Abstract
Background
The role of extracorporeal membrane oxygenation (ECMO) for patients with refractory respiratory failure due to coronavirus 2019 (COVID‐19) is still unclear even now over a year into the pandemic. ECMO is becoming more commonplace even at smaller community hospitals. While the advantages of venovenous (VV) ECMO in acute respiratory distress syndrome (ARDS) from COVID‐19 have not been fully determined, we believe the benefits outweighed the risks in our patient population. Here we describe all patients who underwent VV ECMO at our center.
Methods
All patients placed on ECMO at our center since the beginning of the pandemic, May 5, 2020, until February 20, 2021 were included in our study. All patients placed on ECMO during the time period described above were followed until discharge or death. The primary endpoint was in‐hospital death. Secondary outcomes included discharge disposition, that is, whether patients were sent to a long‐term acute care center (LTAC), inpatient rehabilitation, or went directly home.
Results
A total of 41 patients were placed on VV ECMO for refractory acute respiratory failure. Survival to discharge, the primary end point, was 63.4% (26/41). Inpatient mortality was 36.6% (15/41).
Conclusions
We show here that a successful high‐volume VV ECMO program for ARDS is achievable at even a medium‐size community hospital. We think our success can be replicated by most small‐ and medium‐size community hospitals with cardiothoracic surgery programs and intensivist teams.
Keywords: COVID‐19, critical care, ECMO
1. INTRODUCTION
The role of extracorporeal membrane oxygenation (ECMO) for patients with refractory respiratory failure due to coronavirus 2019 (COVID‐19) is still unclear even now over a year into the pandemic. However, some studies have shown the success of venovenous (VV) ECMO in acute respiratory distress syndrome (ARDS) from COVID‐19. 1 , 2 Unfortunately, for unknown reasons the mortality of VV ECMO for COVID patients has been increasing. 3
ECMO is becoming more commonplace even at smaller community hospitals. 4 The Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure (CESAR) trial has demonstrated the improvement in mortality in patients referred to an ECMO center. 5 While the advantages of VV ECMO in ARDS from COVID‐19 have not been fully determined, we believe the benefits outweighed the risks in our patient population. Here we describe all patients who underwent VV ECMO at our center.
2. METHODS
2.1. Patients
All patients placed on ECMO at our center since the beginning of the pandemic, May 5, 2020 until February 20, 2021 were included in our study. All patients were followed until discharge or mortality. All patients were confirmed SARS‐CoV‐2‐positive by laboratory testing and were in acute respiratory failure. Patients were managed by a multidisciplinary team including anesthesiologists/critical care intensivists, cardiologists, pharmacists, and cardiothoracic surgeons. The study was approved by our institutional review board.
2.2. Outcomes
The primary endpoint was in‐hospital death. All patients placed on ECMO during the time period described above were followed until discharge or death. Secondary outcomes included discharge disposition, that is, whether patients were sent to a long‐term acute care center (LTAC), inpatient rehabilitation, or went directly home. We report hospital length of stay, duration of mechanical ventilation, duration of ECMO, tracheostomy use, need for renal replacement therapy, and complications while on ECMO.
2.3. Statistical analysis
Continuous variables were presented as median with interquartile range (IQR) or mean with standard deviation. However, due to outliers, we believe the central tendency of our data is most accurately reflected by median with IQR. Categorical variables were presented as proportions.
3. RESULTS
3.1. Patients
From May 5, 2020 to February 20, 2021, a total of 41 patients were placed on VV ECMO for refractory acute respiratory failure. Pre‐ECMO course and patient characteristics are summarized in Table 1. Median age was 58 years (IQR, 49−64). Twenty‐four patients (58%) were male. Median body mass index (BMI) was 36.7 (IQR, 33.1−42.7). Fourteen of our patients (34.1%) were morbidly obese with a BMI over 40. Hypertension was the most common comorbidity, present in 31 patients (80.4%) and 9 patients (21.9%) were active smokers.
Table 1.
Patient characteristics and hospital course before ECMO initiation.
| Median age, years (IQR) | 58 (49−64) |
| Mean age, years, mean ± SD (range) | 55.6 ± 11.4 (31−74) |
| Sex, male (%) | 24/41 (58%) |
| Median body mass index, kg/m2 (IQR) | 36.7 (33.1−42.7) |
| Mean body mass index, kg/m2, mean ± SD (range) | 38.9 ± 10 (26.3−81.1) |
| Comorbidities, n (%) | |
| Hypertension | 33/41 (80.4%) |
| Diabetes | 19/41 (46.3%) |
| Chronic obstructive pulmonary disease | 1/41 (2.4%) |
| Coronary artery disease | 6/41 (14.6%) |
| Active smoker | 9/41 (21.9%) |
| Morbidly obese | 14/41 (34.1%) |
| Admissions, n (%) | |
| Direct admission from ED | 22/41 (53.7%) |
| Transfer from outside facility | 19/41 (46.3%) |
| Intubated before transfer | 15/41 (36.5%) |
| Interventions before ECMO (%) | |
| Paralyzed | 21/41 (51.2%) |
| Proned | 24/41 (58.5%) |
| CPR/cardiac arrest | 1/41 (2.4%) |
| Intubated | 34/41 (82.9%) |
| ECMO initiated while on HFNO | 4/41 (9.7%) |
| ECMO initiated while on BiPAP | 3/41 (7.3%) |
| Median hours from intubation to ECMO (IQR) | 22 (7−43) |
| Median days from admission to ECMO (IQR) | 1 (0.5−4) |
| Mean days from admission to ECMO days mean ± SD (range) | 3.6 ± 5.4 (0.5–25) |
| Median P:F ratio of intubated patients before ECMO (IQR) | 64.5 (55.7−84.2) |
| Initial cannulation site, n (%) | |
| Dual lumen single internal jugular cannula | 21/41 (51.2%) |
| Bilateral femoral VV | 18/41 (43.9%) |
| Internal jugular femoral combination | 2/41 (4.8%) |
| Bedside cannulation | 17/41 (41.4%) |
Abbreviations: BiPAP, bilevel positive airway pressure; ECMO, extracorporeal membrane oxygenation; HFNO, high flow nasal oxygen; IQR, interquartile range; VV, venovenous.
Thirty‐four patients were on mechanical ventilation and in ARDS by Berlin criteria when cannulated for ECMO. 6 Median ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2) or P:F ratio was 64.5 (IQR, 55.7−84.2) before ECMO initiation in the mechanically ventilated cohort. Four patients were cannulated on high flow nasal oxygen (HFNO), and three patients were cannulated on bilevel positive airway pressure (BiPAP). Before ECMO, 21 patients (51.2%) were paralyzed and 24 patients (58.5%) were proned.
3.2. ECMO cannulation
Seventeen patients (41.4%) were cannulated at bedside. Eighteen patients (43.9%) were originally cannulated in the femoral−femoral configuration, 21 patients (51.2%) were originally cannulated with a dual lumen single internal jugular (IJ) cannula, and 2 patients were originally cannulated with an IJ and femoral combination configuration.
Cannulations at bedside were performed under ultrasound guidance. Standard J‐wires were used to place a sheath, at which point the wire was exchanged for a heavier stiff wire. The heavy wire was used for dilation and placement of the cannula. A bedside ultrasound as well as fluoroscopy with a portable C‐arm was used to confirm wire placement. After placement, standard portable chest and abdominal films were obtained to confirm cannula positioning.
Seven patients (17.1%) required reconfiguration of ECMO cannulas. Three patients of the 21 originally cannulated with a dual lumen single IJ cannula (3/21, 14.3%) required reconfiguration to femoral−femoral due to drainage and flow issues.
3.3. Outcomes
Survival to discharge, the primary end point, was 63.4% (26/41). Inpatient mortality was 36.6% (15/41). Twelve patients were extubated while on ECMO. Table 2 outlines our ECMO results. Figure 1 outlines the disposition of our patients. Twenty‐one patients were discharged to long‐term acute care hospitals (LTAC) (51%). Ten of the patients sent to LTACs have followed up in our hospital system after discharge from their LTAC; the other 11 patients have not followed up in our hospital system and their current status is unknown. Three patients were discharged to an inpatient rehabilitation facility. Two patients were discharged directly home.
Table 2.
Outcomes of patients with severe acute respiratory distress syndrome with COVID‐19 who were initiated on venovenous ECMO.
| Survival to discharge, n (%) | 26/41 (63.4) |
| Inpatient mortality, n (%) | 15/41 (36.6) |
| Median duration of ECMO, days (IQR) | 19 (13−30) |
| Median duration of ventilation, days (IQR) | 33 (16−41) |
| Median length of stay of survivors, days (IQR) | 37 (25−50.5) |
| Required tracheostomy, n (%) | 19/41 (46) |
| Required reconfiguration of ECMO, n (%) | 7/41 (17) |
| Extubated while on ECMO, n (%) | 12/41 (29.2) |
| Complications | |
| Blood stream infection, n (%) | 7/41 (17.1) |
| Renal replacement therapy, n (%) | 22/41 (53.7) |
| Bleeding, n (%) | 18/41 (43.9) |
| Hemorrhagic brain injury, n (%) | 2/41 (4.8) |
| Switched from heparin to argatroban | 11/41 (26.9) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; IQR, interquartile range.
Figure 1.
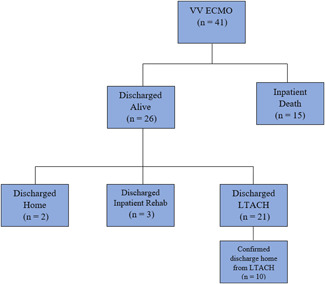
Outcomes of patients initiated on VV ECMO for COVID‐19. ECMO, extracorporeal membrane oxygenation; VV, venovenous.
3.4. Complications
Any bleeding that required at least temporary cessation of anticoagulation was recorded as a bleeding event. Eighteen patients had bleeding events (43.9%). Two patients had hemorrhagic brain injuries diagnosed with CT imaging; both patients died. Nine patients had significant cannula site bleeding that required at least temporary cessation of anticoagulation. Three patients had gastrointestinal bleeding that required transfusion. Eleven patients were switched from heparin to argatroban due to thrombocytopenia. One patient suffered a dislodgement of their dual lumen single right IJ cannula and was emergently converted to femoral−femoral configuration.
4. CONCLUSIONS
We present one of the largest cohorts of patients with refractory COVID‐19 requiring ECMO cannulation at a nonacademic medium‐size (less than 500 available beds) hospital. While the role of ECMO remains unclear for patients with refractory respiratory failure from COVID‐19, we believe our patient population benefited from it. Our mortality rate was substantially lower than the most recent COVID‐19 ECMO mortality from the Extracorporeal Life Support Organization (ELSO) registry. 7 Our mortality rate was also lower than the prepandemic ECMO for ARDS mortality from ELSO, which was approximately 40%. 3
We believe one of the main contributing factors to our success was early cannulation in the ARDS course. Early cannulation was emphasized in the ECMO for COVID‐19: Updated 2021 Guidelines from ELSO. Their criteria based on the ECMO to Rescue Lung Injury in Severe ARDS (EOLIA) trial recommends cannulation after just 3−6 h of persistently low P:F ratios after the failure of optimal medical management, including proning, paralytics, and high PEEP mechanical ventilation. 3 , 8 Our median (IQR) time to cannulation from intubation was 22 (7−43) h. Early involvement of the entire ECMO multidisciplinary team was crucial to our prompt cannulations.
Originally most of the patients were cannulated with bilateral femoral cannulas. As the pandemic progressed, we started using more dual‐lumen single‐cannula right ventricular assist devices (right atrium to pulmonary artery) in the right IJ vein using the ProtekDuo system (TandemLife). We had good results from the dual‐lumen single‐cannula right ventricular assist device, and we believe it is more comfortable for the patient and allows for ambulation. The dual‐lumen single‐cannula right ventricular assist device also provides right‐sided support in cases of right ventricular dysfunction and potentially decreases recirculation issues. ELSO guidelines in 2020 recommended against a single right IJ cannula. 9 However, the ELSO 2021 guidelines changed to more friendly language, saying there “may be a role” for these cannulas. 3
We successfully cannulated 17 patients at bedside, a safe method that has been previously discussed in the literature. 2 Seven patients required reconfiguration of their cannulas. Three of our single right IJ cannulas required conversion to femoral−femoral to improve flow.
The dual‐lumen single IJ cannulas used were 29 french (fr) and 31 fr. Femoral cannulas were 24 fr multistage drainage cannulas and 19−20 fr IJ single‐stage return cannulas when using IJ−femoral configuration. When using femoral−femoral technique we wanted at least 8 cm distance between the drainage and return cannulas to avoid recirculation. Our initial bedside cannulations were done with transesophageal echocardiogram guidance. This was during the early days of the pandemic when personal protection equipment was limited, and the infectiousness of the virus was poorly understood. Now all cannulations are done in the cath lab under fluoroscopy.
Our medium‐size community hospital is part of a local hospital system with four smaller satellite hospitals in our network. A total of 19 of our patients (46.3%) were transferred from outside hospitals in our ECMO referral network. The importance of having an organized referral network within a region was highlighted by the most recent ELSO guidelines. 3 Our intensivist team that was responsible for evaluating and accepting transfers was also leading the multidisciplinary ECMO, and at the time of transfer, the entire multidisciplinary team was involved in the assessment of ECMO. This initial involvement of the whole team allowed us to cannulate and initiate ECMO early which we believe was key to our success.
All patients had transthoracic echocardiogram (TTE) to assess cardiac function. Most patients had TTE before cannulation. Although a few of our patients were emergently cannulated and there was insufficient time for full cardiac work before cannulation. We had no cardiac function cut‐off for Protek duo. Also, none of our patients required venous‐arterial ECMO (VA‐ECMO) for cardiogenic shock. However, if precannulation echocardiogram showed right ventricular dysfunction, then we favored the ProtekDuo system.
As part of our early cannulation strategy, we placed several patients on ECMO while on HFNO or BiPAP. Even though ELSO guidelines currently recommend maximizing traditional therapy before ECMO, that is, intubation, proning, paralytics, and high PEEP ventilation, 3 we believe that by initiating ECMO before invasive mechanical ventilation we may be able to avoid further lung damage. Nevertheless, the four patients we placed on ECMO while on HFNO later required intubation. The mortality of our HFNO cannulation cohort was one of four patients; their total mechanical ventilation time was 2, 4, 15, and 26 days. Therefore, this strategy did seem to decrease total ventilation days, although our sample size is too small to draw any conclusions. Further study in a larger patient population would be necessary to clarify the benefits of this strategy.
Our main contraindications to ECMO were the length of mechanical ventilation, multisystem organ failure, and age. Our oldest patient placed on ECMO was 73 years. Three of the four patients we placed on ECMO over 70 years of age died. Obesity was not considered a contraindication to cannulation, even though a BMI greater than 40 is listed as a relative contraindication in the ELSO guidelines. 9 We cannulated 14 patients with a BMI above 40. The mortality in our morbidly obese cohort was 5/14 (35.7%). We believe this vulnerable patient population can benefit greatly from ECMO as traditional mechanical ventilatory strategies are less effective in the morbidly obese due to decreased lung compliance and decreased forced expiratory volumes. 10 Therefore, we believe that extracorporeal oxygenation is even more important in an obese patient with ARDS. We do acknowledge the obvious technical challenges of cannulating obese patients. However, we have demonstrated that this can be done safely at the bedside in crowded ICU rooms. Our most obese patient, with a BMI of 81, was cannulated at bedside.
We did not prone patients while cannulated on ECMO due to the logistical challenges proning while cannulated involves, although there is some limited literature now available showing the feasibility and benefits of proning while cannulated. 11 , 12 Furthermore, a substantial portion of our patients were obese, adding to these technical challenges. We did ambulate several patients with single right IJ dual‐lumen cannulas. We believe ambulation has similar advantages of recruiting unused alveoli and increases lung compliance. 13 We extubated 12 patients while on ECMO. We believe extubation decreases invasive ventilation injury, increases patient involvement in pulmonary toilette, and promotes easier ambulation. Four patients that were extubated on ECMO required reintubation, and all four patients requiring reintubation while on ECMO eventually died.
All patients while on ECMO were placed on “lung rest” ventilator settings, which consisted of pressure control with a rate of 10, pressure of 10, PEEP of 10, and FiO2 was weaned to 30%. Several of our patients were extubated while on ECMO. When weaning these patients the sweep was set to zero and if the patient was able to maintain acceptable blood gases and oxygen saturations, they would be decannulated. Most patients were decannulated while still on mechanical ventilation. The patient would be placed on lung protective settings. Patient's settings were tidal volumes of 6−8 ml/kg of predicted body weight and high PEEP. Next, the sweep was set to zero and the ventilator was set to a FiO2 of 50% or less. Oxygenation and CO2 levels were monitored for 6−12 h on a sweep of zero. If they were able to tolerate an FiO2 of 50% with a PaO2/FiO2 ratio above 100 mmHg while on lung‐protective ventilation, then they were decannulated.
A continuous heparin infusion was used on all patients. Using the 4T's score for heparin‐induced thrombocytopenia (HIT), if any patient was at “intermediate probability” of HIT we would switch to argatroban. At our hospital argatroban was similar in cost to heparin and we found it easy to titrate. We had more bleeding events than thrombotic events, as do cases registered in the ELSO database. The ELSO guidelines in 2020 recommended, “consider targeting anticoagulation at the higher end of normal ECMO parameters.” However, new ELSO 2021 guidelines recommend COVID‐19 ECMO patients should be anticoagulated similar to other patients in ARDS. 3 , 9
Our data show that a medium‐size (less than 500 available total beds) community hospital can effectively handle a large number of ECMO patients. We were able to achieve this because of our intensivist team and 24‐h advance practice provider coverage. The rapid training of medical intensive care unit (MICU) nursing staff to become ECMO competent was also particularly important to our outcomes. We had limited perfusionist involvement in the day‐to‐day ECMO care as we have only a few perfusionists covering our entire hospital and all cardiothoracic cases. We used the LifeSPARC pump (TandemLife) at our institution. Its simplicity of use allowed our MICU nurses to quickly become comfortable with the set‐up and were able to adjust settings as necessary. Some literature shows that nurse‐led ECMO management has no difference in survival compared to perfusion‐led ECMO management and may have cost benefits. 14
ECMO was becoming more common in small‐ and medium‐size hospitals even before the COVID‐19 pandemic. 4 Data have shown that higher prepandemic ECMO volume does not result in lower mortality for COVID‐19 ECMO patients. 1 Furthermore, Sanaiha et al. 4 showed in their prepandemic analysis that small‐ and medium‐size hospital ECMO programs had a statistically significant lower mortality in these patients. Our mortality was considerably lower than the predicted survival of ventilated COVID‐19 patients. 15 , 16 , 17 Although the role of ECMO in patients with COVID‐19 has still not been established, we believe our cannulated patients were unlikely to survive without ECMO support. Our mortality was lower than national trends despite a patient population that was older, more obese, and therefore at a higher risk for death from COVID‐19 infection. We show here that a successful high‐volume VV ECMO program for ARDS is achievable at even a medium‐size community hospital.
AUTHOR CONTRIBUTIONS
James Lee West: Data analysis/interpretation. Andrew Nutting: Data analysis/interpretation. Brock Daughtry: Data analysis/interpretation. Andrew M. Frey: Data analysis/interpretation. Clara T. Nicolas: Drafting article. Rayan Saab: Review of article. David H. Sibley: Review of article. Joshua Smith: Review of article. Ronald Roan: Review of article. Michael Crain: Review of article.
ETHICS STATEMENT
Informed patient consent statement is not available since our study was a retrospective review of results. The study was approved by our Institutional Review Board.
West JL, Nutting A, Daughtry B, et al. Coronavirus 2019 (COVID‐19) venovenous extracorporeal oxygenation: single community hospital results and insights. J Card Surg. 2022;37:2009‐2014. 10.1111/jocs.16514
REFERENCES
- 1. Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E. Extracorporeal membrane oxygenation support in COVID‐19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shih E, DeMaio MJ, Squiers JJ, et al. Venovenous extracorporeal membrane oxygenation for patients with refractory coronavirus disease 2019 (COVID‐19): multicenter experience of referral hospitals in a large health care system. J Thorac Cardiovasc Surg. 2020;163(3):1071‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Badulak J, Antonini MV, Stead CM, et al. Extracorporeal membrane oxygenation for COVID‐19: updated 2021 guidelines from the Extracorporeal Life Support Organization. ASAIO J. 2021;67:485‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanaiha Y, Bailey K, Downey P, et al. Trends in mortality and resource utilization for extracorporeal membrane oxygenation in the United States: 2008–2014. Surgery. 2019;165:381‐388. [DOI] [PubMed] [Google Scholar]
- 5. Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351‐1363. [DOI] [PubMed] [Google Scholar]
- 6. The ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526‐2533. [DOI] [PubMed] [Google Scholar]
- 7.Full COVID‐19 Registry Dashboard. Accessed October 6, 2021. https://www.elso.org/Registry/FullCOVID19RegistryDashboard.aspx
- 8. Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965‐1975. 10.1056/NEJMoa1800385 [DOI] [PubMed] [Google Scholar]
- 9. Shekar K, Badulak J, Giles P, et al. Extracorporeal Life Support Organization Coronavirus Disease 2019 Interim Guidelines: a consensus document from an International Group of Interdisciplinary Extracorporeal Membrane Oxygenation Providers. ASAIO J. 2020;66(7):707‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peters U, Dixon AE. The effect of obesity on lung function. Expert Rev Respir Med. 2018;12:755‐767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giani M, Martucci G, Madotto F, et al. Prone positioning during venovenous extracorporeal membrane oxygenation in acute respiratory distress syndrome. A multicenter cohort study and propensity‐matched analysis. Ann Am Thorac Soc. 2021;18:495‐501. [DOI] [PubMed] [Google Scholar]
- 12. Garcia B, Cousin N, Bourel C, et al. Prone positioning under VV‐ECMO in SARS‐CoV‐2‐induced acute respiratory distress syndrome. Crit Care. 2020;24:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katz S, Arish N, Rokach A, Zaltzman Y, Marcus E‐L. The effect of body position on pulmonary function: a systematic review. BMC Pulm Med. 2018;18:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dhamija A, Kakuturu J, Schauble D, et al. Outcome and cost of nurse‐led versus perfusionist‐led extracorporeal membrane oxygenation. Ann Thorac Surg. 2021;113:1127‐1134. 10.1016/j.athoracsur.2021.04.095 [DOI] [PubMed] [Google Scholar]
- 15. Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID‐19 requiring invasive mechanical ventilation. A meta‐analysis. Am J Respir Crit Care Med. 2021;203:54‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roedl K, Jarczak D, Thasler L, et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: a multicentric study in Germany. Aust Crit Care. 2021;34:167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]


