Abstract
Background
COVID‐19‐associated pulmonary aspergillosis (CAPA) is a major complication of critically ill COVID‐19 patients, with a high mortality rate and potentially preventable. Thus, identifying patients at high risk of CAPA would be of great interest. We intended to develop a clinical prediction score capable of stratifying patients according to the risk for CAPA at ICU admission.
Methods
Single centre retrospective case–control study. A case was defined as a patient diagnosed with CAPA according to 2020 ECMM/ISHAM consensus criteria. 2 controls were selected for each case among critically ill COVID‐19 patients.
Results
28 CAPA patients and 56‐matched controls were included. Factors associated with CAPA included old age (68 years vs. 62, p = .033), active smoking (17.9% vs. 1.8%, p = .014), chronic respiratory diseases (48.1% vs. 26.3%, p = .043), chronic renal failure (25.0% vs. 3.6%, p = .005), chronic corticosteroid treatment (28.6% vs. 1.8%, p < .001), tocilizumab therapy (92.9% vs. 66.1%, p = .008) and high APACHE II at ICU admission (median 13 vs. 10 points, p = .026). A score was created including these variables, which showed an area under the receiver operator curve of 0.854 (95% CI 0.77‐0.92). A punctuation below 6 had a negative predictive value of 99.6%. A punctuation of 10 or higher had a positive predictive value of 27.9%.
Conclusion
We present a clinical prediction score that allowed to stratify critically ill COVID‐19 patients according to the risk for developing CAPA. This CAPA score would allow to target preventive measures. Further evaluation of the score, as well as the utility of these targeted preventive measures, is needed.
Keywords: CAPA, COVID‐19, critically ill, score
1. INTRODUCTION
The coronavirus disease 19 (COVID‐19) pandemic has affected more than 250 million people, with more than 5 million deaths due to this condition. 1 One third of hospitalised patients with COVID‐19 will develop severe acute respiratory distress syndrome (ARDS), which usually leads to intensive care unit (ICU) admission and mechanical ventilation. 2
COVID‐19‐associated pulmonary aspergillosis (CAPA) has recently been recognised as a major complication of critically ill COVID‐19 patients. 3 It is estimated that 10%‐20% of ICU‐admitted COVID‐19 patients eventually develop CAPA. 4 , 5 Recent studies have shown that cumulative incidence may vary from 5% or less to rates close to 40%, with higher incidence in patients needing mechanical ventilation. 6 Additionally, this entity is a life‐threatening condition, with mortality rates usually exceeding 60% despite appropriate antifungal treatment. 5 , 7 Accordingly, some authors have proposed the use of antifungal prophylaxis 8 , 9 or the implantation of screening protocols along with pre‐emptive treatment when Aspergillus spp. is isolated from respiratory samples. 10 Applying these special measures to all critically ill COVID‐19 patients, including patients at low risk for CAPA, could result in patient's harm due to secondary effects of inappropriate antifungal therapy 11 , 12 and lack of efficiency. Thus, identifying patients at high risk of CAPA in order to target the aforementioned measures would be of great interest. However, to date, studies that attempted to identify risk factors for CAPA development are scarce and show contradictory results. 4 , 13 , 14 Furthermore, none of these studies has resulted in a score capable to predict CAPA development at ICU admission.
We intend to identify factors associated with the occurrence of CAPA among critically ill COVID‐19 patients and to develop a clinical score capable of stratifying them according to the risk for CAPA at ICU admission.
2. PATIENTS AND METHODS
We performed a single centre retrospective case–control study. Our hospital is a 613‐bed tertiary teaching hospital in Madrid, with a catchment area of 550.000 inhabitants, with 22 ICU beds, that increased to 64 ICU beds during the first waves of the COVID‐19 pandemic.
Cases were identified from a prospective cohort that includes all patients diagnosed with invasive fungal infection at our centre since January 2018. A case was defined as a patient diagnosed with CAPA between March 2020 and August 2021, which corresponded to the first 5 waves in our setting. CAPA was defined according to 2020 ECMM/ISHAM criteria. 15 Cases were classified either as possible, probable or proven CAPA.
Controls were selected from a prospective cohort including all ICU‐admitted patients with ARDS due to COVID‐19. Controls were matched to cases by admission date. 2 controls were selected for each case. As one of our main objectives was to identify risk factors for CAPA development, which could include patient's age, sex, comorbidities and COVID‐19 severity, we decided not to match controls according to these variables.
Data were collected from electronic medical records and managed using REDCap electronic capture tools, 16 with a license provided to Puerta de Hierro‐Segovia de Arana Research Institute (IDISPHSA for its Spanish abbreviation). 17 Data collected using REDCap platform were anonymised and included demographics, comorbidities, microbiological data and outcomes.
The study was approved by the hospital ethics committee (protocol identification PI‐10/22, reference 07/117290.9/22). Since this was a retrospective, non‐interventional study and only required collection of previously generated and anonymised data, informed consent was not required.
2.1. Laboratory and microbiological procedure
Galactomannan (GM) qualitative detection was performed by sandwich chemiluminescent immunoassay (CLIA) Aspergillus Galactomannan Ag VIRCLIA@ monotest (Vircell, Granada, Spain). According to manufacter's instructions, a result equal or greater than 0.20 was considered positive, both in serum and in bronchoalveolar lavage. This cut‐off point has been validated against the well‐established indexes of GM Platelia, BioRad. 18
Respiratory samples for fungal cultures were grown in Sabouraud‐gentamicin‐chloramphenicol agar and antifungal susceptibility tests were performed by broth microdilution at the national reference centre (Carlos III Health Institute, National Microbiology Centre, Majadahonda, Spain). In some samples, direct visualisation by KOH stain was performed at the request of the attending physician.
All tests were performed according to manufacturer´s instructions.
No CAPA screening protocol was implemented during the study period and the tests were ordered at the discretion of the attending physician. Fibrobronchoscopy and bronchoalveolar lavage were performed under specific request of attending physician.
2.2. Definitions
ARDS was defined according to Berlin's criteria. 19 The acute physiology and chronic health evaluation (APACHE II score 20 ) punctuation was calculated at ICU admission except from patients not admitted to ICU, where APACHE II was calculated at the time of severe ARDS development. The waves’ period was considered as follows: first wave from February to July 2020; second wave from August to November 2020; third wave from December 2020 to February 2021; fourth wave from March to May 2021; and fifth wave from June to August 2021.
2.3. Primary and secondary objectives
Our primary objective was to identify risk factors for CAPA among critically ill COVID‐19 patients. Our secondary objective was to develop and validate a clinical prediction score for the occurrence of CAPA among critically ill COVID‐19 patients based on data available at ICU admission. We also sought to describe characteristics of CAPA patients and factors associated with poor outcome.
2.4. Data analysis
Data are presented as median and interquartile range (IQR) for quantitative variables, and as absolute and percentage value for qualitative variables. For the primary and secondary purposes, inferential statistical analysis was done using chi‐square test and Fisher exact test (when necessary) for qualitative variables and Mann–Whitney's U for quantitative ones. A 180‐day mortality analysis was performed by means of Cox regression and Kaplan–Meier curves. Hazard ratios (HR) with 95% confidence intervals (CI) are provided.
To create the score, variables clinically and statistically significant in the previous univariate analysis were included in a multivariate logistic regression model in order to estimate each patient's predicted probability of developing CAPA. 21 , 22 As we did not intend to seek for independent‐associated factors, we did not establish a limit of covariate based on the number of cases. 22 , 23 , 24 , 25 If the variables included in showed to improve the predictive model, they were considered for the final predicted probability calculation. Afterwards, the predicted probability was transformed into a punctuation score based on the beta‐coefficient from this model. The score was subsequently applied to all patients. Calibration of the score was tested by the Hosmer‐Lemeshow goodness‐to‐fit test. 25 Discrimination of the score was measured by the area under the receiving operator curve (AUC‐ROC) with the 95% CI. A bootstrap procedure was employed for internal validation. The score was applied to 1000 bootstrap samples, and the optimism was calculated. Then, the bootstrapped corrected AUC‐ROC was computed. A value of AUC‐ROC of 0.5 indicates random predictions, and a value of 1 indicates perfect discrimination. A model with an AUC‐ROC roughly above 0.7 is considered to be useful to perform predictions of individual subjects. 25 Cut‐off points along with sensitivity, specificity and likelihood ratios are provided. Negative and positive predictive values were calculated applying the score to a population with a prevalence similar to that of our ICU cohort.
Bilateral p values below .05 were considered statistically significant. All statistical analyses were performed using SPSS version 25 software (SPSS Inc, IBM, Chicago, Illinois, United States).
3. RESULTS
Between March 2020 and August 2021, 28 CAPA patients were prospectively identified in our centre, including 22 ICU‐admitted patients, representing 6.04% (22/364) patients admitted to ICU with severe ARDS due to COVID‐19. In addition to the 22 that were at the ICU ward, 3 others were on non‐invasive mechanical ventilation at an intensive respiratory care unit and the remaining 3 were severe ARDS COVID‐19 cases receiving the highest possible oxygen supplementation at conventional hospitalisation. Of note, all of the 6 patients not admitted to ICU had developed critical COVID‐19 with severe ARDS and had criteria for ICU admission. Therefore, they fulfilled ECMM/ISHAM 2020 consensus criteria for CAPA. However, due to healthcare system overload (and in particular the lack of available ICU beds), as well as age and basal comorbidity of some patients, other patients with potentially better survival opportunities were prioritised over them for ICU admission. Six (21.4%) CAPA patients were diagnosed during the first, 6 (21.4%) during the second wave, 5 (17.9%) during the third, 6 (21.4%) during the fourth wave and 5 (17.9%) during the fifth wave. Accordingly, 56‐matched controls were selected.
3.1. CAPA characteristics
Characteristics of the 28 CAPA patients included in the study are shown in Table 1.
TABLE 1.
Factors associated with CAPA
| Variable | CAPA (n = 28) | Control (n = 56) | p | Missing |
|---|---|---|---|---|
| Comorbidity | ||||
| Age (years) | 68 (65–72) | 62 (52–71) | .033 | 0 |
| Sex (female) | 21.4% (6) | 30.4% (17) | 0.446 | 0 |
| Active smoking | 17.9% (5) | 1.8% (1) | .014 | 11 |
| Arterial hypertension | 64.3% (18) | 44.6% (25) | 0.108 | 0 |
| Diabetes mellitus | 39.3% (11) | 17.9% (10) | .059 | 0 |
| Chronic respiratory disease | 48.1% (13) | 26.3% (15) | .043 | 0 |
| COPD | 28.6% (8) | 10.7% (6) | .060 | 0 |
| Asthma | 3.6% (1) | 7.1% (4) | 0.661 | 0 |
| Other | 21.4% (6) | 10.7% (6) | 0.202 | 0 |
| Chronic cardiac failure | 21.4% (6) | 8.9% (5) | 0.168 | 0 |
| Ischaemic heart disease | 21.4% (6) | 5.4% (3) | .054 | 0 |
| Chronic renal failure | 25.0% (7) | 3.6% (2) | .005 | 0 |
| Liver cirrhosis | 7.1% (2) | 0 | 0.108 | 0 |
| Solid malignancy | 7.1% (2) | 10.7% (6) | 0.713 | 0 |
| Prior immunocompromise | ||||
| Any IC condition | 42.9% (12) | 19.6% (11) | .037 | 0 |
| Haematological malignancy | 14.3% (4) | 3.6% (2) | 0.172 | 0 |
| Solid organ transplantation | 17.9% (5) | 1.8% (1) | .014 | 0 |
| Autoimmune disease | 14.3% (4) | 12.5% (7) | 1.000 | 0 |
| Previous chronic corticoid | 28.6% (8) | 1.8% (1) | <.001 | 0 |
| Other previous IS treatments | 28.6% (8) | 10.7% (6) | .060 | 0 |
| COVID‐19 presentation and management prior to CAPA diagnosis | ||||
| Neutropenia | 14.3% (4) | 1.8% (1) | .042 | 0 |
| Confirmed bacterial coinfection | 57.1% (16) | 44.6% (25) | 0.356 | 0 |
| Viral coinfection other than CMV | 7.1% (2) | 1.8% (1) | 0.547 | 0 |
| Renal replacement therapy | 35.7% (10) | 10.7% (6) | .008 | 0 |
| Vasopressor drug therapy | 42.9% (12) | 39.3% (22) | 0.816 | 0 |
| APACHE II | 13 (9–18) | 10 (8–13) | .026 | 2 |
| Any corticoid treatment | 100% | 100% | 1.000 | 0 |
| Corticoid pulses | 46.4% (13) | 28.6% (16) | .085 | 0 |
| Tocilizumab | 92.9% (26) | 66.1% (37) | .008 | 0 |
| 1 dose | 43.5% (10/23) | 86.1% (31/36) | .001 | 4 |
| 2 or more doses | 56.5% (13/23) | 13.9% (5/36) | ||
| Anakinra | 10.7% (3) | 8.9% (5) | 1.000 | 0 |
| Remdesivir | 14.3% (4) | 5.4% (3) | 0.215 | 0 |
| Antibiotics | 96.4% (27) | 91.1% (51) | 0.658 | 0 |
| Blood components transfusion | 51.9% (14) | 21.8% (12) | .011 | 0 |
| Outcomes | ||||
| In‐hospital mortality | 60.7% (17) | 14.3% (8) | <.001 | 0 |
| CAPA‐associated death | 76.5% (13/17) | – | – | 0 |
| COVID‐associated death | 88.2% (15/17) | 87.5% (7/8) | 1.000 | 0 |
| ICU length of stay | 57 (28–85) | 18 (13–38) | .010 | 27 |
| Hospital length of stay | 66 (43–88) | 33 (22–58) | .003 | 25 |
Qualitative variables are expressed as percentage (absolute number). Quantitative variables are expressed as median (interquartile range).
Abbreviations: CAPA, COVID‐associated pulmonary aspergillosis; COPD, Chronic obstructive pulmonary disease; IC, Immunocompromised.
Median time from hospital admission to CAPA diagnosis was 21 days (IQR 11–41), and median time from ICU admission to CAPA was 11 days (IQR 6‐42).
Sixteen patients (57.1%) were classified as probable CAPA and 12 (42.8%) as possible CAPA. Comparison between probable and possible cases is shown in Table S1. Bronchoalveolar lavage culture was only available in 2 out of 12 possible CAPA and none of them had GM performed in this sample. There were no cases of proven CAPA. Evidence of tracheobronchitis was noted in 4 out of 14 patients with available bronchoscopy (28.6%). In 23.1% (6/26) cases serum galactomannan was positive and 1 (3.6%) had extra‐pulmonary organ involvement: the case consisted of a endogenous fungal endophthalmitis in the context of an extensive probable CAPA (without microbiological confirmation of the ocular involvement).
Aspergillus fumigatus complex was the most frequently isolated species (64.3%, n = 18), followed by Aspergillus niger complex (14.3%, n = 4). There were isolated cases of Aspergillus terreus and Aspergillus flavus (3.6% each). In one case (3.6%), the Aspergillus species was not identified and in 3 cases (10.7%) there was not a culture growth. In 20 cases, an antifungigram was available: only 1 case (5.0%) was resistant to amphotericin B (A terreus, intrinsic resistance), and 1 case (5.0%) of voriconazole and isavuconazole resistance was detected in a patient with no species identification. CAPA patients had an in‐hospital mortality rate of 60.7% (n = 15), while controls had an in‐hospital mortality of 14.3% (n = 8) (p < .001).
3.2. Factors associated with CAPA
When comparing patients with and without CAPA regarding baseline characteristics (Table 1), CAPA patients were older (median age 68 years (IQR 65–72) vs. 62 (52–71), p = .033) and more frequently active smokers (17.9% vs. 1.8%, p = .014). CAPA patients had more often chronic respiratory diseases (48.1% vs. 26.3%, p = .043), chronic renal failure (25.0% vs. 3.6%, p = .005) and prior immune‐compromise (42.9% vs. 19.6%, p = .037). Specifically, they were more frequently under chronic corticosteroid treatment prior to hospital admission (28.6% vs. 1.8%, p < .001).
With respect to COVID‐19 complications and management, CAPA patients had received more frequently tocilizumab (92.9% vs. 66.1%, p = .008) and had a higher APACHE II at ICU admission (median 13 (IQR 9–18) vs. 10 (8–13), p = .026).
3.3. CAPA prediction score development and validation
Among factors associated with CAPA, age, active smoking, chronic respiratory diseases, chronic renal failure, previous chronic corticosteroid treatment, tocilizumab therapy for COVID‐19 and APACHE II at ICU admission were selected for the multivariate logistic regression model. The model is summarised in Table 2. The calculated score is shown in Table 3.
TABLE 2.
Multivariate logistic regression model for developing of the CAPA risk score
| Variable | OR | 95% CI | Beta‐coefficient | Points |
|---|---|---|---|---|
| Age (per 5 years) | 1.35 | 0.60–3.00 | 0.493 | 64–69 years: 2 |
| >/= 70 years: 3 | ||||
| Active smoking | 3.58 | 1.67–7.70 | 1.26 | 3 |
| Chronic respiratory disease | 1.26 | 0.33–4.78 | 0.23 | 2 |
| Chronic renal failure | 2.67 | 0.35–20.31 | 1.98 | 4 |
| Prior chronic corticoid | 49.61 | 5.41–149.1 | 5.00 | 5 |
| Tocilizumab | 20.96 | 1.56–278.9 | 3.04 | 4 |
| APACHE II (per 3 points) | 1.64 | 0.71–3.76 | 0.297 | 10–12:1 |
| >/=13:2 |
All 7 variables included in the regression model improved the predictive model, so all of them were considered to calculate the score punctuation. In order to calculate the score punctuation, beta‐coefficients of categorical variables were transformed into points using the following rule: beta‐coefficient lower than 0.5:2 points; beta‐coefficient 0.5‐1.5:3 points; beta‐coefficient 1.6‐4:4 points; beta‐coefficient greater than 4:5 points. In the case of non‐categorical variables (age and APACHE II), punctuation was divided in 3 groups, the first with no punctuation: a second group with a low punctuation and a third with high punctuation. Due to higher beta‐coefficient for age compared to APACHE II, age was given more weight to the score.
TABLE 3.
CAPA risk score punctuation
| Variable | Points | |
|---|---|---|
| Years | 64–69 years | 2 |
| >/= 70 years | 3 | |
| Active smoking | 3 | |
| Chronic respiratory disease | 2 | |
| Chronic renal failure | 4 | |
| Chronic corticoid treatment | 5 | |
| Tocilizumab treatment | 4 | |
| APACHE II at ICU admission | 10–12 | 1 |
| >/= 13 | 2 | |
| Total | 0–23 | |
The score presented a good calibration, with a p‐value in the goodness‐to‐fit Hosmer‐Lemeshow test of 0.565. The area under ROC was 0.861 (95% CI 0.78–0.93, p < .001, Figure 1). In the internal validation, after 1000 bootstrap samples, the optimism estimated was 0.047, with a bootstrapped corrected area under ROC was 0.854 (95% CI 0.77–0.92). Figure 2 shows bootstrap samples area under ROC values histogram.
FIGURE 1.
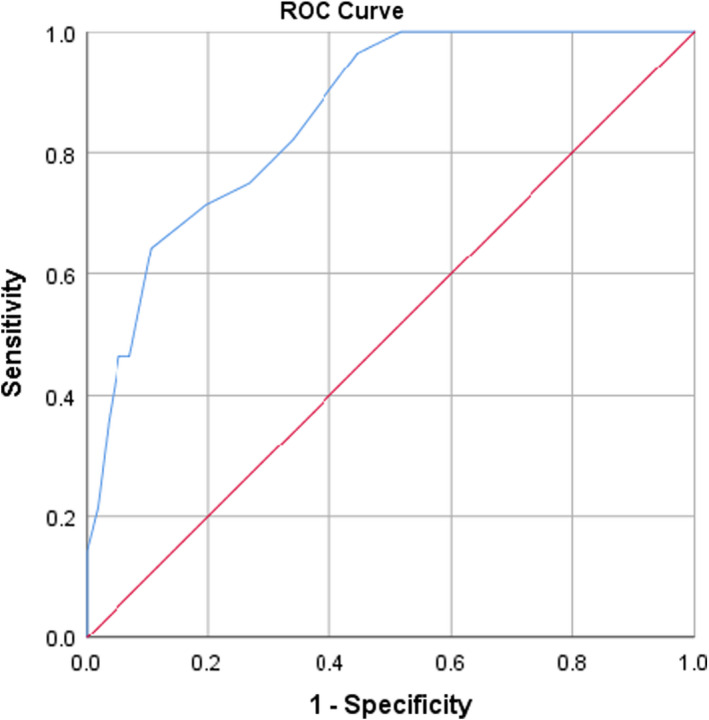
CAPA risk score receiver operator curve. The AUC was 0.861 (95% CI 0.78–0.93, p < .001)
FIGURE 2.
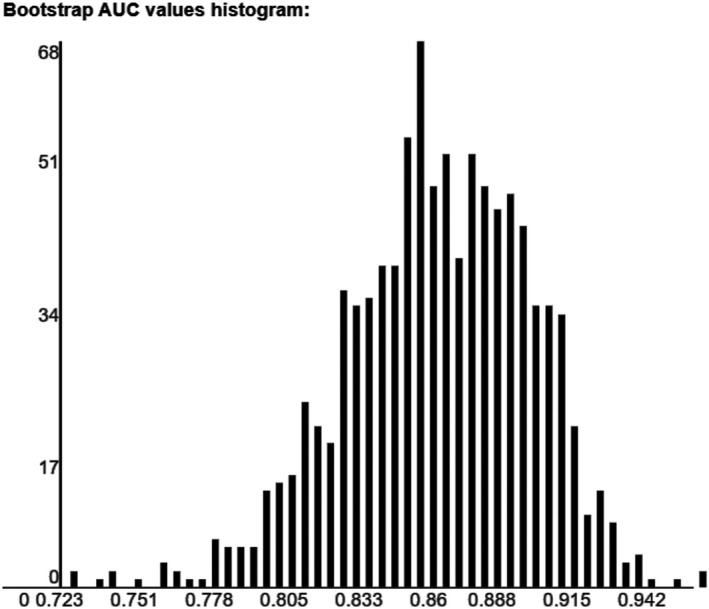
Histogram of distribution of area under the receiver operator curve (AUC) of 1000 bootstrapped samples. The optimism estimated was 0.047, with a corrected AUC of 0.854 (95% CI 0.77–0.92)
Cut‐off points are provided in Table 4. A score below 6 points had a sensitivity of 96.4% (95% CI 89.1%–100%) and specificity of 55.4% (95% CI 41.9%–68.8%). The negative likelihood ratio (NLR) was 0.06, and the positive likelihood ratio (PLR) was 2.16. Applying this cut‐off to a population with a prevalence similar to ours would result in a negative predictive value (NPV) of 99.6% and a positive predictive value (PPV) of 12.2%.
TABLE 4.
Proposed cut‐off points for CAPA risk stratification based on the score
| Cut‐off point | S (%) | E (%) | +LR | −LR | PPV | VPN |
|---|---|---|---|---|---|---|
| Score >/= 5 points | 100% | 48.2% | 1.96 | 0 | 11.0% | 100% |
| Score >/=6 points | 96.4% | 55.4% | 2.16 | 0.06 | 12.2% | 99.6% |
| Score >/= 10 points | 64.3% | 89.3% | 6.01 | 0.40 | 27.9% | 97.5% |
| Score >/= 12 points | 46.4% | 94.6% | 8.67 | 0.57 | 35.6% | 96.5% |
Abbreviations: +LR, positive likelihood ratio; E, specificity; −LR, negative likelihood ratio; PPV, positive predictive value; PV, negative predictive value; S, sensitivity.
A score equal or over 10 points had a sensitivity of 64.3% (95% CI 45.4%–83.2%) and specificity of 89.3% (95% CI 80.9%–97.6%). The PLR was 6.01 and NLR was 0.40. Applying this cut‐off to a population with a prevalence similar to ours would result in a PPV of 27.9% and a NPV of 97.5%.
Hence, when applying this score to critically ill COVID‐19 patients in our population, we would be able to identify 3 groups of patients according to CAPA risk: 1‐ Very low risk for CAPA: patients with a score inferior to 6 (predicted risk lower to 0.5%); 2‐ Intermediate risk for CAPA: patients with a score between 6 and 9 (predicted risk 5%‐10%); 3‐ High risk for CAPA: patients with a score equal or greater than 10 (predicted risk greater than 25%). However, if the same score is applied in other population with a high prevalence of CAPA (ie, 15%), we would identify 2 groups of patients: 1‐ Very low risk for CAPA: patients with score inferior to 6 (predicted risk lower than 1%); 2‐ High risk for CAPA: patients with score equal or greater than 6 (predicted risk greater than 30%).
3.4. Factors associated with mortality among CAPA patients
Table 5 summarises baseline characteristics, COVID‐19 complications, management and CAPA characteristics between survivors and non‐survivors. Classification as probable CAPA according to ECMM/ISHAM criteria (vs. possible CAPA) was associated with higher in‐hospital mortality (81.3% vs. 33.3%, respectively, p = .019). Moreover, among patients with probable CAPA, those with positive serum GM had a higher 180‐day mortality than those with a negative value (HR 3.88, 95% CI 1.16–12.82). Thus, incorporating serum GM to ECMM/ISHAM classification allowed to discriminate patients according to mortality risk. Survival analysis is shown in Figure 3.
TABLE 5.
Factors associated with in‐hospital mortality among patients with COVID‐associated pulmonary aspergillosis
| Variable | Total (n = 28) | Survivor (n = 11) | Non‐survivor (n = 17) | p | |
|---|---|---|---|---|---|
| Comorbidity | |||||
| Age (years) | 68 (65–72) | 71 (64–74) | 68 (56–72) | .161 | |
| Sex (female) | 21.4% (6) | 27.3% (3) | 17.6% (3) | .653 | |
| Active smoking | 17.9% (5) | 27.3% (3) | 11.8% (2) | .738 | |
| Chronic respiratory disease | 48.1% (13) | 45.5% (5) | 47.1% (8) | 1.000 | |
| Chronic cardiac failure | 21.4% (6) | 27.3% (3) | 17.6% (3) | .653 | |
| Chronic renal failure | 25.0% (7) | 36.4% (4) | 17.6% (3) | .381 | |
| Haematological cancer | 14.3% (4) | 18.2% (2) | 11.8% (2) | 1.000 | |
| Solid organ transplant | 17.9% (5) | 9.1% (1) | 23.5% (4) | .329 | |
| Chronic corticoid | 28.6% (8) | 18.2% (2) | 35.3% (6) | .419 | |
| Other IS treatments | 28.6% (8) | 18.2% (2) | 35.3% (6) | .419 | |
| COVID‐19 presentation and management prior to CAPA diagnosis | |||||
| Bacterial coinfection | 57.1% (16) | 45.5% (5) | 64.7% (11) | .441 | |
| RRT | 35.7% (10) | 18.2% (2) | 47.1% (8) | .226 | |
| Vasopressor drug | 42.9% (12) | 27.3% (3) | 52.9% (9) | .172 | |
| APACHE II | 13 (9–18) | 9 (7–13) | 15 (11–20) | .017 | |
| Corticoid pulses | 46.4% (13) | 36.4% (4) | 52.9% (9) | .460 | |
| Tocilizumab | 92.9% (26) | 100% (11) | 88.2% (15) | .505 | |
| Blood transfusion | 51.9% (14) | 54.5% (6) | 50.0% (8) | 1.000 | |
| Aspergillosis radiology and clinical presentation | |||||
| Days from admission | 21 (11–41) | 23 (10–57) | 19 (12–38) | .280 | |
| Respiratory worsening | 85.7% (24) | 90.9% (10) | 82.4% (14) | .635 | |
| Refractory fever | 17.9% (5) | 27.3% (3) | 11.8% (2) | .353 | |
| Haemoptysis | 28.6% (8) | 18.2% (2) | 35.3% (6) | .419 | |
| Tracheobronchitis | 20.0% (4/12) | 60.0% (3/5) | 14.3% (1/7) | .031 | |
| Solitary nodule | 14.3% (4) | 9.1% (1) | 17.6% (3) | .635 | |
| Multiple nodules | 25.0% (7) | 18.2% (2) | 29.4% (5) | .668 | |
| Cavitary nodule (s) | 25.0% (7) | 27.3% (3) | 23.5% (4) | 1.000 | |
| Alveolar infiltrate | 67.9% (19) | 81.8% (9) | 58.8% (10) | .197 | |
| Positive serum GM | 23.1% (6/26) | 0% (0/11) | 40.0% (6/15) | .018 | |
| Aspergillosis microbiology | |||||
| A fumigatus complex | 64.3% (18) | 63.6% (7) | 64.7% (11) | .963 | |
| A niger complex | 14.3% (4) | 18.2% (2) | 11.8% (2) | ||
| Other species | 10.7% (3) | 0 | 17.7% (3) | ||
| No culture growth | 10.7% (3) | 18.2% (2) | 5.9% (1) | ||
| Aspergillosis classification | |||||
| ECMM/ISHAM | Probable | 57.1% (16) | 27.3% (3) | 76.5% (13) | .019 |
| Possible | 42.9% (12) | 72.7% (8) | 23.5% (4) | ||
| Treatment and outcomes | |||||
| Combination therapy | 39.3% (11) | 36.4% (4) | 41.2% (7) | 1.000 | |
| Voriconazole | 50.0% (14) | 36.4% (4) | 41.2% (7) | 1.000 | |
| Isavuconazole | 39.3% (14) | 54.5% (6) | 47.1% (8) | 1.000 | |
| Amphotericin B | 35.7% (10) | 27.3% (3) | 41.2% (7) | .368 | |
Abbreviations: GM, galactomannan; IC, immunosuppressive; RRT, Renal replacement therapy.
FIGURE 3.
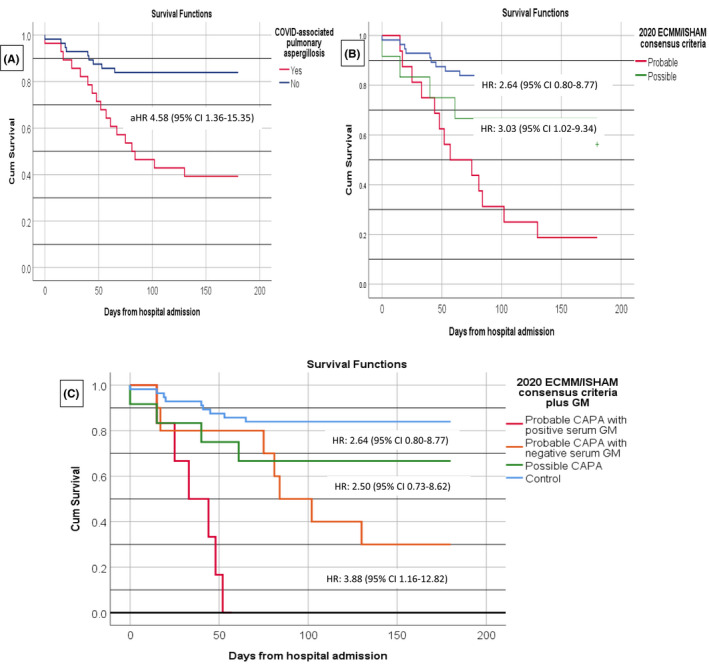
Kaplan–Meier survival curves of 180‐day mortality among different population of COVID‐associated pulmonary aspergillosis (CAPA) and controls. Survival analysis was made by means of Cox regression. Hazards ratios (HR) with their 95% confidence interval (CI) are presented. Figure 1A represents survival curve among patients with CAPA and ICU controls. Adjusted HR (aHR) was obtained after adjusting for age, smoking, chronic respiratory disease, immunocompromised status, prior chronic corticoid treatment, chronic renal failure, renal replacement therapy, APACHE II at ICU admission and blood component transfusion. Figure 1B represents survival curve according to 2020 ECMM/ISHAM consensus criteria CAPA classification and Figure 1C survival curve according to 2020 ECMM/ISHAM consensus criteria plus serum galactomannan (GM). Only patients with probable CAPA had positive serum GM
Other factors such as age, prior immune‐compromise, chronic respiratory diseases and radiological findings were not associated with mortality in CAPA patients. All patients received antifungal treatment, except for one patient who died 24 h after CAPA diagnosis. No specific antifungal drug (ie, voriconazole, isavuconazole or amphotericin B) was associated with improved outcomes. Antifungal combination (vs. monotherapy) was not associated with an inferior mortality (63.6% vs. 58.8%, p = 1.000).
4. DISCUSSION
In the present study, several baseline characteristics determined at ICU admission such as age, active smoking, chronic respiratory diseases, prior immuno‐compromise, chronic corticosteroid, APACHE II and tocilizumab treatment were associated with the development of CAPA in COVID‐19 patients. A clinical prediction score based on these characteristics was developed that allowed to stratify critically ill COVID‐19 patients in low, intermediate or high risk for CAPA at the time of ICU admission. CAPA mortality in the present series was associated with CAPA classification according to ECMM/ISHAM consensus criteria (possible vs. probable CAPA) and serum GM levels.
Previous studies have identified risk factors for CAPA, including age, prior respiratory diseases, chronic renal failure, chronic corticosteroid use, neutropenia, COVID‐19 severity and treatment of the COVID‐19 episode with corticosteroid or tocilizumab. 4 , 13 , 26 , 27 , 28 In the present study, we could validate most of these factors. However, we could not corroborate the association between corticosteroid treatment for COVID‐19 and CAPA, since, due to local protocols, corticosteroids were extensively used in COVID‐19 patients very early in the pandemic. Additionally, we found associations that had not previously been described between immune‐compromise or solid organ transplantation and CAPA development. Our institution cares for a high percentage of patients with these conditions, compared to other cohorts, 4 , 29 , 30 which may have facilitated to unveil these associations.
Based on the aforementioned risk factors, we constructed a clinical prediction score, the CAPA score that reliably stratified critically ill COVID‐19 patients according to their risk for CAPA development. To the best of our knowledge, this is the first published score effective to predict CAPA risk. In our population, a patient with a CAPA score below 6 points would have a risk for CAPA inferior to 0.5%. Additionally, a patient with a CAPA score value equal or greater than 10 would present a CAPA risk over 25%.
Although the prevalence of CAPA in our ICU‐admitted patients (6.04%) is similar to other works 27 and is consistent with studies in our setting 31 and autopsy studies, 32 some other authors have noted a higher prevalence, 4 , 12 , 28 , 33 up to 30%. 34 These differences could be related to regional variations in incidence. 35 Using the same cut‐off of 6 points in a high prevalence population to identify patients at low risk would remain adequate, as it would identify patients with a risk of developing CAPA of approximately 1%. Of note, in populations with a higher prevalence of CAPA, the cut‐off of 6 points would be enough to identify patients at high risk, since patients with 6 or more points would present a risk greater than 40%.
Some studies suggest the efficacy of antifungal prophylaxis in preventing CAPA, 8 , 9 while other authors consider systematic screening in all patients. 10 However, all those studies have failed to demonstrate a benefit of these measures, in terms of survival, in an unselected population. This could potentially be related to adverse effects of antifungal treatment 10 , 23 , 24 , 25 in patients with low risk of CAPA. Applying the CAPA score developed in the present study would allow to obviate these measures in patients with low risk for CAPA (ie, CAPA score below 6), and select patients at a higher risk who could benefit most from them (ie, patients with CAPA score equal or greater than 10 in a setting with low CAPA incidence, or CAPA score equal or greater than 6 in settings with high CAPA incidence). Nevertheless, the CAPA score needs external validation in other populations and further evaluation in order to assess the efficacy of the targeted approach based on the stratification in reducing CAPA cases and improving survival in critically ill COVID‐19 patients. Additionally, other risk factors outside the score should be taken into account, such as CMV replication. 37
Moreover, we intended to identify factors associated with mortality in CAPA patients. We found that the classification according to ECMM/ISHAM consensus criteria along with serum galactomannan allows to distinguish patients with a different mortality risk: possible CAPA, probable CAPA with negative serum GM and probable CAPA with positive serum GM. The prognostic value of serum GM among probable CAPA (according to ECMM/ISHAM classification) was noted in a previous study. 38 Our results support the hypothesis that the term CAPA encompasses a complex entity with various phases of invasion and damage 3 , 15 , 36 , 39 and that different biomarker profiles may correspond to different stages of the disease. 38
Ours is a single centre retrospective study and has the inherent limitations of this design. One of the major limitations of our study is the relative small sample size, which may have limited the power of the statistical analysis. Another limitation is that there was no CAPA screening protocol in our institution, and, consequently, a respiratory fungal culture was not available for every control patient, so it is not possible to exclude that a small proportion of the controls could actually have CAPA. However, the prevalence of CAPA in our institution is similar to that found in similar settings 31 as well as in recent systematic reviews, 5 , 26 suggesting that the majority of CAPA cases were identified. Additionally, in the same way that other studies on CAPA, it is difficult to distinguish between Aspergillus spp. colonisation and invasive infection in critically COVID‐19 patients, given that histologic samples are rarely available and clinical‐radiological features are often overlapping and non‐specific. Though, we tried to mitigate this limitation by systematically applying 2020 ECMM/ISHAM consensus criteria for CAPA diagnosis and classification. Another limitation is that not all cases were admitted to the ICU, with 6 CAPA patients outside ICU unit. However, all of them were critical COVID‐19 with severe ARDS and fulfilled ICU admission criteria, though unfortunately were not admitted to ICU due to healthcare system overload. Finally, our score needs validation in external, prospective and larger samples. In spite of these limitations, we believe that our real‐life results are of interest and can inspire further studies with larger figures.
In conclusion, we have developed and internally validated a clinical prediction score that allowed to stratify critically ill COVID‐19 patients according to the risk for developing CAPA. Accordingly, at ICU admission, patients could be classified as low risk (score inferior to 6), intermediate risk (score between 6 and 9) and high risk (score equal or greater than 10). This CAPA score would enable targeting preventive measures such as periodic screening or antifungal prophylaxis to patients at high risk who could benefit most from them, while avoiding these measures in patients at low risk. Further evaluation of the score, as well as the usefulness of preventive measures in patients at high risk, is warranted.
CONFLICTS OF INTEREST
AFC declares personal fees for lectures/presentations/educational events outside the present manuscript. All other authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Jorge Calderon Parra: Conceptualization (lead); Data curation (equal); Formal analysis (equal); Methodology (equal); Project administration (equal); Software (lead); Writing – original draft (equal); Writing – review & editing (lead). Ana Fernández‐Cruz: Conceptualization (lead); Formal analysis (equal); Investigation (equal); Methodology (lead); Project administration (lead); Supervision (lead); Validation (lead); Writing – original draft (lead); Writing – review & editing (lead). Patricia Mills‐Sanchez: Conceptualization (equal); Data curation (equal); Investigation (equal). Victor Moreno‐Torres: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Writing – review & editing (equal). Sandra Tejhado‐Bravo: Writing – review & editing (equal). Isabel Sánchez‐Romero: Methodology (equal); Writing – review & editing (equal). Barbara Balandín‐Moreno: Supervision (equal); Writing – review & editing (equal). Marina Calvo‐Salvador: Writing – review & editing (equal). Francisca Portero‐Azorín: Methodology (equal); Writing – review & editing (equal). Sarela García‐Masedo: Methodology (equal); Writing – review & editing (equal). Elena Muñez Rubio: Writing – review & editing (equal). Antonio Ramos‐Martinez: Methodology (equal); Supervision (equal); Validation (equal); Writing – review & editing (equal).
Supporting information
Table S1
Calderón‐Parra J, Mills‐Sanchez P, Moreno‐Torres V, et al; the HUPH IFI Study Group . COVID‐19‐associated pulmonary aspergillosis (CAPA): Risk factors and development of a predictive score for critically ill COVID‐19 patients. Mycoses. 2022;65:541–550. doi: 10.1111/myc.13434
Funding information
No funding was received for this article.
Contributor Information
Jorge Calderón‐Parra, Email: jorge050390@gmail.com.
Ana Fernández‐Cruz, Email: anafcruz999@gmail.com.
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Casas‐Rojo JM, Antón‐Santos JM, Millán‐Núñez‐Cortés J, et al. Clinical characteristics of patients hospitalized with COVID‐19 in Spain: results from the SEMI‐COVID‐19 Registry. Rev Clin Esp. 2020;220(8):480‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verweij PE, Brüggemann RJM, Azoulay E, et al. Taskforce report on the diagnosis and clinical management of COVID‐19 associated pulmonary aspergillosis. Intensive Care Med. 2021;47(8):819‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prattes J, Wauters J, Giacobbe DR, et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients—a multinational observational study by the European Confederation of Medical Mycology. Clin Microbiol Infect. 2021;S1198743X21004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitaka H, Kuno T, Takagi H, Patrawalla P. Incidence and mortality of COVID‐19‐associated pulmonary aspergillosis: a systematic review and meta‐analysis. Mycoses. 2021;64(9):993‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salmanton‐García J, Sprute R, Stemler J, et al. COVID‐19‐associated pulmonary aspergillosis, March‐August 2020. Emerg Infect Dis. 2021;27(4):1077‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iqbal A, Ramzan M, Akhtar A, et al. COVID‐Associated pulmonary aspergillosis and its related outcomes: a single‐center prospective observational study. Cureus. 2021;13(8):e16982. doi: 10.7759/cureus.16982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hatzl S, Reisinger AC, Posch F, et al. Antifungal prophylaxis for prevention of COVID‐19‐associated pulmonary aspergillosis in critically ill patients: an observational study. Crit Care. 2021;25(1):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Ackerbroeck S, Rutsaert L, Roelant E, Dillen K, Wauters J, Van Regenmortel N. Inhaled liposomal amphotericin‐B as a prophylactic treatment for COVID‐19‐associated pulmonary aspergillosis/aspergillus tracheobronchitis. Crit Care Lond Engl. 2021;25(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grootveld R, Paassen J, Boer MGJ, et al. Systematic screening for COVID‐19 associated invasive aspergillosis in ICU patients by culture and PCR on tracheal aspirate. Mycoses. 2021;64(6):641‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schouten J, De Waele J, Lanckohr C, et al. Antimicrobial stewardship in the ICU in COVID‐19 times: the known unknowns. Int J Antimicrob Agents. 2021;58(4): 106409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pasquier G, Bounhiol A, Robert Gangneux F, et al. A review of significance of Aspergillus detection in airways of ICU COVID‐19 patients. Mycoses. 2021;64(9):980‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu J, Yang X, Lv Z, et al. Risk factors for invasive Aspergillosis in patients admitted to the intensive care unit with Coronavirus Disease 2019: a multicenter retrospective study. Front Med. 2021;8: 753659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koehler P, Cornely OA, Böttiger BW, et al. COVID‐19 associated pulmonary aspergillosis. Mycoses. 2020;63(6):528‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID‐19‐associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149‐e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95: 103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouza E, Alonso R, Vega MDM, Alguacil M, Machado M, Girones IG, et al. Comparison of the performance of two galactomannan detection tests: Platelia Galactomannan (Bio‐Rad) and Galactomannan Virclia (Vircell) [Internet]. Abstract presented at: European Congress of Clinical Microbiology and Infectious Diseases 2020; 2020. Available from: https://markterfolg.de/ESCMID/Abstractbook2020.pdf
- 19. Definition Task Force ARDS, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526‐2533. [DOI] [PubMed] [Google Scholar]
- 20. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818‐829. [PubMed] [Google Scholar]
- 21. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361‐387. [DOI] [PubMed] [Google Scholar]
- 22. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives†. Eur J Cardiothorac Surg. 2018;53(6):1112‐1117. [DOI] [PubMed] [Google Scholar]
- 23. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710‐718. [DOI] [PubMed] [Google Scholar]
- 24. van Smeden M, de Groot JAH, Moons KGM, et al. No rationale for 1 variable per 10 events criterion for binary logistic regression analysis. BMC Med Res Methodol. 2016;16(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hosmer DW, Lemeshow S. Chapter 5. Applied Logistic Regression, 2nd edn. Wiley; 160‐164. [Google Scholar]
- 26. Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among COVID‐19 intubated patients: a prospective study. Clin Infect Dis off Publ Infect Dis Soc Am. 2020;73(11):ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dellière S, Dudoignon E, Fodil S, et al. Risk factors associated with COVID‐19‐associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2021;27(5):790.e1‐790.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gregoire E, Pirotte BF, Moerman F, et al. Incidence and risk factors of COVID‐19‐associated pulmonary Aspergillosis in intensive care unit‐A monocentric retrospective observational study. Pathog Basel Switz. 2021;10(11):1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID‐19‐associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID‐19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63(8):766‐770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yusuf E, Seghers L, Hoek RAS, van den Akker JPC, Bode LGM, Rijnders BJA. Aspergillus in critically Ill COVID‐19 patients: a scoping review. J Clin Med. 2021;10(11):2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Machado M, Valerio M, Álvarez‐Uría A, et al. Invasive pulmonary aspergillosis in the COVID‐19 era: An expected new entity. Mycoses. 2021;64(2):132‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kula BE, Clancy CJ, Nguyen MH, Schwartz IS. Invasive mould disease in fatal COVID‐19: a systematic review of autopsies. Lancet Microbe 2021;2:e405‐e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lahmer T, Kriescher S, Herner A, et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID‐19 pneumonia: results from the prospective AspCOVID‐19 study. PLoS One. 2021;16(3):e0238825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chong WH, Neu KP. Incidence, diagnosis and outcomes of COVID‐19‐associated pulmonary aspergillosis (CAPA): a systematic review. J Hosp Infect. 2021;113:115‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prattes J, Koehler P, Hoenigl M, Wauters J, Giacobbe DR, Lagrou K. COVID‐19 associated pulmonary aspergillosis: regional variation in incidence and diagnostic challenges. Intensive Care Med. 2021;47(11):1339‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fekkar A, Neofytos D, Nguyen M‐H, Clancy CJ, Kontoyiannis DP, Lamoth F. COVID‐19‐associated pulmonary aspergillosis (CAPA): how big a problem is it? Clin Microbiol Infect. 2021;27(9):1376‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Calderón‐Parra J, Moreno‐Torres V, Mills‐Sanchez P, et al. Association of COVID‐19‐associated pulmonary Aspergillosis with cytomegalovirus replication: a case‐control study. J Fungi. 2022;8(2):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ergün M, Brüggemann RJM, Alanio A, et al. Aspergillus test profiles and mortality in critically Ill COVID‐19 patients. J Clin Microbiol. 59(12):e01229‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamoth F, Lewis RE, Walsh TJ, Kontoyiannis DP. Navigating the uncertainties of COVID‐19 associated aspergillosis (CAPA): A comparison with influenza associated aspergillosis (IAPA). J Infect Dis. 2021;224(10):1631‐1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


