Abstract
Acute coagulopathy, specific placental pathology, and an increased risk of fetal death have been reported in pregnant women with COVID‐19; however, the association between coagulopathy and fetal death remains unknown. We report two pregnant women with COVID‐19 who showed acute coagulopathy prior to fetal death. Both pregnant women presented with thrombocytopenia after testing positive for SARS‐CoV‐2 (days 5 and 7). They had mild symptoms, but coagulopathy progressed, and their fetuses died on day 9 at 27 and 22 weeks of pregnancy. Their coagulability improved after delivery. Placental histology in both cases showed intervillous infiltration of histiocytes, necrosis of trophoblasts, and intervillous fibrin deposition, which were consistent with previously reported pathological findings related to SARS‐CoV‐2. In the management of pregnant women with COVID‐19, thrombocytopenia may be a predictive marker of fetal death following coagulopathy and placental inflammatory changes due to SARS‐CoV‐2 infection.
Keywords: hematologic and clotting, obstetric infections, placental pathology, stillbirth
Introduction
The coronavirus disease 2019 (COVID‐19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has spread to pregnant women worldwide. The impact of COVID‐19 on pregnant women was unclear at the beginning of the pandemic but is now becoming more apparent. Recently published studies and systematic reviews have reported the increased risk of stillbirth associated with maternal SARS‐CoV‐2 infection, 1 , 2 , 3 and the characteristic pathological findings of placentas delivered from pregnant women with COVID‐19 are becoming clear. 4 However, although acute coagulopathy has been reported in pregnant women with COVID‐19, 5 the association between coagulopathy and fetal death remains unknown.
Here, we report two pregnant women with COVID‐19 whose fetuses died following the onset of acute coagulopathy and whose placentas showed characteristic pathological findings due to COVID‐19. Then, we discuss the clinical course of fetal death following coagulopathy in pregnant women with COVID‐19. Both patients provided approval of the report, and their anonymity is protected.
Cases
Case 1
A 28‐year‐old Japanese woman (gravida 3, para 2) who had undergone two cesarean sections was pregnant with her third child and had a platelet count of 270 × 109/L (reference range for pregnant women [RR], 6 155 to 409 × 109/L) at 10 weeks and 3 days of pregnancy. On presentation at a general hospital, she had a fever of 39°C at 26 weeks and 0 days of pregnancy and was positive for SARS‐CoV‐2 (day 0). At 26 weeks and 4 days of pregnancy (day 4), she visited an outpatient obstetrics clinic, and a cardiotocogram showed uterine contractions and no abnormalities in the fetal heartbeat. At 27 weeks and 0 days of pregnancy (day 7), she was admitted to the respiratory unit of the general hospital because her fever rose to 40°C, and she developed a cough, nasal discharge, and abnormal taste. Blood examination revealed that platelet count, prothrombin time (PT), activated partial thromboplastin time (APTT), and D‐dimer were 90 × 109/L, 12.3 s (RR, 9.5–13.5 s), 46.9 s (RR, 24.2–38.1 s), and 9.1 μg/mL (RR, <1.29 μg/mL), respectively (Figure 1). Obstetrical examination, ultrasonography, and cardiotocogram were not performed during hospitalization. She felt decreased fetal movement at 3 a.m. at 27 weeks and 3 days of pregnancy (day 9) and was examined by an obstetrician at 7 a.m., who confirmed that the fetus had died. Blood examination revealed that the platelet count, PT, APTT, fibrinogen, and D‐dimer were 44 × 109/L, 13.6 s, 53.1 s, 44 mg/dL (RR, 291–538 mg/dL), and 48.3 μg/mL, respectively (Figure 1). She was diagnosed with disseminated intravascular coagulation (DIC) and was therefore referred to Kumamoto University Hospital.
FIGURE 1.
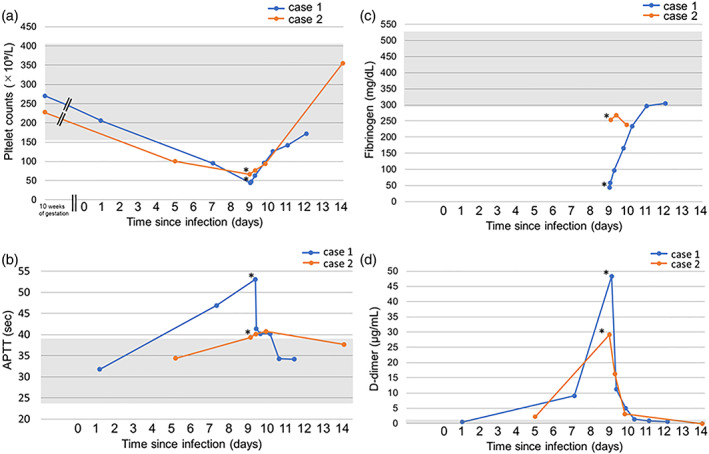
Temporal change in laboratory parameters for coagulation. (a) Platelet count: Platelet count continues to decrease after SARS‐CoV‐2 infection compared to the values at 10 weeks of gestation and increases after delivery in both cases. (b) Activated partial thromboplastin time (APTT): APTT is transiently prolonged with a peak on the day of delivery, similar to the trends in platelet count, in both cases. (c) Fibrinogen: Fibrinogens were below the normal levels for pregnant women in both cases. (d) D‐dimer: D‐dimer transiently increased with a peak on the day of delivery, similar to the trends in platelet count and APTT, in both cases. Asterisks indicate the time of fetal death. Gray zones indicate the normal range of each parameter 6
There were no findings suggestive of placental abruption or HELLP syndrome. As the cause of progressive DIC was unknown, the patient underwent a cesarean section after blood transfusion of 8 units of fresh frozen plasma (FFP) and 20 units of platelet concentrates (PC) on day 9. A 1040‐g stillborn baby showing a normal appearance was delivered, and the amount of blood loss was 790 g during the operation. Two units of red cell concentrate, 12 units of FFP, and 20 units of PC were required throughout the intraoperative and postoperative periods, and her coagulability improved. In the placenta, significant infiltration of histiocytes into the intervillous space, degeneration and necrosis of trophoblasts, and frequent fibrin deposition were histologically observed (Figure 2a, b). The presence of SARS‐CoV‐2 was confirmed in the syncytiotrophoblasts of the villi (Figure 2c).
FIGURE 2.
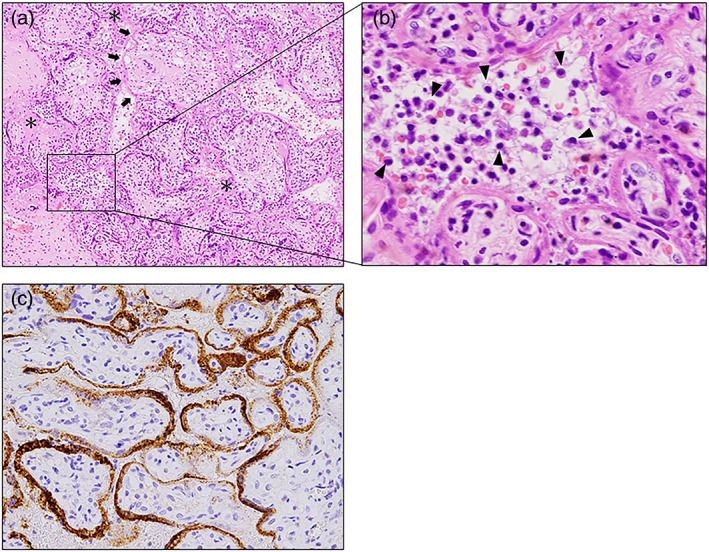
Histological findings of the placenta in case 1. (a) Intervillous fibrinoid depositions are frequently observed (asterisks). Necrosis of trophoblasts that were homogeneously stained with eosin is also observed (arrows). Hematoxylin–eosin (HE), ×20. (b) Variable polymorphous inflammatory infiltrates composed mainly of histocytes are observed in the intervillous spaces (arrowheads). HE, magnified view of the square in a, ×400. (c) Immunohistochemical staining for the SARS‐CoV‐2 spike protein using anti‐SARS‐CoV/SARS‐Cov‐2 (COVID‐19) spike antibody (1A9). The expression of SARS‐CoV‐2 spike protein is observed in the syncytiotrophoblasts of the villi ×100
Case 2
A 35‐year‐old Japanese woman (gravida 3, para 2) was pregnant with her third child and had a platelet count of 235 × 109/L at 10 weeks and 2 days of pregnancy. She had no symptoms and was screened for COVID‐19 because she had been in contact with a colleague with COVID‐19. Her test result at a public health institution was positive for SARS‐CoV‐2 at 20 weeks and 5 days of pregnancy (day 0), and she was directed to be hospitalized in a general hospital that does not have an obstetrics department. At 21 weeks and 3 days of pregnancy (day 5), she complained of a fever of 39°C, joint pain, headache, and fatigue and received blood examination. Her platelet count, PT, APTT, and D‐dimer were 107 × 109/L, 11.9 s, 34.9 s, and 2.8 μg/mL, respectively (Figure 1). She felt decreased fetal movement, and her attending physician confirmed a fetal heartbeat using transabdominal ultrasonography at 21 weeks and 5 days of pregnancy (day 7). Because she began labor at 22 weeks and 0 days of pregnancy (day 9), she was referred to a maternity hospital. Fetal death was confirmed, and her platelet count was 90 × 109/L. She was referred to Kumamoto University Hospital because of coagulation abnormalities of unknown cause.
The patient vaginally delivered a 365‐g stillborn baby showing a normal appearance immediately upon arrival. Blood examination revealed that platelet count, PT, APTT, fibrinogen, and D‐dimer were 73 × 109/L, 11.3 s, 39.7 s, 255 mg/dL, and 29.6 μg/mL, respectively (Figure 1). Coagulation abnormalities improved without the need for treatment after delivery. The placenta histologically showed infiltration of histiocytes into the intervillous space, degeneration and necrosis of trophoblasts, and prominent fibrin deposition (Figure 3).
FIGURE 3.
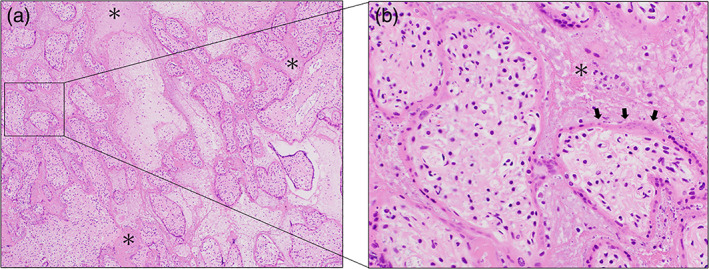
Histological findings of the placenta in case 2. (a) Deposits of fibrinoids are prominently observed in the intervillous spaces (asterisks) HE, ×20. (b) Fibrin deposition (asterisks) and diffuse damage of trophoblasts with necrosis (arrows) are observed in the intervillous spaces. HE, magnified view of the square in a, ×400
Discussion
Here, we first report two similar cases of pregnant women with COVID‐19 who demonstrated thrombocytopenia and subsequent placental inflammatory changes resulting in fetal death in sequence. These findings suggest that coagulopathy may be induced by the placental inflammatory changes caused by SARS‐CoV‐2.
COVID‐19 during pregnancy has been reported to increase maternal and neonatal complications, including preeclampsia, preterm birth, gestational diabetes, and fetal growth restriction. 1 Furthermore, COVID‐19 has recently been reported to be associated with a more than a twofold increased risk of stillbirth. 1 , 2 , 3 In a study of 3527 pregnant women with COVID‐19 conducted in England, stillbirth was reported with a statistically significant increased odds ratio of 2.21 (95% CI: 1.58 to 3.11). 2 A review of 42 articles including 7590 pregnant women with COVID‐19 also showed that the rate of stillbirths was significantly high (odds ratio 2.11, 95% CI: 1.14 to 3.90). 3
It is obvious that stillbirths are more common in pregnant women with COVID‐19, and the possible association between placental pathology and poor pregnancy outcomes is gradually becoming evident. In the analysis of nine placentas that were positive for SARS‐CoV‐2 by immunohistochemistry and PCR, intervillous inflammatory infiltrates composed mainly of histiocytes, villous trophoblast necrosis, and intervillous fibrinoid deposition were specific pathological findings of placental infection with SARS‐CoV‐2. 7 These pathological features were localized to diffuse, and their range was associated with neonatal mortality. 7 Another study suggested that two pathological findings of the three described findings, namely, histiocytic intervillositis and villous trophoblast necrosis, were specific to infection of syncytiotrophoblasts by SARS‐CoV‐2. In this study, the placentas of five stillborn cases were all characterized by a marked increase in intervillous fibrin deposits, which suggests that intervillous fibrin deposits may cause imperfect placental circulation leading to fetal death. 4 In general, intervillous fibrin deposits tend to be associated with maternal autoimmune diseases and are accompanied by severe fetal growth restriction and a high risk of fetal death. 8 In the present two cases, placental changes induced by COVID‐19 might have caused fetal death.
The clinical features of coagulation abnormalities, including DIC and thrombotic microangiopathy, that occur in the general population with COVID‐19 are gradually becoming clear 9 ; however, few articles have described coagulopathies related to pregnant women with COVID‐19. Therefore, the clinical course of coagulation abnormalities in pregnant women with COVID‐19 remains unknown. One article reported that two pregnant women with COVID‐19 presented with acute progressive coagulopathy with thrombocytopenia, elevated D‐dimer, and decreased fibrinogen at 35 weeks of gestation. Both women underwent a cesarean section and delivered a healthy newborn 3 days after testing positive for SARS‐CoV‐2, and the coagulopathy improved after delivery. 5 Another article reported that delivery was planned for two pregnant women with COVID‐19 because of progressive thrombocytopenia at 35 and 37 weeks of gestation. They delivered a healthy newborn, and their placental pathology showed histiocytic intervillositis, villous trophoblast necrosis, and fibrin deposition. However, a relation between thrombocytopenia and placental changes was not discussed in the article. 4 In the present two cases, coagulation disturbance improved after delivery of the placenta, which suggests that placental histological changes may result in maternal coagulopathy. Laboratory parameters for coagulation, including platelet counts, APTT, fibrinogen, and D‐dimer, showed abnormal values at the time of fetal death. However, these four parameters were not measured every day after the infection in both cases; therefore, which parameter could be the most predictive marker remains unknown. From the limited data available, the platelet counts were the first to clearly deviate from the reference range in both cases after testing positive for SARS‐CoV‐2 (Figure 1). Therefore, thrombocytopenia could be a predictive marker of possible changes in placental function and fetal status when managing pregnant women with COVID‐19.
Specific placental changes related to COVID‐19 are thought to be the result of an immune response rather than direct infection with SARS‐CoV‐2. In the analysis of 28 placentas that were obtained from mothers with COVID‐19, including 44% asymptomatic mothers, only 13% were positive for SARS‐CoV‐2. In addition, placental NK and T cells were activated, and interferon‐related genes were highly expressed in two placentas that were negative for SARS‐CoV‐2 through single‐cell transcriptomic analyses. 10 Regardless of the severity of the clinical symptoms, an immune response may occur at the maternal‐fetal interface, and placental functions and fetal status can be changed if the time of the immune response is longer. In the present two cases, fetal death may have been due to the long observation time of 10 days after testing positive for SARS‐CoV‐2 despite the decrease in platelets.
In conclusion, when managing pregnant women with COVID‐19, thrombocytopenia may be a predictive marker of fetal death following coagulopathy and placental inflammatory changes due to SARS‐CoV‐2 infection. Maternal platelets as well as fetal status should be evaluated at least once after testing positive for SARS‐CoV‐2 in the outpatient clinic, even if the clinical symptoms are mild.
Conflict of Interest
None declared.
Author Contributions
Akihito Sagara and Munekage Yamaguchi collected and analyzed data and wrote the manuscript. Yoshiki Mikami made the pathological diagnosis. Akihito Sagara, Munekage Yamaguchi, Takeshi Motohara, Takashi Ohba, and Eiji Kondoh were involved in diagnosis and treatment of the patients. Eiji Kondoh provided supervision. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID‐19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gurol‐Urganci I, Jardine JE, Carroll F. Maternal and perinatal outcomes of pregnant women with SARS‐CoV‐2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021;225(5):522.e1–522.e11. 10.1016/j.ajog.2021.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei SQ, Bilodeau‐Bertrand M, Liu S, Auger N. The impact of COVID‐19 on pregnancy outcomes: a systematic review and meta‐analysis. CMAJ. 2021;193:e540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwartz DA, Baldewijns M, Benachi A. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID‐19) and intrauterine maternal‐fetal severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) transmission in live‐born and stillborn infants. Arch Pathol Lab Med. 2021;145:517–28. [DOI] [PubMed] [Google Scholar]
- 5. Vlachodimitropoulou Koumoutsea EV, Vivanti AJ, Shehata N. COVID‐19 and acute coagulopathy in pregnancy. J Thromb Haemost. 2020;18:1648–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abbassi‐Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009;114:1326–31. [DOI] [PubMed] [Google Scholar]
- 7. Garrido‐Pontnou M, Navarro A, Camacho J, et al. Diffuse trophoblast damage is the hallmark of SARS‐CoV‐2‐associated fetal demise. Mod Pathol. 2021;34:1704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devisme L, Chauvière C, Franquet‐Ansart H, et al. Perinatal outcome of placental massive perivillous fibrin deposition: a case–control study. Prenat Diagn. 2017;37:323–8. [DOI] [PubMed] [Google Scholar]
- 9. Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID‐19. Lancet Haematol. 2020;7:e438–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu‐Culligan A, Chavan AR, Vijayakumar P, Irshaid L, Courchaine EM, Milano KM, et al. SARS‐CoV‐2 infection in pregnancy is associated with robust inflammatory response at the maternal‐fetal interface. medRxiv. 2021. 10.1101/2021.01.25.21250452 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


