Abstract
Background
Persistent symptoms of SARS‐CoV‐2 are prevalent weeks to months following the infection. To date, it is difficult to disentangle the direct from the indirect effects of SARS‐CoV‐2, including lockdown, social, and economic factors.
Objective
The study aims to characterize the prevalence of symptoms, functional capacity, and quality of life at 12 months in outpatient symptomatic individuals tested positive for SARS‐CoV‐2 compared to individuals tested negative.
Methods
From 23 April to 27 July 2021, outpatient symptomatic individuals tested for SARS‐CoV‐2 at the Geneva University Hospitals were followed up 12 months after their test date.
Results
At 12 months, out of the 1447 participants (mean age 45.2 years, 61.2% women), 33.4% reported residual mild to moderate symptoms following SARS‐CoV‐2 infection compared to 6.5% in the control group. Symptoms included fatigue (16% vs. 3.1%), dyspnea (8.9% vs. 1.1%), headache (9.8% vs. 1.7%), insomnia (8.9% vs. 2.7%), and difficulty concentrating (7.4% vs. 2.5%). When compared to the control group, 30.5% of SARS‐CoV‐2 positive individuals reported functional impairment at 12 months versus 6.6%. SARS‐CoV‐2 infection was associated with the persistence of symptoms (adjusted odds ratio [aOR] 4.1; 2.60–6.83) and functional impairment (aOR 3.54; 2.16–5.80) overall, and in subgroups of women, men, individuals younger than 40 years, those between 40–59 years, and in individuals with no past medical or psychiatric history.
Conclusion
SARS‐CoV‐2 infection leads to persistent symptoms over several months, including in young healthy individuals, in addition to the pandemic effects, and potentially more than other common respiratory infections. Symptoms impact functional capacity up to 12 months post infection.
Keywords: epidemiology, functional impairment, infectious diseases, inflammation, internal medicine, persistent symptoms, post‐COVID, SARS

Introduction
Post‐acute sequelae of SARS‐CoV‐2 infection (PASC), long COVID, or post‐COVID syndrome [1, 2, 3] have now been shown to be prevalent to varying degrees following the infection. Prevalence varies between 10% and 30% [4, 5, 6, 7] in the first few months following the infection, and up to 70% [8] depending on the study population. Taking lessons from history and past infections [9], symptoms may persist for months to years in some patients, as already described in conditions following the severe acute respiratory syndrome (SARS) outbreak in 2003 [10], the Middle East respiratory syndrome (MERS) in 2012 [11], and other viruses such as the Epstein–Barr virus [12], or bacteria such as Coxiella burnetii [12]. To date, few studies have assessed the prevalence of symptoms at 12 months after SARS‐CoV‐2 infection and most were limited to post‐hospitalized settings only [13, 14, 15]. The long‐term prevalence of symptoms in outpatient settings has been described at several weeks to months post infection [5, 6, 16] but the information is still missing on the chronicity and prolonged evolution of these symptoms, as well as the burden of disease.
Additionally, only a few studies using patient records have compared post SARS‐CoV‐2 symptoms to the general population of adults tested negative for the infection [17, 18, 19], potentially shedding light on the direct versus indirect effects of the virus due to lockdown and pandemic‐related measures. A recent study assessing PASC among seropositive versus seronegative healthcare workers showed a higher prevalence of symptoms lasting at least 2 months in the seropositive group, with disruption of their work, social, and home life [20]. Determining the consequences due to the virus in addition to those due to postponed healthcare [21], socioeconomic disruption [22], or the mental health burden of the pandemic [23] is extremely important. This has led many in the scientific community to call on studies comparing sequelae post SARS‐CoV‐2 infection with control groups, beyond information from patient records only [9]. Control groups have been difficult to establish as the available testing (reverse transcriptase polymerase chain reaction [RT‐PCR] or antigenic testing in the acute phase, serology in the later phases) could lead to false negatives owing in part to the time of testing, access to test, and immune response [24].
In this study, we describe the prevalence of symptoms, functional capacity, productivity, and quality of life at 12 months in individuals tested positive for SARS‐CoV‐2 compared to individuals tested negative during the same time period (defined as the control group). The study hypothesis postulates that SARS‐CoV‐2 positive individuals suffer more from persistent symptoms leading to functional impairment on top of the pandemic effects when compared to SARS‐CoV‐2 negative individuals. We also assess the association between SARS‐CoV‐2 infection and the long‐term persistence of symptoms.
Methods
From 23 April to 27 July 2021, an online questionnaire was sent to all adults, 12 months after being tested for SARS‐CoV‐2 infection (RT‐PCR test between 15 March 2020 and 30 July 2020) at the outpatient testing center of the Geneva University Hospitals (Switzerland). Overall, 3515 adults had a valid mobile phone number or email address, and were contacted for follow‐up. Individuals tested positive for SARS‐CoV‐2 were considered as the group of interest for SARS‐CoV‐2 infection and those tested negative for SARS‐CoV‐2 were considered as the control group. All individuals gave consent and the study was approved by the Cantonal Research Ethics Commission of Geneva, Switzerland (protocol number 2021‐00389). Only individuals with a laboratory‐confirmed test date were included in this study.
The questionnaire was distributed via REDCap, and a personal link was sent to each participant via email or SMS in case an email address was not available. The follow‐up questionnaire included questions about baseline characteristics, comorbidities, self‐rated health, symptoms at the time of testing, evolution of symptoms since testing, current symptoms over the past 2 weeks, symptom intensity and frequency when present, treatment, number of contacts with the healthcare system (hospitalizations and visits to the primary care physician or other specialists), functional capacity, productivity, and quality of life.
Age categories were defined as “below 40,” “40–59 years,” and “60 years and above” on the basis of previous studies suggesting that middle age may be a predictor of persistent symptoms [16]. Symptoms at testing were categorized as asymptomatic (self‐reported “no symptoms”), paucisymptomatic (self‐reported “very few symptoms” at testing), or symptomatic (self‐reported “had several symptoms” at testing). Symptoms’ intensity in the 2 weeks preceding the questionnaire was assessed using validated scales when possible—the Chalder fatigue scale [25] and the Eastern Cooperative Oncology Group (ECOG) performance scale [26] for fatigue, the modified Medical Research Council scale for dyspnea [27], the Insomnia Severity Index for sleeping disorders [28], the Hospital Anxiety and Depression (HAD) scale for psychiatric conditions [29], and a Likert scale with self‐reported options of mild, moderate, or severe for all reported symptoms. The bimodal scoring of the Chalder fatigue scale [25] was used and scores were rated as 0–3 (no fatigue) and 4 or more (fatigue present). Symptoms’ frequency in the 2 weeks preceding the questionnaire was assessed using a Likert scale with self‐reported options of never, rarely, often, or always. Quality of life was assessed using the 12‐item Short Form survey (SF‐12) questionnaire [30], and physical component score (PCS) and mental component score (MCS) were calculated based on the answers; these scores generally have means of 50 and standard deviations (SDs) of 10 in the general US population [30]. A score of 50 or less on the PCS can be used to indicate a physical condition, and a score of 42 or less on the MCS can be used to indicate potential clinical depression [31, 32]. Functional capacity was assessed using the Sheehan Disability Scale [33], assessing functional impairment in three domains—professional, social, and family life—using a 10‐point visual scale with 0 (no impairment at all), 1–3 (mild impairment), 4–6 (moderate impairment), 7–9 (marked impairment), and 10 (extreme impairment), as well as numerical values for days lost and days with reduced productivity due to functional impairment [33] in the week preceding the survey. Self‐rated health was assessed using the first question of the SF‐12 questionnaire [30] consisting of a Likert scale of excellent, very good, good, fair, and poor; answers were later categorized into two categories: 0 (poor to fair) and 1 (good to excellent). See Supporting Information for the complete survey instrument.
Data were collected using REDCap v11.0.3 and analyzed using the statistical software Stata, version 16.0 (StataCorp). Descriptive analyses included percentages with comparisons using chi‐square tests or Fisher's exact test when appropriate. A p‐value <0.05 was considered significant. Prevalence estimates of each symptom were adjusted for the time from infection, age, sex, education, profession, working in healthcare setting, smoking, physical activity, COVID‐19 vaccination status, symptoms at presentation, hospitalization, and the following comorbidities when present prior to testing: overweight or obese, hypertension, respiratory disease, cardiovascular disease, diabetes, immunosuppression, hypothyroidism, anemia, migraine, tension headache, sleeping disorder, anxiety, depression, any psychiatric condition, irritable bowel syndrome, chronic pain syndrome, and chronic fatigue.
Logistic regression models were used to evaluate associations between SARS‐CoV‐2 infection and persistent symptoms at 12 months, as well as SARS‐CoV‐2 infection and functional impairment at 12 months. Only participants reporting onset of new symptoms at testing were included in the regression analysis, in order to ensure that symptoms were new in nature. The outcome of persistence of symptoms at 12 months was defined as having at least one symptom in the past 2 weeks preceding the questionnaire. The outcome of functional impairment was defined as having mild, moderate, or severe functional impairment using the Sheehan Disability Scale [33]. Multivariable regression models were used to calculate adjusted odds ratios (aORs) with a 95% confidence interval. aORs were adjusted for time from infection, age, sex, education, profession, working in healthcare setting, smoking, physical activity, COVID‐19 vaccination status, symptoms at presentation, hospitalization, and the following comorbidities when present prior to testing: overweight or obese, hypertension, respiratory disease, cardiovascular disease, diabetes, immunosuppression, hypothyroidism, anemia, migraine, tension headache, sleeping disorder, anxiety, depression, any psychiatric condition, irritable bowel syndrome, chronic pain syndrome, and chronic fatigue. Additional analyses were conducted to determine the association between SARS‐CoV‐2 infection and the persistence of symptoms or functional impairment at 12 months across subgroups of sex, age, and individuals without any past medical or psychiatric history. The subgroup analysis for individuals without any past medical or psychiatric history aimed to mitigate any potential bias from underlying conditions that could lead to similar persistent symptoms independent of SARS‐CoV‐2 infection.
Results
Overall, 3515 persons received the questionnaire—n = 703 (20%) with a positive test result and 2812 (80%) with a negative test result. Of those invited to participate, 1639 persons answered the questionnaire (46.6% response rate), with a mean age of 45.4 years (SD, 14.6), 60.8% were women, and 29.2% had a positive SARS‐CoV‐2 test, compared to nonresponders, who had a mean age of 47.8 years (SD, 15.9) (p < 0.001) and of whom 56.2% were women (p < 0.001). Of the 1639 persons answering the questionnaire, 192 reported a positive test between the initial test and the follow‐up and were excluded because of lack of a laboratory‐confirmed test date and result. Ultimately, 1447 persons were included in the analysis (Fig. 1).
Fig. 1.
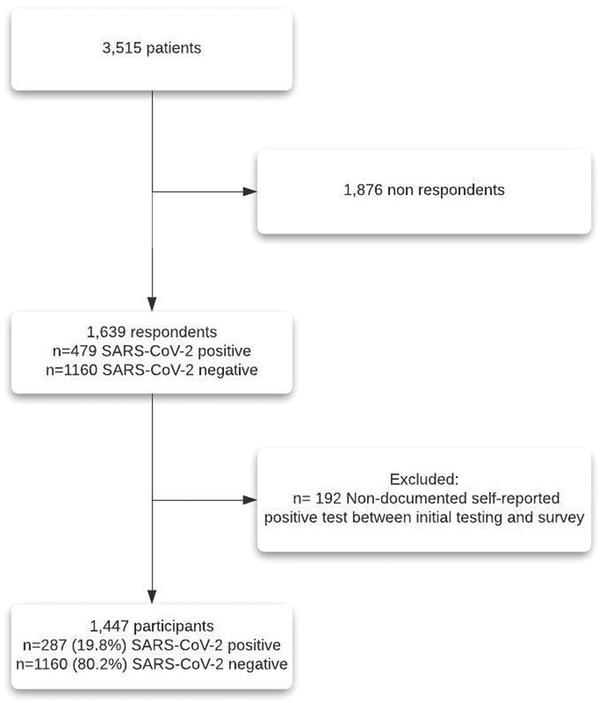
Flowchart.
The mean age of the included participants was 45.2 years (SD, 14.5) and 61.2% were women. When considering baseline characteristics (Table 1), 50.5% of participants had never smoked and 41.8% did not have any comorbidities prior to the infection. Of the 1447 participants, n = 287 (19.8%) had a laboratory‐confirmed SARS‐CoV‐2 infection and n = 1160 (80.2%) had a negative RT‐PCR test, n = 194 (13.4%) self‐reported hospitalization since their test date, with n = 33 (11.5%) reporting their hospitalization was due to SARS‐CoV‐2 infection. The median time from testing to survey was 367 days (interquartile range [IQR], 349–378) overall, 388 days (IQR, 372–399) for individuals who tested positive, and 364 days (IQR, 346–374) for individuals who tested negative.
Table 1.
Overall characteristics of participants tested positive and negative for SARS‐CoV‐2
| SARS‐CoV‐2 | SARS‐CoV‐2 | |||
|---|---|---|---|---|
| Total | Positive | Negative | ||
| (n = 1447) | (n = 287) | (n = 1160) | p‐Value | |
| Age ± SD (years) | 45.2 ± 14.5 | 44.2 ± 13.2 | 45.5 ± 14.8 | 0.179 |
| Characteristic | N (%) | N (%) | N (%) | |
| Age categories | 0.001 | |||
| Below 40 | 599 (41.4) | 115 (40.1) | 484 (41.7) | |
| 40–59 years | 589 (40.7) | 139 (48.4) | 450 (38.8) | |
| 60 years and above | 259 (17.9) | 33 (11.5) | 226 (19.5) | |
| Sex | 0.160 | |||
| Male | 561 (38.8) | 101 (35.2) | 460 (39.7) | |
| Female | 886 (61.2) | 186 (64.8) | 700 (60.3) | |
| Employment status | 0.009 | |||
| Salaried | 997 (68.9) | 220 (76.7) | 777 (67.0) | |
| Retired | 131 (9.0) | 12 (4.2) | 119 (10.3) | |
| Student | 118 (8.1) | 20 (7.0) | 98 (8.5) | |
| Independent | 76 (5.3) | 13 (4.5) | 63 (5.4) | |
| Looking after home/family | 39 (2.7) | 6 (2.1) | 33 (2.8) | |
| Unemployed | 39 (2.7) | 6 (2.1) | 33 (2.8) | |
| Disability | 27 (1.9) | 3 (1.0) | 24 (2.1) | |
| Other | 20 (1.4) | 7 (2.4) | 13 (1.1) | |
| Education | 0.017 | |||
| Primary | 72 (5.0) | 21 (7.3) | 51 (4.4) | |
| Apprenticeship | 165 (11.4) | 23 (8.0) | 142 (12.2) | |
| Secondary | 202 (14.0) | 46 (16.0) | 156 (13.5) | |
| Tertiary | 902 (62.3) | 170 (59.2) | 732 (63.1) | |
| Other | 75 (5.2) | 22 (7.7) | 53 (4.6) | |
| Prefer not to answer | 31 (2.1) | 5 (1.7) | 26 (2.2) | |
| Profession | <0.001 | |||
| Never worked | 91 (6.3) | 21 (7.3) | 70 (6.0) | |
| Unskilled workers | 94 (6.5) | 10 (3.5) | 84 (7.2) | |
| Skilled workers | 256 (17.7) | 39 (13.6) | 217 (18.7) | |
| Highly skilled workers | 338 (23.4) | 103 (35.9) | 235 (20.3) | |
| Professional—managers | 415 (28.7) | 59 (20.6) | 356 (30.7) | |
| Other | 226 (15.6) | 52 (18.1) | 174 (15.0) | |
| Prefer not to answer | 27 (1.9) | 3 (1.0) | 24 (2.1) | |
| Smoking status | <0.001 | |||
| Never smoked | 731 (50.5) | 162 (56.4) | 569 (49.0) | |
| Current smoker | 296 (20.5) | 32 (11.1) | 264 (22.8) | |
| Previous smoker, stopped independent of infection | 378 (26.1) | 78 (27.2) | 300 (25.9) | |
| Previous smoker, stopped due to infection | 2 (0.1) | 2 (0.7) | 0 (0.0) | |
| Prefer not to answer | 40 (2.8) | 13 (4.5) | 27 (2.3) | |
| Physical activity | 0.785 | |||
| None | 236 (16.3) | 46 (16) | 190 (16.4) | |
| Partial | 710 (49.1) | 141 (49.1) | 569 (49.1) | |
| Regular | 489 (33.8) | 99 (34.5) | 390 (33.6) | |
| Prefer not to answer | 12 (0.8) | 1 (0.3) | 11 (0.9) | |
| Hospitalization since test | 194 (13.4) | 33 (11.5) | 161 (13.9) | 0.262 |
| Vaccination | <0.001 | |||
| No | 458 (31.6) | 145 (50.5) | 313 (27.0) | |
| 2 doses | 714 (49.4) | 84 (29.3) | 630 (54.3) | |
| 1 dose | 262 (18.1) | 54 (18.8) | 208 (17.9) | |
| Prefer not to answer | 13 (0.9) | 4 (1.4) | 9 (0.8) | |
| Symptoms at presentation | <0.001 | |||
| Asymptomatic | 331 (22.9) | 22 (7.7) | 309 (26.7) | |
| Paucisymptomatic | 691 (47.7) | 221 (77.0) | 470 (40.5) | |
| Had several symptoms | 419 (29.0) | 44 (15.3) | 375 (32.3) | |
| Prefer not to answer | 6 (0.4) | 0 (0.0) | 6 (0.5) | |
| Comorbidities | ||||
| None | 605 (41.8) | 132 (46.0) | 473 (40.8) | 0.109 |
| Overweight | 213 (14.7) | 41 (14.3) | 172 (14.8) | 0.817 |
| Sleep disorders | 178 (12.3) | 31 (10.8) | 147 (12.7) | 0.388 |
| Hypertension | 157 (10.9) | 25 (8.7) | 132 (11.4) | 0.193 |
| Anxiety | 127 (8.8) | 21 (7.3) | 106 (9.1) | 0.329 |
| Migraine | 123 (8.5) | 25 (8.7) | 98 (8.4) | 0.886 |
| Irritable bowel syndrome | 99 (6.8) | 14 (4.9) | 85 (7.3) | 0.141 |
| Chronic fatigue syndrome | 91 (6.3) | 20 (7.0) | 71 (6.1) | 0.596 |
| Depression | 89 (6.2) | 16 (5.6) | 73 (6.3) | 0.650 |
| Respiratory disease | 88 (6.1) | 15 (5.2) | 73 (6.3) | 0.498 |
| Tension headache | 62 (4.3) | 10 (3.5) | 52 (4.5) | 0.455 |
| Anemia | 61 (4.2) | 11 (3.8) | 50 (4.3) | 0.718 |
| Memory disorders | 58 (4.0) | 19 (6.6) | 39 (3.4) | 0.012 |
| Tendinitis | 57 (3.9) | 9 (3.1) | 48 (4.1) | 0.435 |
| Obesity | 55 (3.8) | 15 (5.2) | 40 (3.4) | 0.158 |
| Cardiovascular disease | 54 (3.7) | 10 (3.5) | 44 (3.8) | 0.805 |
| Attention disorders | 44 (3.0) | 12 (4.2) | 32 (2.8) | 0.209 |
| Diabetes | 43 (3.0) | 9 (3.1) | 34 (2.9) | 0.855 |
| Hypothyroidism | 43 (3.0) | 7 (2.4) | 36 (3.1) | 0.553 |
| Immunosuppression | 29 (2.0) | 1 (0.3) | 28 (2.4) | 0.025 |
| Cancer | 20 (1.4) | 3 (1.0) | 17 (1.5) | 0.585 |
| Chronic pain syndrome | 20 (1.4) | 4 (1.4) | 16 (1.4) | 0.985 |
| Deep vein thrombosis | 19 (1.3) | 3 (1.0) | 16 (1.4) | 0.656 |
| Hyperthyroidism | 15 (1.0) | 2 (0.7) | 13 (1.1) | 0.526 |
| Rheumatoid arthritis | 11 (0.8) | 3 (1.0) | 8 (0.7) | 0.535 |
| Multiple sclerosis | 10 (0.7) | 0 (0.0) | 10 (0.9) | 0.114 |
| Renal disease | 9 (0.6) | 0 (0.0) | 9 (0.8) | 0.134 |
| Fibromyalgia | 9 (0.6) | 1 (0.3) | 8 (0.7) | 0.510 |
| Dysmenorrhea | 8 (0.6) | 1 (0.3) | 7 (0.6) | 0.602 |
| Reactive arthritis | 8 (0.6) | 2 (0.7) | 6 (0.5) | 0.713 |
Note: SARS‐CoV‐2 status was determined as per the result of the reverse transcriptase polymerase chain reaction test in symptomatic outpatient individuals.
Abbreviation: SD, standard deviation.
At 12 months, an estimated 33.4% of individuals post SARS‐CoV‐2 infection still had at least one symptom compared to 6.5% in the control group (p < 0.001). Significant differences according to SARS‐CoV‐2 infection were seen in the prevalence of fatigue and several neurologic, psychiatric, upper respiratory, and cardiac symptoms (Table 2). The number of symptoms at 12 months was increased following a documented SARS‐CoV‐2 infection with 7.8% of individuals with SARS‐CoV‐2 infection reporting 5–10 symptoms at 12 months compared to 1.1% of individuals in the control group (p < 0.001). Table 2 shows the adjusted prevalence of each symptom at 12 months following testing.
Table 2.
Prevalence and number of symptoms in individuals who tested positive and individuals who tested negative for SARS‐CoV‐2 at 12 months a , b
| SARS‐CoV‐2 positive | SARS‐CoV‐2 negative | ||
|---|---|---|---|
| (n = 287) | (n = 1160) | ||
| Symptom | % (95% CI) | % (95% CI) | p‐Value |
| Any symptom | 33.4 (31.3–35.5) | 6.5 (6.1–6.8) | <0.001 |
| Fatigue | 16.0 (14.4–17.5) | 3.1 (2.7–3.3) | <0.001 |
| Loss or change in smell | 10.0 (8.6–11.5) | 1.7 (1.6–1.9) | <0.001 |
| Loss or change in taste | 10.3 (7.8–12.8) | 1.5 (1.2–1.9) | <0.001 |
| Dyspnea | 8.9 (6.6–11.2) | 1.1 (0.9–1.3) | <0.001 |
| Headache | 9.8 (8.4–11.2) | 1.7 (1.5–2.0) | <0.001 |
| Insomnia | 8.9 (5.4–12.3) | 2.7 (1.6–3.9) | <0.001 |
| Difficulty concentrating/loss of memory | 7.4 (5.8–9.1) | 2.5 (2.0–3.0) | <0.001 |
| Mental exhaustion | 6.9 (5.0–8.8) | 1.4 (1.1–1.7) | <0.001 |
| Paresthesia | 5.4 (3.1–7.8) | 1.9 (1.5–2.3) | <0.001 |
| Dizziness/lack of equilibrium | 6.4 (4.4–8.5) | 1.8 (1.0–2.5) | <0.001 |
| Cough | 6.5 (3.4–9.7) | 3.2 (1.8–4.6) | 0.033 |
| Chest pain | 6.0 (2.9–9.2) | 4.0 (2.3–5.7) | 0.214 |
| Palpitations | 3.8 (1.6–6.1) | 1.6 (0.6–2.5) | 0.032 |
| Myalgia | 7.3 (6.2–8.4) | 1.7 (1.5–1.9) | <0.001 |
| Arthralgia | 2.9 (2.0–3.9) | 2.0 (1.7–2.4) | 0.050 |
| Digestive symptoms (nausea, vomiting, diarrhea, abdominal pain) | 4.0 (3.0–4.9) | 1.6 (1.2–2.0) | <0.001 |
| Number of symptoms | |||
| None | 67.8 (65.8–69.7) | 93.6 (93.2–93.9) | <0.001 |
| 1 symptom | 10.6 (10.1–11.1) | 2.7 (2.6–2.9) | <0.001 |
| 2 symptoms | 4.8 (4.5–5.0) | 1.0 (0.9–1.1) | <0.001 |
| 3 symptoms | 5.0 (4.7–5.3) | 0.9 (0.8–1.0) | <0.001 |
| 4 symptoms | 2.8 (2.6–3.0) | 0.5 (0.4–0.5) | <0.001 |
| 5–10 symptoms | 7.8 (7.2–8.5) | 1.1 (1.1–1.2) | <0.001 |
| ≥11 symptoms | 1.1 (1.0–1.2) | 0.1 (0.1–0.1) | <0.001 |
Abbreviation: CI, confidence interval.
“Any symptom” was defined as the presence of any one symptom of those listed in the survey instrument.
Estimates of prevalence were adjusted for time from infection, age, sex, education, profession, working in healthcare setting, smoking, physical activity, COVID‐19 vaccination, symptoms at presentation, hospitalization, and the following comorbidities present prior to testing: overweight or obese, hypertension, respiratory disease, cardiovascular disease, diabetes, immunosuppression, hypothyroidism, anemia, migraine, tension headache, sleeping disorder, anxiety, depression, any psychiatric condition, irritable bowel syndrome, chronic pain syndrome, and chronic fatigue.
Functional impairment was more important in individuals who tested positive compared to those tested negative for SARS‐CoV‐2, and so were the numbers of days lost and days with reduced productivity in the week preceding the follow‐up (Table 3. After adjustment, an estimated 30.5% of individuals who tested positive reported functional impairment at 12 months from the infection versus 6.6% of individuals who tested negative (p < 0.001). In the week preceding the questionnaire, participants reported to have experienced one or more days of reduced productivity in the week preceding the questionnaire in 11.8% of cases compared to 3.9% in the control group (p < 0.001).
Table 3.
Functional impairment of individuals who tested positive compared to individuals who tested negative for SARS‐CoV‐2 at 12 months using the Sheehan Disability Scale a [33]
| SARS‐CoV‐2 | SARS‐CoV‐2 | |||
|---|---|---|---|---|
| Total | Positive | Negative | ||
| (n = 1447) | (n = 287) | (n = 1160) | ||
| % (95% CI) | % (95% CI) | % (95% CI) | p‐Value | |
| Functional impairment | ||||
| No impairment | 88.8 (88.1–89.5) | 69.5 (67.5–71.7) | 93.4 (93.1–93.8) | <0.001 |
| Mild impairment | 5.9 (5.6–6.2) | 14.5 (13.7–15.3) | 3.8 (3.6–4.0) | <0.001 |
| Moderate impairment | 4.2 (3.9–4.5) | 12.2 (11.2–13.2) | 2.2 (2.1–2.4) | <0.001 |
| Severe impairment | 1.1 (1.0–1.2) | 3.6 (3.2–4.0) | 0.5 (0.4–0.6) | <0.001 |
| ≥1 day lost in the past week | 2.8 (2.2–3.3) | 4.8 (3.4–6.2) | 2.3 (1.7–2.8) | 0.002 |
| ≥1 day of reduced productivity in the past week | 5.5 (5.0–6.1) | 11.8 (10.1–13.5) | 3.9 (3.4–4.4) | <0.001 |
Abbreviation: CI, confidence interval.
Estimates were adjusted for time from infection, age, sex, education, profession, working in healthcare setting, smoking, physical activity, COVID‐19 vaccination status, symptoms at presentation, hospitalization, and the following comorbidities present prior to testing: overweight or obese, hypertension, respiratory disease, cardiovascular disease, diabetes, immunosuppression, hypothyroidism, anemia, migraine, tension headache, sleeping disorder, anxiety, depression, any psychiatric condition, irritable bowel syndrome, chronic pain syndrome, and chronic fatigue.
SARS‐CoV‐2 infection was positively associated with the persistence of symptoms at 12 months (aOR 4.21; 2.60–6.83). The association was significant in the subgroups of women, men, individuals younger than 40 years, and those between 40–59 years of age. The association was also significant for individuals without any past medical or psychiatric conditions (Fig. 2). SARS‐CoV‐2 infection was positively associated with functional impairment at 12 months (aOR 3.54; 2.16–5.80). The association was significant in the subgroups of women, men, in individuals younger than 40 years, and in those between 40–59 years of age. The association was also significant for individuals without any past medical or psychiatric conditions (Fig. 2).
Fig. 2.
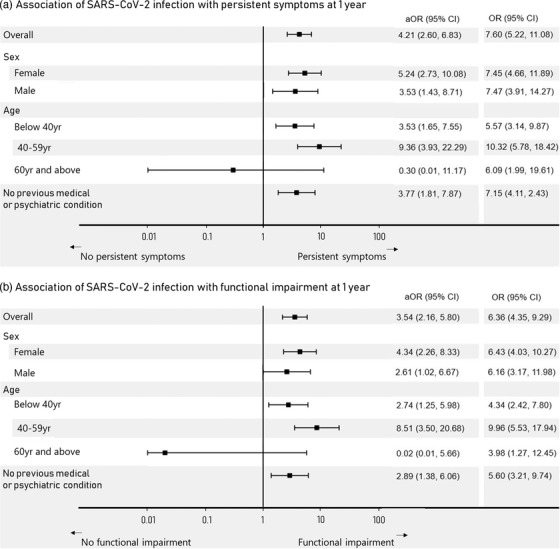
Associations between laboratory‐confirmed SARS‐CoV‐2 infection and the persistence of symptoms and functional impairment at 1 year stratified by sex, age categories, and pre‐existing medical conditions (n = 1245). The persistence of symptoms was defined as the presence of any one symptom within the 2 weeks before the questionnaire. Functional impairment was assessed using the Sheehan Disability Scale [33]. Odds ratios were adjusted for time from infection, age, sex, education, profession, working in healthcare setting, smoking, physical activity, COVID‐19 vaccination status, symptoms at presentation, hospitalization, and the following comorbidities present prior to testing: overweight or obese, hypertension, respiratory disease, cardiovascular disease, diabetes, immunosuppression, hypothyroidism, anemia, migraine, tension headache, sleeping disorder, anxiety, depression, any psychiatric condition, irritable bowel syndrome, chronic pain syndrome, and chronic fatigue. aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
Self‐rated health was reported as very good to excellent in 47.4% of individuals at 12 months post SARS‐CoV‐2 infection, as good in 38.8% of individuals, and as fair to poor in 13.8% of individuals (Table 4). The mean weighted physical health score using the SF‐12 questionnaire was 49.7 (SD, 7.9) for individuals post SARS‐CoV‐2 infection compared to 50.9 (SD, 7.8) for the control group (p = 0.017). The mean weighted mental health score using the SF‐12 questionnaire was 41.8 (SD, 5.5) for individuals post SARS‐CoV‐2 infection compared to 41.0 (SD, 5.9) for the control group (p = 0.041). Additionally, the mean HAD anxiety score was 5.5 (SD, 3.9) for individuals post SARS‐CoV‐2 infection compared to 6.2 (SD, 3.9) for the control group (p = 0.008). The mean HAD depression score was 3.4 (SD, 3.6) for individuals post SARS‐CoV‐2 infection versus 3.7 (SD, 3.5) for the control group (p = 0.189). Further details are presented in Table 4.
Table 4.
Quality of life and physical and mental health of individuals who tested positive compared to individuals who tested negative for SARS‐CoV‐2 at 12 months a , b
| SARS‐CoV‐2 | SARS‐CoV‐2 | |||
|---|---|---|---|---|
| Total | Positive | Negative | ||
| (n = 1447) | (n = 287) | (n = 1160) | ||
| Quality of life—SF12 | % (95% CI) | % (95% CI) | % (95% CI) | p‐Value |
| Current self‐rated health | ||||
| Excellent | 17.8 (17.0–18.6) | 14.4 (12.9–15.8) | 18.6 (17.7–19.5) | <0.001 |
| Very good | 34.8 (34.1–35.4) | 33.0 (31.3–34.6) | 35.2 (34.5–35.9) | 0.007 |
| Good | 36.0 (35.2–36.8) | 38.8 (37.2–40.5) | 35.4 (34.5–36.3) | <0.001 |
| Fair | 10.4 (9.8–11.0) | 12.7 (11.1–14.2) | 9.9 (9.2–10.5) | <0.001 |
| Poor | 0.9 (0.8–1.1) | 1.1 (0.9–1.4) | 0.9 (0.7–1.0) | 0.096 |
| SF‐12 scores | ||||
| SF‐12 physical component score (mean ± SD) | 50.7 ± 7.8 | 49.7 ± 7.9 | 50.9 ± 7.8 | 0.017 |
| SF‐12 mental component score (mean ± SD) | 41.2 ± 5.9 | 41.8 ± 5.4 | 41.0 ± 5.9 | 0.041 |
| HAD—Anxiety and Depression | % (95% CI) | % (95% CI) | % (95% CI) | |
| HAD Anxiety score ≥8 | 29.0 (27.9–30.0) | 21.9 (19.7–24.1) | 30.7 (29.5–31.9) | <0.001 |
| HAD Depression score ≥8 | 12.8 (12.1–13.5) | 9.9 (8.6–11.3) | 13.4 (12.6–14.3) | 0.001 |
| HAD Anxiety score ≥11 | 11.3 (10.6–12.0) | 8.3 (6.9–9.6) | 12.0 (11.2–12.9) | <0.001 |
| HAD Depression score ≥11 | 4.2 (3.7–4.8) | 4.6 (3.4–5.7) | 4.2 (3.6–4.7) | 0.561 |
| HAD scores | ||||
| HAD Anxiety score (mean ± SD) | 6.1 ± 3.9 | 5.5 ± 3.9 | 6.2 ± 3.9 | 0.008 |
| HAD Depression score (mean ± SD) | 3.7 ± 3.5 | 3.4 ± 3.6 | 3.7 ± 3.5 | 0.189 |
Note: A score of more than 8 on each of the anxiety or depression components of HAD 29 indicates a positive score.
Abbreviations: CI, confidence interval; HAD, Hospital Anxiety and Depression scale; SD, standard deviation; SF‐12, Short Form 12 item.
In SF‐12, a physical component score of 50 or less indicates a physical condition, while a mental component score of 42 may be indicative of clinical depression. 30
Estimates were adjusted for time from infection, age, sex, education, profession, working in healthcare setting, smoking, physical activity, COVID‐19 vaccination status, symptoms at presentation, hospitalization, and the following comorbidities present prior to testing: overweight or obese, hypertension, respiratory disease, cardiovascular disease, diabetes, immunosuppression, hypothyroidism, anemia, migraine, tension headache, sleeping disorder, anxiety, depression, any psychiatric condition, irritable bowel syndrome, chronic pain syndrome, and chronic fatigue.
Discussion
At 12 months, persistent symptoms were more prevalent in individuals who tested positive compared to individuals who tested negative for SARS‐CoV‐2 after adjusting for sociodemographic variables and comorbidities. The increased prevalence is significant for most symptoms with the largest differential seen for fatigue, headache, dyspnea, insomnia, myalgia, difficulty concentrating, and loss/change in taste or smell. SARS‐CoV‐2 infection led to significantly more functional impairment when compared to the control group, as well as up to three times less productivity at 12 months from testing. Increased functional impairment has been suggested in seropositive healthcare workers [20] and in smaller studies including post‐hospitalized patients [34]. Our results show functional impairment in outpatient individuals post SARS‐CoV‐2 infection. The overall PCS on the SF‐12 scale was lower in the SARS‐CoV‐2 positive group compared to the SARS‐CoV‐2 negative group, indicating potentially poorer physical health. The overall MCS was low in both groups, indicating an overall impact on quality of life. The mental score component and the anxiety and depression scale scores were lower in the group of individuals without SARS‐CoV‐2 infection compared to individuals post SARS‐CoV‐2 infection, possibly due to underlying baseline characteristics where the control group reported more anxiety and depression than the SARS‐CoV‐2 group [35]. One hypothesis is that people with more anxiety or depression might have opted to stay home or be less socially active during the pandemic, thus decreasing their risk of SARS‐CoV‐2 infection [36, 37].
The prevalence of persistent symptoms is in line with several recent studies and compares to infections like SARS in 2003 [10] and MERS [11], where symptoms lingered and caused functional impairment for years in a significant proportion of individuals. SARS‐CoV‐2 may, however, cause a higher clinical burden of disease than other respiratory viruses, as suggested in recent studies comparing long‐term sequelae post hospitalization for SARS‐CoV‐2 versus influenza [17, 38, 39]. As influenza is also known to cause potential declines in functional capacity, especially in hospitalized patients [40], a comparison of the functional impairment following SARS‐CoV‐2 infection versus influenza would bring further insight into the overall burden of disease. Mechanisms leading to long‐term symptoms of SARS‐CoV‐2 are still being studied, as well as a potential treatment and preventive approaches [41]. In a recent study, vaccination was associated with a decreased prevalence of symptoms at 28 days post infection [42], potentially due to the fact that vaccinated individuals experienced less symptomatic infections [42].
The importance of this study is showing the differential impact of SARS‐CoV‐2 infection versus other potential upper respiratory infections in symptomatic outpatient individuals, and in addition to the potential general pandemic toll. Additionally, our study shows that symptoms can persist at least 12 months after the infection (alpha strain of SARS‐CoV‐2) and are associated with more functional impairment and loss of productivity when compared to other common upper respiratory infections or pandemic‐related effects in general. Individuals suffering from PASC should be cared for long term, with a multidisciplinary approach addressing the range of their symptoms and any potential impact on their productivity and activities of daily living. These findings also underline the importance of continuing to follow public health recommendations that aim to reduce infection, regardless of age, sex, or underlying conditions in order to avoid persistent symptoms.
Limitations in this study include the nature of the follow‐up with self‐reported items as well as the relatively low response rate, which, although similar to other general survey questionnaires [43], may result in ascertainment bias. Additionally, several of the assessed symptoms, such as fatigue, loss of taste or smell, and others, are subjective and self‐reported in nature in clinical practice as well as in research settings. The time from infection was shorter for participants who tested positive compared to those who tested negative, potentially leading to bias, although with a difference of only 20 days. Time from infection was controlled for in all analyses. The age group of 60 years and older included a smaller number of participants and a low number of SARS‐CoV‐2 positive individuals, thus potentially leading to a lack of power in the multivariable regression models and loss of associations. Additionally, we considered a negative RT‐PCR test as negative for a SARS‐CoV‐2 infection. While it is possible for individuals with a negative RT‐PCR test to still have SARS‐CoV‐2 infection [44], the decision to include only laboratory‐confirmed infection, potentially biasing results towards the null hypothesis, was made to objectively assess the duration of symptoms as narrowly as possible as well as the impact of SARS‐CoV‐2 infection versus other potential factors. Finally, more women participated in the study, and participants were highly educated, skilled workers, raising the issue of generalizability of the results to the general population. Of note, profession and education were controlled for in all analyses, and all participants were tested at the same testing center and had similar access to care, thus mitigating this factor as a potential confounder leading to symptoms’ persistence.
To conclude, SARS‐CoV‐2 infection causes persistent symptoms at 12 months from diagnosis in addition to the COVID‐19 pandemic effects, and more than other potential upper respiratory infections. As the COVID‐19 landscape continues to evolve with new treatment options, vaccination, and new variants, physicians should continue monitoring their patients and encouraging them to avoid infection and re‐infection regardless of age, sex, and underlying conditions in order to reduce the risk of PASC.
Conflict of interest
The authors declare no potential conflict of interest. Furthermore, we confirm that the manuscript has been read and approved by all the named authors, and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that this work was conducted with the ethical approval of the Cantonal Research Ethics Commission of Geneva, Switzerland, and that the approvals are acknowledged in the manuscript.
Author contributions
Mayssam Nehme: Conceptualization; formal analysis; investigation; methodology; writing – original draft; writing – review and editing. Olivia Braillard: Conceptualization; data curation; writing – review and editing. François Chappuis: Conceptualization; writing – original draft; writing – review and editing. Laurent Kaiser: Data curation; writing – review and editing. Idris Guessous: Conceptualization; data curation; funding acquisition; methodology; project administration; resources; supervision; writing – original draft; writing – review and editin. All authors of the CoviCare Study team contributed to the study design and reviewing the manuscript.
Funding
This study is funded by the Leenaards Foundation, the Geneva University Hospitals Private Foundation, and the Private Research Funds of the Division of Primary Care Medicine at the Geneva University Hospitals. The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Supporting information
Supplement 1. Survey instrument
Acknowledgments
Open access funding provided by Universite de Geneve.
[Correction added on 11 May 2022, after first online publication: CSAL funding statement has been added.]
Nehme M, Braillard O, Chappuis F, Courvoisier DS, Kaiser L, Guessous I, et al. One‐year persistent symptoms and functional impairment in SARS‐CoV‐2 positive and negative individuals. J Intern Med. 2022;292:103–115.
Data availability statement
Individual study data that underlie the results reported in this article can be made available to the scientific community after de‐identification and upon submission of a data request application to the investigator board via the corresponding author.
References
- 1. National Institutes of Health (NIH) . When COVID‐19 symptoms linger: new NIH initiative seeks to understand why some people continue to have symptoms long after recovery. https://covid19.nih.gov/news‐and‐stories/when‐COVID‐19‐symptoms‐linger (2021). Accessed 22 Aug 2021.
- 2. National Institute for Health and Care Excellence . NICE, SIGN and RCGP set out further details about the UK guideline on management of the long‐term effects of COVID‐19. https://www.nice.org.uk/news/article/nice‐sign‐and‐rcgp‐set‐out‐further‐details‐about‐the‐uk‐guideline‐on‐management‐of‐the‐long‐term‐effects‐of‐covid‐19 (2020). Accessed 22 Aug 2021.
- 3. World Health Organization . A clinical case definition of post COVID‐19 condition by a Delphi consensus. https://apps.who.int/iris/bitstream/handle/10665/345824/WHO‐2019‐nCoV‐Post‐COVID‐19‐condition‐Clinical‐case‐definition‐2021.1‐eng.pdf (2021). Accessed 10 Oct 2021.
- 4. Nehme M, Braillard O, Alcoba G, Aebischer Perone S, Courvoisier D, Chappuis F, et al. COVID‐19 symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med. 2020;174:723–5. 10.7326/M20-5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logue JK, Franko NM, McCulloch DJ, McDonald D, Magedson A, Wolf CR, et al. Sequelae in adults at 6 months after COVID‐19 infection. JAMA Netw Open. 2021;4(2):e210830. 10.1001/jamanetworkopen.2021.0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nehme M, Braillard O, Chappuis F, Courvoisier DS, Guessous I. Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID‐19 in an outpatient setting. Ann Intern Med. 2021;174:1252–60. 10.7326/M21-0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Office of National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID‐19) infection in the UK: 1 April 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1april2021 (2021). Accessed 22 Aug 2021.
- 8. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32. 10.1016/S0140-6736(20)32656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Auwaerter PG. The race to understand post‐COVID‐19 conditions. Ann Intern Med. 2021;174:1458–9. 10.7326/M21-3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tansey CM, Louie M, Loeb M, Gold WL, Muller MP, de Jager J, et al. One‐year outcomes and health care utilization in survivors of severe acute respiratory syndrome. Arch Intern Med. 2007;167(12):1312–20. 10.1001/archinte.167.12.1312 [DOI] [PubMed] [Google Scholar]
- 11. Lee SH, Shin HS, Park HY, Kim JL, Lee JJ, Lee H, et al. Depression as a mediator of chronic fatigue and post‐traumatic stress symptoms in Middle East respiratory syndrome survivors. Psychiatry Investig. 2019;16(1):59–64. 10.30773/pi.2018.10.22.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hickie I, Davenport T, Wakefield D, Vollmer‐Conna U, Cameron B, Vernon SD, et al. Post‐infective and chronic fatigue sy precipitated by viral and non‐viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575. 10.1136/bmj.38933.585764.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3‐month, 6‐month, 9‐month, and 12‐month respiratory outcomes in patients following COVID‐19‐related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–54. 10.1016/S2213-2600(21)00174-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan X, Huang H, Wang C, Jin Z, Zhang Z, He J, et al. Follow‐up study of pulmonary function among COVID‐19 survivors 1 year after recovery. J Infect. 2021;83(3):381–412. 10.1016/j.jinf.2021.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang L, Yao Q, Gu X, Wang Q, Ren L, Wang Y, et al. 1‐year outcomes in hospital survivors with COVID‐19: a longitudinal cohort study. Lancet. 2021;398(10302):747–58. 10.1016/S0140-6736(21)01755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Carvalho‐Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao‐Tournois C, Laribi S, et al. Follow‐up of adults with noncritical COVID‐19 two months after symptom onset. Clin Microbiol Infect. 2021;27(2):258–63. 10.1016/j.cmi.2020.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al‐Aly Z, Xie Y, Bowe B. High‐dimensional characterization of post‐acute sequelae of COVID‐19. Nature. 2021;594(7862):259–64. 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 18. Lund LC, Hallas J, Nielsen H, Koch A, Mogensen SH, Brun NC, et al. Post‐acute effects of SARS‐CoV‐2 infection in individuals not requiring hospital admission: a Danish population‐based cohort study. Lancet Infect Dis. 2021;21:1373–82. 10.1016/S1473-3099(21)00211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chevinsky JR, Tao G, Lavery AM, Kukielka EA, Click ES, Malec D, et al. Late conditions diagnosed 1–4 months following an initial coronavirus disease 2019 (COVID‐19) encounter: a matched‐cohort study using inpatient and outpatient administrative data—United States, 1 March–30 June 2020. Clin Infect Dis. 2021;73(Suppl 1):S5–16. 10.1093/cid/ciab338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Havervall S, Rosell A, Phillipson M, Mangsbo SM, Nilsson P, Hober S, et al. Symptoms and functional impairment assessed 8 months after mild COVID‐19 among health care workers. JAMA. 2021;325(19):2015–6. 10.1001/jama.2021.5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenbaum L. The untold toll—the pandemic's effects on patients without Covid‐19. N Engl J Med. 2020;382(24):2368–71. 10.1056/NEJMms2009984 [DOI] [PubMed] [Google Scholar]
- 22. Saladino V, Algeri D, Auriemma V. The psychological and social impact of Covid‐19: new perspectives of well‐being. Front Psychol. 2020;11:577684. 10.3389/fpsyg.2020.577684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holmes EA, O'Connor RC, Perry VH, Tracey I, Wessely S, Arseneault L, et al. Multidisciplinary research priorities for the COVID‐19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7(6):547–60. 10.1016/S2215-0366(20)30168-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false‐negative rate of reverse transcriptase polymerase chain reaction‐based SARS‐CoV‐2 tests by time since exposure. Ann Intern Med. 2020;173(4):262–7. 10.7326/M20-1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–53. 10.1016/0022-3999(93)90081-p [DOI] [PubMed] [Google Scholar]
- 26. Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A(7):1135–41. 10.1016/0959-8049(95)00664-8 [DOI] [PubMed] [Google Scholar]
- 27. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–6. 10.1378/chest.93.3.580 [DOI] [PubMed] [Google Scholar]
- 28. Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. 10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 29. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 30. Ware J Jr, Kosinski M, Keller SD. A 12‐item short‐form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 31. Silveira E, Taft C, Sundh V, Waern M, Palsson S, Steen B. Performance of the SF‐36 health survey in screening for depressive and anxiety disorders in an elderly female Swedish population. Qual Life Res. 2005;14(5):1263–74. 10.1007/s11136-004-7753-5 [DOI] [PubMed] [Google Scholar]
- 32. Soh SE, Morello R, Ayton D, Ahern S, Scarborough R, Zammit C, et al. Measurement properties of the 12‐item Short Form Health Survey version 2 in Australians with lung cancer: a Rasch analysis. Health Qual Life Outcomes. 2021;19(1):157. 10.1186/s12955-021-01794-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med. 1997;27(2):93–105. 10.2190/T8EM-C8YH-373N-1UWD [DOI] [PubMed] [Google Scholar]
- 34. Halpin SJ, McIvor C, Whyatt G, Adams A, Harvey O, McLean L, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol. 2021;93(2):1013–22. 10.1002/jmv.26368 [DOI] [PubMed] [Google Scholar]
- 35. Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID‐19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID‐19 cases in the USA. Lancet Psychiatry. 2021;8(2):130–40. 10.1016/S2215-0366(20)30462-4 Erratum in: Lancet Psychiatry. 2021 Jan;8(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fan VS, Dominitz JA, Eastment MC, Locke E, Green P, Berry K, et al. Risk factors for testing positive for SARS‐CoV‐2 in a national US healthcare system. Clin Infect Dis. 2021;73:e3085–94. 10.1093/cid/ciaa1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clark C, Davila A, Regis M, Kraus S. Predictors of COVID‐19 voluntary compliance behaviors: an international investigation. Glob Transit. 2020;2:76–82. 10.1016/j.glt.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donnino MW, Moskowitz A, Thompson GS, Heydrick SJ, Pawar RD, Berg KM, et al. Comparison between patients hospitalized with influenza and COVID‐19 at a tertiary care center. J Gen Intern Med. 2021;36(6):1689–95. 10.1007/s11606-021-06647-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert‐Bitter P, et al. Comparison of the characteristics, morbidity, and mortality of COVID‐19 and seasonal influenza: a nationwide, population‐based retrospective cohort study. Lancet Respir Med. 2021;9(3):251–9. 10.1016/S2213-2600(20)30527-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Macias AE, McElhaney JE, Chaves SS, Nealon J, Nunes MC, Samson SI, et al. The disease burden of influenza beyond respiratory illness. Vaccine. 2021;39(Suppl 1):A6–14. 10.1016/j.vaccine.2020.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crook H, Raza S, Nowell J, Young M, Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021;374:n1648. 10.1136/bmj.n1648 Erratum in: BMJ. 2021 Aug 3;374:n1944. [DOI] [PubMed] [Google Scholar]
- 42. Antonelli M, Penfold RS, Merino J, Sudre CH, Molteni E, Berry S, et al. Risk factors and disease profile of post‐vaccination SARS‐CoV‐2 infection in UK users of the COVID Symptom Study app: a prospective, community‐based, nested, case‐control study. Lancet Infect Dis. 2022;22:43–55. 10.1016/S1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Althubaiti A. Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–7. 10.2147/JMDH.S104807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang Z, Bi Q, Fang S, Wei L, Wang X, He J, et al. Insight into the practical performance of RT‐PCR testing for SARS‐CoV‐2 using serological data: a cohort study. Lancet Microbe. 2021;2(2):e79–87. 10.1016/S2666-5247(20)30200-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. Survey instrument
Data Availability Statement
Individual study data that underlie the results reported in this article can be made available to the scientific community after de‐identification and upon submission of a data request application to the investigator board via the corresponding author.


