Abstract
Background
COVID‐19 patients on mechanical ventilation are at risk to develop invasive aspergillosis. To provide additional data regarding this intriguing entity, we conducted a retrospective study describing risk factors, radiology and prognosis of this emerging entity in a Brazilian referral centre.
Methods
This retrospective study included intubated (≥18 years) patients with COVID‐19 admitted from April 2020 until July 2021 that had bronchoscopy to investigate pulmonary co‐infections. COVID‐19‐associated aspergillosis (CAPA) was defined according to the 2020 European Confederation of Medical Mycology/International Society of Human and Animal Mycosis consensus criteria. The performance of tracheal aspirate (TA) cultures to diagnose CAPA were described, as well as the radiological findings, risk factors and outcomes.
Results
Fourteen patients (14/87, 16%) had probable CAPA (0.9 cases per 100 ICU admissions). The sensitivity, specificity, positive predictive value and negative predictive value of TA for the diagnosis of CAPA were 85.7%, 73.1%, 46.2% and 95% respectively. Most of the radiological findings of CAPA were classified as typical of invasive pulmonary aspergillosis (64.3%). The overall mortality rate of probable CAPA was 71.4%. Age was the only independent risk factor for CAPA [p = .03; odds ratio (OR) 1.072]. CAPA patients under renal replacement therapy (RRT) may have a higher risk for a fatal outcome (p = .053, hazard ratio 8.047).
Conclusions
CAPA was a prevalent co‐infection in our cohort of patients under mechanical ventilation. Older patients had a higher risk to develop CAPA, and a poor prognosis may be associated with RRT.
Keywords: aspergillosis, CAPA, COVID‐19, diagnosis, outcomes, treatment
1. INTRODUCTION
It is estimated that over 10%–20% of the SARS‐CoV‐2‐infected patients require hospitalisation, 1 , 2 and 3%–5% of them end up needing intensive care support. 1
Patients with severe COVID‐19 requiring intensive care are at risk to develop invasive pulmonary aspergillosis (IPA). 3 With a prevalence of up to 30% in some intensive care units, 4 , 5 the COVID‐19‐associated aspergillosis (CAPA) has mortality rates over 40%. 4 , 6 The diagnosis CAPA may be challenging and the radiologic findings may be unspecific and unhelpful for its diagnosis. 7 However, diagnostic criteria have been proposed and have helped researchers to better characterise and study this disease. 8 To bring additional relevant information regarding CAPA, we investigated the cases that occurred at a Brazilian referral centre between April 2020 to July 2021, including two high incidence peaks of SARS‐CoV‐2 infections that occurred in June 2020 and March 2021 (https://covid19.who.int/region/amro/country/br). Moreover, we further analysed the epidemiology, laboratory and radiological findings, as well the risk factors and prognostic features of this intriguing and emergent medical entity.
2. MATERIAL AND METHODS
2.1. Settings and casuistic
This retrospective study was carried out at the Albert Einstein Hospital of São Paulo, a referral Brazilian centre with 650 beds. All consecutive patients (≥18 years) with the diagnosis of COVID‐19 by RT‐PCR admitted to the intensive care unit (ICU) between 1 April 2020 and 31 July 2021 that had bronchoalveolar lavage (BAL) to investigate a fungal or bacterial superinfection were included. Patients with cystic fibrosis and/or lung transplant recipients with previous recurrent airways colonisation or infection by Aspergillus spp. were excluded from the study. Data were collected and held anonymously, and the study was approved by the local ethics Committee (n. 34 882 620.7.0000.0071).
2.2. Laboratory analysis
BALs and tracheal aspirates (TA) were cultured on Sabouraud plus chloramphenicol (BioMérieux, Marcy L’Étoile, France) agar tubes at 30°C and 35°C for up to 21 days. Fungal identification was carried out with micromorphology and by MALDI‐TOF Mass Spectrometry (Microflex Bruker, Bremen, Germany) according to the manufacturer’s instructions.
The galactomannan in BAL and serum specimens were measured with a sandwich enzyme‐linked immunosorbent assay (Platelia Aspergillus™; Bio‐Rad Laboratories, Hercules, California, USA), and quantitative values were provided as optical density index (ODI).
Voriconazole serum levels requested between the second and the fifth day after the first dose 9 were measured with liquid chromatography tandem mass spectrometry as previously described. 10 All laboratory tests have been approved by the College of American Pathologists (CAP) external proficiency testing (CAP number 6 705 801).
2.3. Classification of CAPA
Patients with COVID‐19 with pulmonary infiltrates (entry criterium) were classified as having proven, probable and possible CAPA according to the 2020 European Confederation of Medical Mycology/International Society of Human and Animal Mycosis (ECMM/ISHAM) consensus criteria. 8
2.4. Radiology
CT scan findings were classified as typical of IPA if they met at least one radiologic criteria of IPA proposed by the European Organization for Research and Treatment of Cancer and the Mycosis Study group Education and Research Consortium (EORTC/MSG) criteria: dense, well‐circumscribed lesion(s) with or without halo sign; air crescent sign; cavity; wedged‐shape and segmental or lobar consolidation. 11 Other radiologic findings were described and classified as nonspecific of IPA.
2.5. Epidemiology, risk and prognostic factors
Variables were analysed at ICU admission and included: age, sex, body mass index (BMI), underlying conditions, haematologic or solid cancer, and sequential organ failure assessment (SOFA) score. 12 Additional variables were evaluated during ICU hospitalisation until the diagnosis of CAPA, or until hospital discharge for those without CAPA: antibacterial and/or antifungal exposure, azithromycin exposure, corticosteroid and/or tocilizumab therapy, high‐level corticosteroid exposure defined as dosage higher than or equivalent to prednisone 16 mg/day ≥15 days, 13 renal replacement therapy (RRT), extracorporeal membrane oxygenation (ECMO), heart failure requiring inotropic drugs, hepatic failure, previous candidaemia during hospitalisation. Prognostic factors were also analysed for the proven or probable CAPA cases at diagnosis: age, BMI, baseline diseases, galactomannan serum levels, SOFA score at CAPA diagnosis, heart failure requiring inotropic drugs, RRT, ECMO.
2.6. Statistical analysis
The sensitivity, specificity, positive and negative predictive values were calculated for TA cultures for the diagnosis of proven or probable CAPA.
The total and monthly incidence of CAPA were calculated per 100 ICU admissions of COVID‐19 patients.
Demographic data and clinical characteristics were presented as counts and percentages. For continuous variables, medians and ranges were described. Group comparisons were carried out with Pearson’s chi‐square or Fisher’s exact test where appropriate for categorical variables. For continuous variables, group comparisons were carried out with the Mann–Whitney test.
To investigate the risk factors of CAPA, proven and probable CAPA were matched to consecutive ICU‐hospitalised controls that also underwent bronchoscopy to investigate a superinfection (non‐CAPA patients) in a 1:3 ratio. Binomial logistic regression was used to characterise independent risk factors for the development of CAPA. Variables with p values <0.1 at the univariable level were included in the multivariable models in a forward stepwise method. Results were given as odds ratios (ORs) with 95% confidence intervals (CIs). Survival curves for CAPA and non‐CAPA patients were calculated by Kaplan–Meier curves, and univariable analysis was assessed by the log‐rank test.
Multivariable analyses of factors associated with death among CAPA patients were performed using stepwise multiple logistic regression analysis. Variables with p values <.1 at the univariable level were included in the multivariable model in a forward stepwise method. Results were given as ORs with 95% CIs. A multivariable time‐to‐death analysis was also performed using stepwise Cox regression analysis. Variables with p values <.1 at the univariable level were included in the multivariable model in a forward stepwise method. Results were given as hazard ratios (HRs) with 95% CIs.
All tests were two‐tailed, and p values <.5 were considered statistically significant. Statistical analysis was performed with SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
3. RESULTS
A total of 1604 COVID‐19 patients were admitted to the ICUs during the study period. Among them, 87 (5.4%) had at least one bronchoscopy and BAL sampling during hospitalisation to investigate the aetiology of new pulmonary infiltrates. According to the CAPA proposed criteria, a total of 14 probable (Table 1) and 8 possible cases were diagnosed. Therefore, the incidence of probable CAPA in the study period was 0.9 cases per 100 ICU admissions. The higher incidence of probable CAPA occurred during August 2020 and April 2021, with 2.9 and 2.5 cases per 100 ICU admissions respectively. Of note, among the 14 probable cases, two (14.3%) patients presented tracheal lesions suggestive of invasive fungal infection. Among the eight possible cases, one patient presented tracheal lesions suggestive of invasive fungal infection.
TABLE 1.
Demographic and clinical data of the 14 patients with probable CAPA
| Case n. | Age/sex | Baseline diseases | Duration of ICU hospitalisation until IPA diagnosis | Duration of mechanical ventilation until IPA diagnosis | Duration of hospitalisation after CAPA diagnosis | Aspergillus species | BAL/Serum GM ODI | Antifungal treatment | VAP after IPA | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77/F | Diabetes mellitus, acute myeloid leukaemia | 17 days | 8 days | 49 days | Negative culture | −/0.85 | Voriconazole 42 days | Yes | Alive |
| 2 | 75/M | Obesity, diabetes mellitus, hypertension, coronary artery disease | 20 days | 11 days | 33 days | Negative culture | >10/1.44 | Voriconazole 2 days followed by L‐AMB 31 days | Yes | Dead |
| 3 | 61/M | – | 15 days | 15 days | 31 days | Aspergillus fumigatus | >10/0.3 | Voriconazole 24 days | No | Alive |
| 4 | 72/M | Obesity, diabetes mellitus, hypertension, chronic renal failure, COPD | 38 days | 27 days | 19 days | Aspergillus niger | >10/− | L‐AMB and Isavuconazole 2 days followed by isavuconazole 17 days | Yes | Dead |
| 5 | 61/M | – | 40 days | 40 days | 3 days | Negative culture | 7.8/− | L‐AMB 3 days | No | Dead |
| 6 | 86/M | Diabetes mellitus, hypertension, myeloid sarcoma, coronary artery disease | 26 days | 24 days | 23 days | Aspergillus niger | −/>10 | Voriconazole 15 days | No | Dead |
| 7 | 77/M | Diabetes mellitus, hypertension, COPD, coronary artery disease | 17 days | 9 days | 88 days | Aspergillus fumigatus | 2.22/− | Voriconazole 21 days | No | Alive |
| 8 | 62/M | COPD, heart failure | 6 days | 6 days | 23 days | Negative culture | 1.22/− | Voriconazole 14 days | Yes | Dead |
| 9 | 69/F | – | 31 days | 28 days | 5 days | Negative culture | 1.02/− | – | No | Dead |
| 10 | 61/M | Diabetes mellitus, COPD | 26 days | 24 days | 7 days | Aspergillus fumigatus | 5.7/0.44 | – | No | Dead |
| 11 | 73/M | Myasthenia gravis | 12 days | 12 days | 9 days | Aspergillus fumigatus | 4.7/− | Voriconazole 9 days | No | Dead |
| 12 | 77/M | Diabetes mellitus, COPD | 32 days | 24 days | 28 days | Aspergillus niger | 0.53/0.08 | L‐AMB 10 days followed by voriconazole 18 days | No | Dead |
| 13 | 58/M | Diabetes mellitus, hypertension | 7 days | 6 days | 64 days | Aspergillus fumigatus | 7.58/0.34 | Voriconazole 47 days | No | Alive |
| 14 | 77/M | Diabetes mellitus, non‐Hodgkin’s lymphoma | 10 days | 10 days | 5 days | Negative culture | 0.2/2.38 | Voriconazole 13 days | Yes | Dead |
Abbreviations: BAL, bronchoalveolar lavage; GM, galactomannan; COPD, chronic obstructive pulmonary diseases; ICU, intensive care unit; IPA, invasive pulmonary aspergillosis; L‐AMB, liposomal amphotericin‐B; ODI, optical density index; VAP, ventilator‐associated pneumonia.
Aspergillus fumigatus and Aspergillus niger were found in five (5.7%) and three (3.5%) of the BAL samples respectively. A total of twelve BAL samples from 10 patients had GM ODI values above 1, and four samples from three patients had values above 10. Of note, six (50%) of these samples were culture negative. BAL direct microscopy was requested in 6 probable CAPA cases, and one sample was positive (16.6%). In 33 patients, a total of 48 tracheal aspirates were collected. Fifteen TA samples from thirteen patients had positive cultures for either A fumigatus (n = 8, 16.7%), A niger (n = 5, 10.4%), Aspergillus terreus (n = 1, 2%) or Aspergillus flavus (n = 1, 2%). Seven patients with probable CAPA had TA to investigate pulmonary infections, and six were positive for Aspergillus spp. (85.7%). Among the 26 patients without probable CAPA that were investigated with TA cultures, 7 (26.9%) had positive samples for Aspergillus spp. Of note, one patient with a TA sample positive for A niger had a serum GM ODI above 10 (Table 1). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of TA for the diagnosis of probable CAPA were 85.7%, 73.1%, 46.2% and 95% respectively. Seventy‐three serum GM samples from 43 patients were collected to investigate CAPA. Among the 14 patients with probable CAPA, eight were investigated with serum GM and three (37.5%) were positive. In two cases, serum positive GM samples were the only microbiological criteria for CAPA (Table 1). Among 35 patients without probable CAPA, 31 had GM samples with ODI below 0.5, and four had isolate specimens with ODI ranging from 0.53 to 1.04. Of note, one of these false‐positive samples (ODI 1.04) was related to a patient receiving oral probiotics containing Bifidobacterium.
Among the fourteen patients with probable CAPA, 9 (64.3%) had one or more typical radiologic criteria of invasive pulmonary aspergillosis: pulmonary cavity (n = 7), dense well‐circumscribed lung lesions with or without halo sign (n = 5), air crescent sign (n = 4) or wedged‐shape segmental consolidation (n = 1). The other 5 patients had nonspecific radiological signs of invasive pulmonary aspergillosis, including ground glass opacities, reticular pattern and consolidations, or bronchopneumonic opacities (Figure 1).
FIGURE 1.
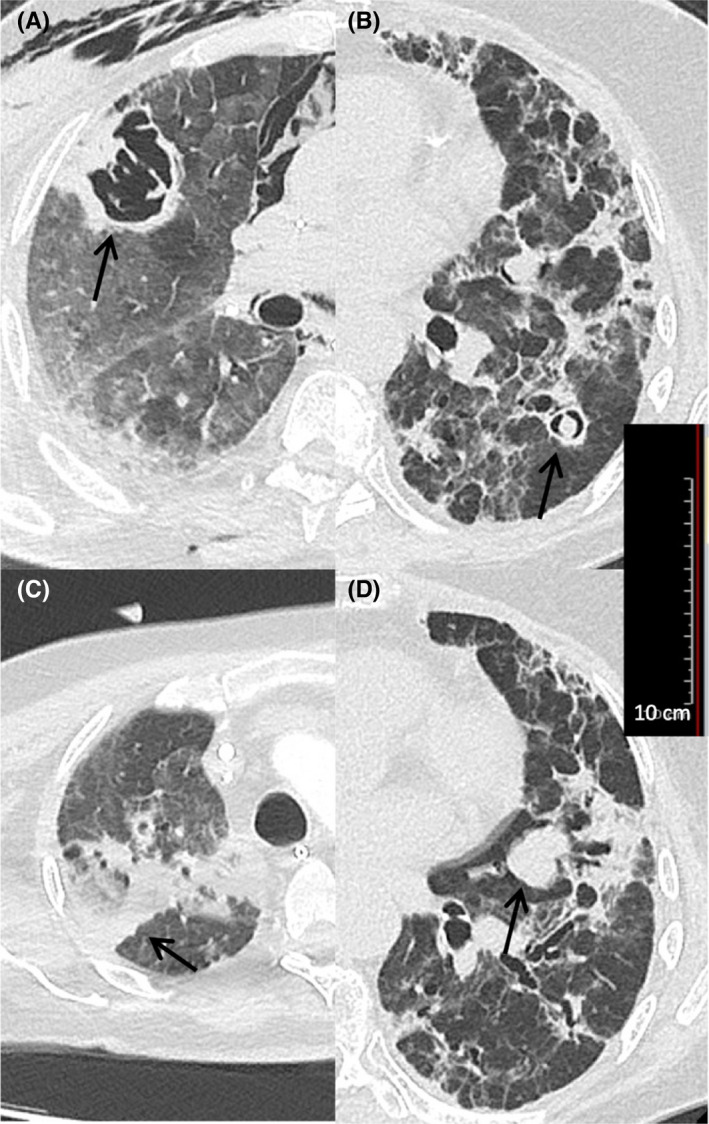
Examples of typical radiologic criteria of invasive pulmonary aspergillosis according to the EORTC/MSG criteria (arrows). (A) Pulmonary cavity (Case 2, see Table 1); (B) air crescent sign (Case 1, see Table 1); (C) wedged‐shape segmental consolidation with reversed halo sign(Case 3, see Table 1); (D) dense well‐circumscribed lesion without halo sign (Case 10, see Table 1)
By analysing the data retrieved by the patients that had probable CAPA, most of the patients were males (85.7%), with a median age of 72 years (interquartile range 62 to 77 years). The median day of hospitalisation until the development of CAPA was 19 days (interquartile range 13 to 30 days). Five of the probable CAPA cases had chronic pulmonary obstructive disease (COPD; 35.7%); eight had diabetes mellitus (57.1%). Only two (14%) patients had received tocilizumab before developing aspergillosis. All patients except one received high‐level corticosteroids before developing CAPA. Twelve (84.7%) probable CAPA cases received antifungal therapy, and nine (64.2%) had voriconazole for at least 48h. More details regarding the antifungal therapy are provided in Table 1. Among the patients that were treated with voriconazole, seven had therapeutic drug monitoring (TDM) and three (42.9%) had serum levels below 1 mg/L. Of note, five patients (35.7%) developed bacterial pneumonia after the diagnosis of CAPA. The overall mortality rate of the probable CAPA cases was 71.4% (n = 10/14), compared to 50% (n = 4/8) of the possible CAPA cases (p =.38).
By comparing the data retrieved from the patients with probable CAPA and controls (Table 2), we found by univariate analysis that older patients were at higher risk to develop aspergillosis (median 72.4 years vs. 62 years, p =.03), and COPD was more prevalent in the aspergillosis group (36% vs. 12%, p =.04). In multivariate analysis (Table 2), age was the independent factor associated with the development of CAPA (p =.03; OR = 1.072, CI95% = 1.006–1.141). The Kaplan–Meier survival curves derived from CAPA and non‐CAPA patients (Figure 2) showed no statistically difference between the two groups (p =.439).
TABLE 2.
Analysis of potential conditions associated with CAPA
| Parameters | CAPA (n = 14) | Non‐CAPA (n = 42) | Univariable analysis p value | Multivariable analysis p value (OR, CI95%) |
|---|---|---|---|---|
| Demographics | ||||
| Median age (interquartile range) | 72 (62–77) | 62 (54–71) | 0.015 | 0.03 (1.072, 1.006–1.141) |
| Male sex (%) | 12 (84) | 34 (81) | 0.16 | |
| Baseline conditions | ||||
| Median BMI (interquartile range) | 27 (24–28) | 28 (26–30) | 0.13 | |
| Diabetes mellitus (%) | 8 (57) | 14 (33) | 0.11 | |
| Hypertension (%) | 7 (50) | 22 (52) | 0.87 | |
| Heart disease (%) | 5 (36) | 6 (14) | 0.08 | |
| Chronic renal failure (%) | 1 (7) | 4 (10) | 0.79 | |
| Chronic obstructive pulmonary disease (%) | 5 (36) | 5 (12) | 0.04 | |
| Cancer (%) | 3 (21) | 4 (10) | 0.34 | |
| SOFA score at ICU admission, median (interquartile range) | 4 (2–5) | 3 (2–4) | 0.58 | |
| External conditions (%) | ||||
| Azithromycin exposure | 7 (50) | 17 (40) | 0.53 | |
| Previous candidaemia | 1 (7) | 2 (5) | 0.45 | |
| Antifungal exposure | 10 (71) | 34 (81) | 0.45 | |
| High level corticosteroid exposure | 13 (93) | 40 (95.2) | 0.73 | |
| Tocilizumab exposure | 2 (14) | 8 (19) | 0.69 | |
| Renal replacement therapy | 10 (71) | 26 (62) | 0.52 | |
| ECMO | 1 (7) | 10 (24) | 0.17 |
Abbreviations: BMI, body mass index; ECMO, extracorporeal membrane oxygenation; SOFA, sequential organ failure assessment. Statistically significant p values were highlighted in bold.
FIGURE 2.
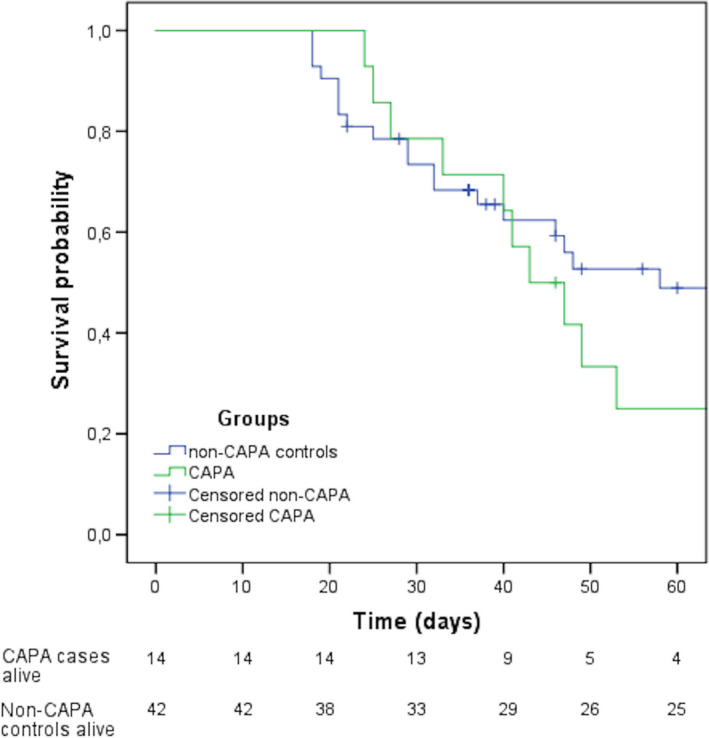
Kaplan–Meier survival curves obtained from CAPA and non‐CAPA patients (P = .439)
The prognostic factors among the 14 patients with probable CAPA were analysed and are summarised in Table 3. Of note, among the 10 patients that deceased, six patients were treated with voriconazole and five had TDM. Three of the five patients (60%) had voriconazole serum levels below 1 mg/L. Among the four survivals, two had received voriconazole and had serum levels were between 1 and 5 mg/L. By multivariate analysis, RRT was the only independent variable associated with poor prognosis (p = .03, OR = 27, CI95% = 1.260–578.354). The time‐to‐death analysis provided similar data compared to the logistic regression model. However, the Cox regression model showed a p value of 0.053 for RRT as a poor prognostic factor, with a HR of 8.047 (CI 95% 0.970–66.796).
TABLE 3.
Prognostic factors among patients with probable CAPA
| Parameters | Dead (n = 10) | Alive (n = 04) | Univariable analysis p value | Multivariable analysis p value (OR, CI95%) |
|---|---|---|---|---|
| Age, median (interquartile range) | 72 (64–76) | 69 (60–77) | 0.71 | |
| BMI, median (interquartile range) | 27 (25–28) | 24 (23–26) | 0.35 | |
| Diabetes mellitus (%) | 6 (60) | 2 (50) | 0.68 | |
| Cancer (%) | 3 (30) | 1 (25) | 1 | |
| SOFA score at CAPA diagnosis, median (interquartile range) | 3 (1–4) | 4 (3–6) | 0.51 | |
| Serum galactomannan ODI>1 (%) | 3 (30) | 0 (0) | 0.5 | |
| Heart Failure requiring inotropic drugs | 4 (40) | 0 (0) | 0.25 | |
| Renal replacement therapy | 9 (90) | 1 (25) | 0.04 | 0.03 (27, 1.260–578.354) |
| ECMO | 1 (10) | 0 (0) | 1 |
Abbreviations: BMI, body mass index; ECMO, extracorporeal membrane oxygenation; SOFA, sequential organ failure assessment; ODI, optical density index. Statistically significant p values were highlighted in bold.
4. DISCUSSION
The rates of reported CAPA are highly variable, ranging from 0% to 30%, 6 , 14 , 15 and separating colonised from infected patients is difficult in some cases. 3 , 16 Moreover, autopsy studies have also shown conflicting results that mirror the reported incidences. 15 , 17 Local factors such as the prevalence of comorbidities (e.g., COPD), the environment (e.g., use or lack of high‐efficiency particulate air filters) and level of immunosuppression (e.g., tocilizumab exposure), among others, may influence the incidence of CAPA of each centre. 4 , 8 , 14
The description and validation of CAPA risk factors will be helpful for its future management. However, only a few risk factors for CAPA have emerged until now, including older patients and tocilizumab exposure. 18 Indeed, a higher tocilizumab exposure would have led to more cases of CAPA in our centre. Studies previous to the COVID‐19 pandemic have shown that COPD patients under mechanical ventilation have a higher risk to develop invasive aspergillosis. 19 Our results showed a trend towards COPD as a risk factor for CAPA. Indeed, a recent meta‐analysis has pointed out that COPD may be also a relevant risk factor for developing CAPA. 20 These findings suggest that in the context of COVID‐19 and mechanical ventilation, older and/or COPD patients with new pulmonary infiltrates that appeared despite broad‐spectrum antibiotics should be investigated for IA.
The diagnosis of CAPA is challenging, and the combination of different diagnostic tools may be necessary for its diagnosis. 21 If only tracheal aspirate cultures were considered for CAPA diagnosis, several cases would have been treated unnecessarily in our centre. Serum galactomannan was requested for 43 patients in our cohort. Although serum GM for CAPA diagnosis may produce false‐positive results, occasionally related to the prescription of nutritional supplements containing Bifidobacterium, 22 positive results may be related to angioinvasion that has been reported in some CAPA cases. 23 , 24 Therefore, serum GM may be useful for the diagnosis of CAPA if requested in scenarios of high pre‐test probability in patients without ongoing known risk factors for false‐positive results.
We provided additional data that illustrate the radiologic findings of CAPA. Our findings are different from some other cohorts where most of the patients lacked typical radiological findings of IPA. 3 , 25 , 26 Typical lesions such as nodules with halo sign were seen in our cohort, corroborating that CAPA may evolve to angioinvasion. Indeed, CAPA is a complex disease presenting a continuum that progresses from respiratory colonisation, to tissue damage and ultimately, to angioinvasion. 27
Voriconazole has been widely used in some centres for the treatment of CAPA, 4 , 5 , 18 and serum levels have been reported to be very erratic among critically ill COVID‐19 patients. 28 In our cohort, a high proportion of the patients receiving voriconazole showed low serum levels. Isavuconazole is another therapeutic option for the treatment of invasive aspergillosis, and some centres have reported successful treatment of CAPA with this drug. 18 However, low isavuconazole serum levels have been reported in patients under RRT. 29 In our cohort, 10 CAPA patients were under RRT during the azole treatment. Therefore, critically ill COVID‐19 patients receiving either voriconazole or isavuconazole may need TDM for the optimal management of CAPA.
Regarding the outcomes, CAPA has shown high mortality rates in different centres, usually above 40%. 18 , 20 We compared the mortality rates of CAPA and non‐CAPA patients, and, differently from other studies, we found no statistical difference in the outcome. Our study included only COVID‐19 patients that underwent bronchoscopy for the investigation of a superinfection. Most of the non‐CAPA patients had a bacterial infection diagnosed (data not shown), which may explain the poor outcomes of both groups. A high mortality rate has also been reported for ventilator‐associated pneumonia by multidrug‐resistant bacteria in COVID‐19 patients. 30 A high proportion of critically ill COVID‐19 patients develops acute kidney injury. 31 In this context, renal failure has been associated with poor prognosis in COVID‐19, 31 and consequently, it may also negatively impact the outcomes of CAPA. In our cohort, 90% of the patients with probable CAPA that deceased had severe renal failure requiring RRT. Continuous research to investigate strategies to mitigate the acute kidney injury in COVID‐19 is necessary to achieve a better global prognosis for these patients. 32
Our study has limitations that need to be pointed out. The lack of an institutional protocol for the diagnosis of CAPA limited the evaluation of TA performance. Not all patients that had bronchoscopy for the investigation of a superinfection had concurrent TA collected. Thus, prospective studies with protocols that include an adequate gold standard method (i.e., lung tissue histology with invasive hyphae) to investigate CAPA are necessary to better evaluate the performance of the different methods for its diagnosis. Moreover, this single‐centre study included a relatively low number of probable CAPA patients. The inclusion of more cases would have helped to point out additional risk and prognostic factors of this relevant disease.
In conclusion, CAPA was prevalent co‐infection in patients under mechanical ventilation. Aspergillus‐positive TA should trigger prompt bronchoscopy for the investigation of CAPA. Serum GM may be useful for management of CAPA when requested and interpreted adequately. Older patients have a higher risk to develop CAPA, and poor prognosis may be associated with renal failure requiring replacement therapy.
AUTHOR CONTRIBUTIONS
João Nobrega de Almeida Júnior: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (lead); Writing – review & editing (lead). André Doi: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Project administration (equal); Supervision (equal); Validation (equal); Writing – review & editing (equal). Maria Julia Watanabe: Data curation (equal); Formal analysis (lead); Investigation (equal). Maira Maluf: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing – review & editing (equal). Cecília Leon Calderon: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing – review & editing (equal). Moacyr Silva: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Writing – review & editing (equal). Jacyr Pasternak: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing – review & editing (equal). Paula Célia Koga: Data curation (equal); Investigation (equal); Project administration (equal); Writing – review & editing (equal). Kelly Aline Santiago: Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing – review & editing (equal). Luis Fernando Aranha: Formal analysis (equal); Investigation (equal); Methodology (equal); Writing – review & editing (equal). Gilberto Szarf: Data curation (equal); Formal analysis (equal); Methodology (equal); Writing – review & editing (equal). Gustavo Teles: Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing – review & editing (equal). Renée Filippi: Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing – review & editing (equal). Vitor Paes: Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Writing – review & editing (equal). Marina Baeta: Data curation (equal); Formal analysis (equal); Methodology (equal); Writing – review & editing (equal). Nelson Hamerschlak: Formal analysis (equal); Investigation (equal); Project administration (equal); Writing – review & editing (equal). Cristóvão Mangueira: Conceptualization (equal); Methodology (equal); Project administration (lead); Writing – review & editing (equal). Marines Martino: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Project administration (lead); Resources (lead); Supervision (lead); Writing – review & editing (equal).
de Almeida Jr JN, Doi AM, Watanabe MJL, et al. COVID‐19‐associated aspergillosis in a Brazilian referral centre: Diagnosis, risk factors and outcomes. Mycoses. 2022;65:449–457. doi: 10.1111/myc.13433
DATA AVAILABILITY STATEMENT
Data available on request due to privacy/ethical restrictions.
REFERENCES
- 1. Auld SC, Caridi‐Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with coronavirus disease 2019*. Crit Care Med. 2020;48:e799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakamichi K, Shen JZ, Lee CS, et al. Hospitalization and mortality associated with SARS‐CoV‐2 viral clades in COVID‐19. Sci Rep. 2021;11:4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koehler P, Cornely OA, Böttiger BW, et al. COVID‐19 associated pulmonary aspergillosis. Mycoses. 2020;63:528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among COVID‐19 intubated patients: a prospective study. Clin Infect Dis. 2020:ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salmanton‐García J, Sprute R, Stemler J, et al. COVID‐19‐associated pulmonary aspergillosis, March‐August 2020. Emerg Infect Dis. 2021;27:1077‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dellière S, Dudoignon E, Fodil S, et al. Risk factors associated with COVID‐19‐associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2020;27(5):790.e1‐790.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verweij PE, Gangneux J‐P, Bassetti M, et al. Diagnosing COVID‐19‐associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e53‐e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID‐19‐associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149‐e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdul‐Aziz MH, Alffenaar J‐WC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med. 2020;46:1127‐1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alffenaar JWC, Wessels AMA, van Hateren K, Greijdanus B, Kosterink JGW, Uges DRA. Method for therapeutic drug monitoring of azole antifungal drugs in human serum using LC/MS/MS. J Chromatogr B. 2010;878:39‐44. [DOI] [PubMed] [Google Scholar]
- 11. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis‐related problems” of the European Society of intensive care medicine. Crit Care Med. 1998;26(11):1793‐1800. [DOI] [PubMed] [Google Scholar]
- 13. Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:1828‐1838. [DOI] [PubMed] [Google Scholar]
- 14. Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID‐19‐associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID‐19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63:766‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flikweert AW, Grootenboers MJJH, Yick DCY, et al. Late histopathologic characteristics of critically ill COVID‐19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. 2020;59:149‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rutsaert L, Steinfort N, Van Hunsel T, et al. COVID‐19‐associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fortarezza F, Boscolo A, Pezzuto F, et al. Proven COVID‐19‐associated pulmonary aspergillosis in patients with severe respiratory failure. Mycoses. 2021;64:1223‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prattes J, Wauters J, Giacobbe DR, et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients‐a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect. 2021;S1198‐743X(21)00474–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J. 2007;30:782‐800. [DOI] [PubMed] [Google Scholar]
- 20. Chong WH, Saha BK, Neu KP. Comparing the clinical characteristics and outcomes of COVID‐19‐associate pulmonary aspergillosis (CAPA): a systematic review and meta‐analysis. Infection. 2022;50(1):43‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dellière S, Dudoignon E, Voicu S, et al. Combination of mycological criteria: a better surrogate to identify COVID‐19 associated pulmonary aspergillosis patients and evaluate prognosis? J Clin Microbiol. 2022;JCM0216921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mennink‐Kersten MASH, Ruegebrink D, Klont RR, et al. Bifidobacterial lipoglycan as a new cause for false‐positive platelia aspergillus enzyme‐linked immunosorbent assay reactivity. J Clin Microbiol. 2005;43:3925‐3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santana MF, Pivoto G, Alexandre MAA, et al. confirmed invasive pulmonary aspergillosis and COVID‐19: the value of postmortem findings to support antemortem management. Rev Soc Bras Med Trop. 2020;53:e20200401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agrawal S, Phulware RH. COVID‐19 associated pulmonary aspergillosis (CAPA) in a pregnant woman. Autops Case Rep. 2021;11:e2021313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dupont D, Menotti J, Turc J, et al. Pulmonary aspergillosis in critically ill patients with Coronavirus Disease 2019 (COVID‐19). Med Mycol. 2021;59:110‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Machado M, Valerio M, Álvarez‐Uría A, et al. Invasive pulmonary aspergillosis in the COVID‐19 era: an expected new entity. Mycoses. 2021;64:132‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verweij PE, Brüggemann RJM, Azoulay E, et al. Taskforce report on the diagnosis and clinical management of COVID‐19 associated pulmonary aspergillosis. Intensive Care Med. 2021;47:819‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le Daré B, Boglione‐Kerrien C, Reizine F, Gangneux J‐P, Bacle A. Toward the personalized and integrative management of voriconazole dosing during COVID‐19‐associated pulmonary aspergillosis. Crit Care. 2021;25:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zurl C, Waller M, Schwameis F, et al. Isavuconazole treatment in a mixed patient cohort with invasive fungal infections: outcome, tolerability and clinical implications of isavuconazole plasma concentrations. J Fungi (Basel). 2020;6:E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ippolito M, Misseri G, Catalisano G, et al. Ventilator‐associated pneumonia in patients with COVID‐19: a systematic review and meta‐analysis. Antibiotics (Basel). 2021;10:545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nadim MK, Forni LG, Mehta RL, et al. COVID‐19‐associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16:747‐764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yende S, Parikh CR. Long COVID and kidney disease. Nat Rev Nephrol. 2021;17:792‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


