89–0.97 | <.001 |
| Lung disease (yes) | 0.85 | 0.44–1.66 | .643 |
| Age, 10 years | 1.37 | 0.93–2.01 | .107 |
| (iv) multivariate (adjusted) model including bilirubin | |||
| Bilirubin, >ULN | 2.18 | 1.15–4.13 | .017 |
| Liver disease (present vs. absent) | 1.65 | 0.86–3.14 | .130 |
| Age, 10 years | 1.49 | 0.81–2.73 | .186 |
| Sex (male) | 1.52 | 0.83–2.79 | .199 |
| Creatinine, mg × dl−1 | 1.07 | 0.91–1.26 | .431 |
| Albumin, g × L−1 | 0.93 | 0.89–0.96 | <.001 |
| Lung disease (yes) | 0.81 | 0.42–1.55 | .525 |
p‐values depicting statistically significant differences are presented as bold values.
FIGURE 3.
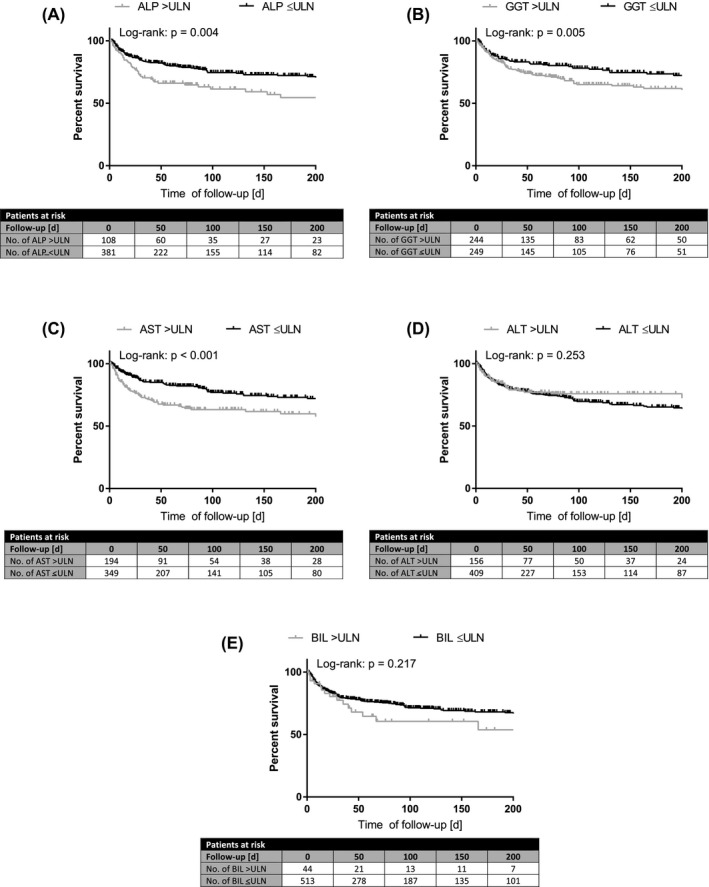
Overall survival binary for elevated/non‐elevated plasma levels of (A) alkaline phosphatase (ALP), (B) gamma‐glutamyl transferase (GGT), (C) aspartate transaminase (AST), (D) alanine aminotransferase (ALT) and (E) bilirubin at blood withdrawal after the first positive SARS‐CoV‐2 PCR. Survival comparison by log‐rank test
Shorter survival in 40–69 years old patients was linked to elevated AST (n = 93/247; p = .004), GGT (n = 128/235; p = .003) and BIL (n = 31/246; p = .005; Table‐S5). Importantly, elevated AST (aHR: 1.78; 95% CI: 1.04–3.06; p = .037) and BIL (aHR: 2.18; 95% CI: 1.15–4.13; p = .017) independently predicted mortality in 40–69 years old patients, while elevated GGT did not (aHR: 0.88; 95% CI: 0.45–1.74; p = .719).
In ≥70 years old patients, only increased AST levels were linked to a shorter time of survival (n = 74/203; p = .034). Assessed by univariate Cox regression analysis, AST >ULN was associated with increased mortality (HR: 1.62; 95% CI: 1.03–2.54; p = .037, Table‐S7). However, after adjustment for age, albumin and lung disease, elevated AST did not predict mortality in this cohort (aHR: 1.36; 95% CI: 0.80–2.32; p = .259).
4. DISCUSSION
In this study, we thoroughly characterized the patterns and trajectories of liver abnormalities in a large Austrian cohort of patients with COVID‐19. Importantly, we identified the patient cohort of 40–69 years of age as a particularly vulnerable group for COVID‐19‐associated liver injury and liver‐related death due to COVID‐19. Moreover, in accordance with previous studies, 18 we identified increased levels of AST and BIL around the time of the first positive SARS‐CoV‐2 PCR test as an independent risk factor for mortality.
Both parameters of hepatocellular and of cholestatic liver injury progressively increased following SARS‐CoV‐2 infection, which is in line with previous studies. 12 , 18 Subsequently, the liver chemistries, including AST and GGT regressed to pre‐COVID infection levels. Interestingly, ALP levels often remained elevated, suggesting prolonged/persistent cholestatic injury in a considerable number of patients with COVID‐19.
Similar to previous studies, 12 , 18 , 20 we observed COVID‐19‐related liver injury at blood withdrawal after the first positive SARS‐CoV‐2 PCR test in approximately 10% of patients. Interestingly, in our cohort pronounced elevation of liver chemistries (i.e. AST/ALT >3xULN and ALP/GGT/BIL >2xULN) were particularly frequent in 40–69 years old patients. Consequently, this was also the patient group with the highest rate of COVID‐19‐related liver injury (16.0%) – even if D‐dimer and CRP levels as established parameters for COVID‐19 severity 5 , 23 , 24 did not differ between 40–69 years old and ≥70 years old patients. However, the higher rate of obesity (44.4%) in the 40–69 years old patients suggests that there could be a considerable proportion of patients with hepatic steatosis, that is undiagnosed NAFLD, in this age group that could predispose to COVID‐19‐associated liver injury.
Interestingly, the rate of cholestatic liver injury (i.e. elevated ALP/GGT/BIL) was particularly high in our cohort (45.0% at the time of the first blood withdrawal after the positive PCR test), as compared to previous reports. 12 , 13 , 18 However, most data on cholestatic liver injury was derived from Asian patients, while large European and American studies often neither assessed ALP, nor GGT, nor BIL. 25 , 26 Thus, there may be differences in the proportion of elevation in these parameters of cholestasis between Asian and European patients. One may speculate that prolonged cholestatic injury is an “early” or “mild” version of the full spectrum of cholestatic disease described as COVID‐19‐associated sclerosing cholangitis after severe SARS‐CoV‐2 infection, that may even require liver transplantation. 3 , 27 , 28 , 29 Further studies should assess the best strategy to follow up patients with ALP elevation after COVID‐19.
Mortality, as well as COVID‐19‐related mortality, was highest among the oldest patient cohort (≥70 years), which confirms older age as an important risk factor for mortality in COVID‐19. 4 , 5 , 26 , 30 , 31 Correspondingly, the hospital admission rate was also highest in SARS‐CoV‐2‐infected patients ≥70 years of age. In contrast, there was not a single death recorded for COVID‐19 patients 18–39 years of age.
While overall mortality increased with age, liver‐related mortality was highest in patients aged 40–69 years old. This fits the observation of more frequent liver injury in this patient cohort and indicates that abnormalities of liver chemistries translate into worse liver‐related outcomes. Thus, 40–69 years old patients with abnormal liver chemistries at COVID‐19 diagnosis should be closely monitored, since they are at a particularly high risk for a severe course and worse outcomes.
Stratified for the severity of pre‐existing liver disease, liver‐related mortality was highest in dACLD patients followed by non‐ACLD and cACLD patients. This is in line with previous studies, which indicated an increased risk of liver‐related complications particularly in dACLD patients. 32 , 33 However, importantly, we also demonstrated that a small percentage (1.7%) of patients without pre‐existing liver disease also died of liver‐related causes, confirming that severe liver function impairment and acute liver failure is a rare but significant complication of COVID‐19. 3
Our observed association of elevated AST and BIL levels at COVID‐19 diagnosis with mortality is in line with previous studies 18 , 34 reporting the prognostic value of AST and BIL in patients with COVID‐19. Especially elevated AST at the first blood withdrawal after the first SARS‐CoV‐2 PCR test seems to predict a severe course of COVID‐19, 35 , 36 since AST was associated with more frequent hospital admission, ICU admission and intubation in all age strata. In 40–69 years old patients, it was also linked to higher overall mortality, COVID‐related death and liver‐related death.
Our study also has limitations: firstly, due to the retrospective study design, selection bias cannot be ruled out. Second, not all parameters were available for all patients at all time points. However, the findings of this study are in line with the existing literature and the missing data is mostly attributable to patients without hospital admission. Thus, we are confident that our data is reflecting the clinical scenario of hospitalized patients with COVID‐19. Thirdly, due to our monocentric study design, external validation of our results is required. Of note, this study mostly included unvaccinated patients infected with early variants of SARS‐CoV‐2. In the light of recent developments, further studies are needed to re‐evaluate the prevalence and prognostic impact of liver chemistry elevation in vaccinated patients or patients infected with the latest variants of the virus.
In conclusion, our large‐scaled Austrian COVID‐19 cohort study identified 40–69 years old patient as a particular risk group for liver injury of both hepatocellular and cholestatic patterns and for liver‐related mortality. Elevated AST and BIL levels at COVID‐19 diagnosis were an independent predictor of mortality, especially in patients aged 40–69 years.
DECLARATION
L.H., K.H., M.A., GS, BS, M.J., G.S., E.E., R.S., M.B. and D.L. declare no conflict of interest. B.Simbrunner received travel support from AbbVie and Gilead. D.B. has received travel support from AbbVie and Gilead. B.Scheiner has received travel support from Abbvie, Gilead and Ipsen. O.K. has received honoraria and research grants from Philips The Surgical Company. M.T. received grant support from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD, Takeda and Ultragenyx, honoraria for consulting from Albireo, Boehringer Ingelheim, BiomX, Falk, Genfit, Gilead, Intercept, MSD, Novartis, Phenex, Regulus and Shire, speaker fees from Bristol‐Myers Squibb, Falk, Gilead, Intercept and MSD, as well as travel support from AbbVie, Falk, Gilead and Intercept and is the co‐inventor of patents on the medical use of 24‐norursodesoxycholic acid. M.M. served as a speaker and/or consultant and/or advisory board member for AbbVie, Bristol‐Myers Squibb, Collective Acumen, Gilead and W. L. Gore & Associates and received travel support from AbbVie, Bristol‐Myers Squibb and Gilead. T.R. received grant support from AbbVie, Boehringer‐Ingelheim, Gilead, MSD, Philips Healthcare, Gore; speaking honoraria from AbbVie, Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from AbbVie, Bayer, Boehringer‐Ingelheim, Gilead, Intercept, MSD, Siemens; and travel support from Boehringer‐Ingelheim, Gilead and Roche.
Supporting information
Appendix S1
Hartl L, Haslinger K, Angerer M, et al. Age‐adjusted mortality and predictive value of liver chemistries in a Viennese cohort of COVID‐19 patients. Liver Int. 2022;42:1297‐1307. doi: 10.1111/liv.15274
Funding information
This study was supported by the Medical Scientific Fund of the Mayor of the City of Vienna to TR (MA 40‐GMWF‐485569‐2020).
Handling Editor: Luca valenti
REFERENCES
- 1. Atzrodt CL, Maknojia I, McCarthy RDP, et al. A guide to COVID‐19: a global pandemic caused by the novel coronavirus SARS‐CoV‐2. Febs J. 2020;287(17):3633‐3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324(8):782‐793. [DOI] [PubMed] [Google Scholar]
- 3. Nardo AD, Schneeweiss‐Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID‐19. Liver Int. 2021;41(1):20‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UKpatients in hospital with covid‐19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. Bmj. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323(18):1775‐1776. [DOI] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garrido I, Liberal R, Macedo G. Review article: COVID‐19 and liver disease‐what we know on 1st may 2020. Aliment Pharmacol Ther. 2020;52(2):267‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bertolini A, van de Peppel IP, Bodewes F, et al. Abnormal liver function tests in patients with COVID‐19: relevance and potential pathogenesis. Hepatology. 2020;72(5):1864‐1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yadav DK, Singh A, Zhang Q, et al. Involvement of liver in COVID‐19: systematic review and meta‐analysis. Gut. 2021;70(4):807‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kulkarni AV, Kumar P, Tevethia HV, et al. Systematic review with meta‐analysis: liver manifestations and outcomes in COVID‐19. Aliment Pharmacol Ther. 2020;52(4):584‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parasa S, Desai M, Thoguluva Chandrasekar V, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta‐analysis. JAMA Netw Open. 2020;3(6):e2011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amin M. COVID‐19 and the liver: overview. Eur J Gastroenterol Hepatol. 2021;33(3):309‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID‐19 and the liver. J Hepatol. 2020;73(5):1231‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai Q, Huang D, Ou P, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742‐1752. [DOI] [PubMed] [Google Scholar]
- 18. Ding ZY, Li GX, Chen L, et al. Association of liver abnormalities with in‐hospital mortality in patients with COVID‐19. J Hepatol. 2020;75:742‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh A, Premkumar M, Singh V. Liver injury in COVID‐19 ‐ the culprit may not be COVID‐19! J Hepatol. 2021;75(3):739‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests. J Hepatol. 2020;73(3):566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Liu S, Liu H, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol. 2020;73(4):807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112(1):18‐35. [DOI] [PubMed] [Google Scholar]
- 23. Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID‐19 patients: a review. Allergy. 2021;76(2):428‐455. [DOI] [PubMed] [Google Scholar]
- 24. Hartl L, Jachs M, Simbrunner B, Bauer DJM, Semmler G, Gompelmann D, Szekeres T, Quehenberger P, Trauner M, Mandorfer M, Scheiner B, Reiberger T Cirrhosis‐associated RAS – inflammation ‐ coagulation axis anomalies: parallels to severe COVID‐19. J Pers Med. 2021;11(12):1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the new York City area. Jama. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roth NC, Kim A, Vitkovski T, et al. Post‐COVID‐19 cholangiopathy: a novel entity. Am J Gastroenterol. 2021;116(5):1077‐1082. [DOI] [PubMed] [Google Scholar]
- 28. Durazo FA, Nicholas AA, Mahaffey JJ, et al. Post‐Covid‐19 cholangiopathy‐a new indication for liver transplantation: a case report. Transplant Proc. 2021;53(4):1132‐1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS‐CoV‐2 infection. BMJ Case Rep. 2020;13(11):e237984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Severe Outcomes Among Patients with Coronavirus Disease . (COVID‐19) ‐ United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020. 2019;69(12):343‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Marjot T, Moon AM, Cook JA, et al. Outcomes following SARS‐CoV‐2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74(3):567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sarin SK, Choudhury A, Lau GK, et al. Pre‐existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; the APCOLIS study (APASL COVID‐19 liver injury Spectrum study). Hepatol Int. 2020;14(5):690‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lei F, Liu YM, Zhou F, et al. Longitudinal association between markers of liver injury and mortality in COVID‐19 in China. Hepatology. 2020;72(2):389‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID‐19 cases: a systematic literature review and meta‐analysis. J Infect. 2020;81(2):e16‐e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis. 2020;73(11):e4208‐e4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


