Introduction
I was deeply honored to be recognized with the 2018 MSACL Award for Distinguished Contribution to Clinical Mass Spectrometry and was privileged to be allowed to share my perspectives in an award lecture entitled “The Triple Quadrupole: Innovation, Serendipity and Persistence”. And I am excited to be able to reprise that topic in this paper for JMSACL.
The growth of mass spectrometry applications in the clinical laboratory has been enabled in part by the widespread availability of computer-controlled tandem mass spectrometers, particularly the triple quadrupole. More broadly, one can hardly name a significant advancement in science that was not made possible by the development of a tool to see something or measure something, and that includes everything from litmus paper to microscopes to giant telescopes on mountain tops to the triple quadrupole. I welcome this opportunity to celebrate the analytical instruments and techniques that make possible great science, including modern clinical chemistry. Here I explore the evolution of tandem mass spectrometry (MS/MS) including a personal perspective on the conceptualization and development of the triple quadrupole, with an emphasis on the role of innovation, serendipity, and persistence. I hope that this perspective offers readers insight into the development of analytical methods and instruments in clinical chemistry and in a world of other application areas.
When thinking about the development of a new analytical instrument or method, I find it useful to refer to an editorial published 50 years ago by Herb Laitinen, the Editor of Analytical Chemistry, as illustrated in Table 1 [1].
Table 1.
The seven ages of an analytical method, from an Analytical Chemistry editorial by Herb Laitinen [1].
| The Seven Ages of an Analytical Method |
|---|
|
|
|
|
|
|
|
Note that the idea of seven ages (of an analytical method) was borrowed by Herb from the seven ages of man, as enumerated by William Shakespeare in As You Like It, Act II, Scene VII – “All the world’s a stage, and all the men and women merely players; they have their exits and their entrances; and one man in his time plays many parts, his acts being seven ages”.
In his typical insightful fashion, Herb provided a roadmap for the evolution of new instruments and methods. The discussion below follows these seven ages for the development of analytical tandem mass spectrometry.
The seven ages of analytical MS/MS
1st age – Conception of fundamental principles
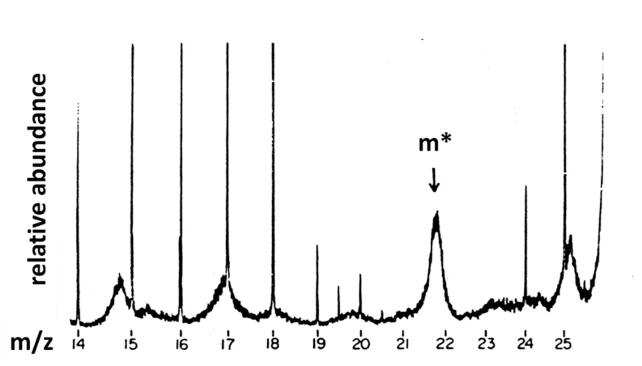
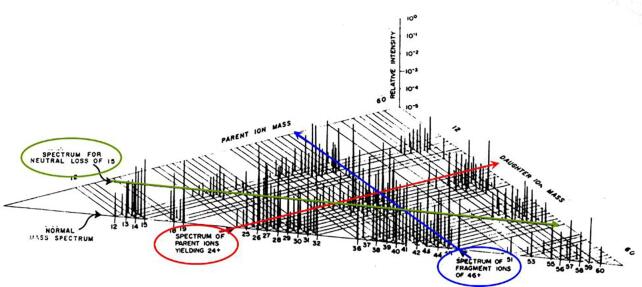
The fundamental principles of MS/MS were first shown in the observation of “metastable peaks” (very broad peaks at non-integer m/z values) in high resolution mass spectra obtained on sector instruments, as shown in Fig. 1 [2]. These diffuse peaks, much broader than other peaks and falling at non-integer values of m/z, arise from unimolecular decomposition of ions in the field-free regions of a magnetic sector mass spectrometer (which may include an electric sector). Whereas a stable ion () would make it all the way from the ion source to the detector intact, and an unstable ion would have enough internal energy to decompose (to form a fragment ion ) in the ion source before being accelerated into the mass analyzer, a metastable ion would decompose after being accelerated to form a fragment ion, , and a neutral, m3:
Fig. 1.
Part of a mass spectrum between m/z 14 and 26 showing a variety of diffuse (metastable) peaks; adapted from [2].
If the sector mass analyzer simply separated ions according to their mass-to-charge ratio, m/z, then this fragment ion would show up the same as a fragment ion formed in the ion source (m2+). But magnetic and electric sectors do not sperate ions simply by m/z; rather, the ion velocity, v, is also involved. Magnetic sectors separate ions according to their momentum, mv/z, and electric sectors separate ions according to their kinetic energy, ½mv2/z. Thus, an ion m1+ which fragments to form m2+ before passing through a magnetic sector will fall at an apparent m/z (m*) which is determined by its mass (which is now m2) and its velocity (which is essentially the same as the velocity of m1, assuming that the unimolecular dissociation did not have much effect on the ion velocity). Experimentally, it is observed that a fraction of the internal energy of m1+ may be converted into translational degrees of freedom of the products m2+ + m3, resulting in peaks that are significantly broadened in velocity, and therefore in momentum and kinetic energy. This results in a broad peak that will pass through the magnetic sector at an apparent m/z value m* = m22/ m1, as shown in Fig. 1.
Although these metastable peaks provided confirmation of a particular fragmentation path (m1+ → m2+), they were often considered more of a nuisance than an asset, and indeed some sector instruments included “metastable suppressors” to remove them from the mass spectrum (possible since they had less than full kinetic energy) [3]. But these metastable peaks also offered insight into the fundamentals of ion fragmentation and spawned the development of ion kinetic energy spectroscopy (IKES) and mass-analyzed ion kinetic energy spectroscopy (MIKES) to explore those insights [4]. As one example, MIKES revealed various shaped (including dish-top) metastable peaks for the loss of NO from substituted nitrobenzene ions [4]. It was also appreciated that some of these ions arose not from unimolecular dissociation but rather from collision-induced dissociation (CID) with background gas in the vacuum system. Early experiments to enhance CID included Keith Jennings loosening a flange or baking the flight tube to increase the pressure and increase collisions [5]; it has been rumored that John Beynon simply drilled a small hole into his vacuum chamber in his impatience to observe CID. Ultimately, collision cells were added to these instruments to enable improved CID and to study ion–molecule reactions [4]. While one could write a treatise on fundamental studies using MS/MS (and indeed there are many available [5], [6]), our focus here is on analytical MS/MS, so let’s move on.
2nd age – Experimental validation of analytical potential
Although tandem mass spectrometry (note that the term MS/MS had not yet been coined) was of significant interest for the fundamental information it provided [5], how was it discovered by analytical chemists, and how was its analytical potential validated?
Graham Cooks at Purdue and others recognized the potential of these metastable peaks for direct mixture analysis, without prior sample clean-up or separation. For example, Rich Kondrat and Graham employed MIKES for direct analysis of alkaloids in plant tissue without sample preparation or chromatography [7]. In one application, leaves and stems from poison hemlock (Conium macula L.) were analyzed for the alkaloid coniine. Plant material was simply crushed and introduced into the ion source together with ammonium acetate as a source of the reagent gas NH3. The MIKE spectrum of m/z 128 identified the ion as protonated coniine based on its fragmentation pattern and by comparison with the MIKE spectrum of the same ion in a coniine standard. As the authors stated [7]:
“Perhaps the single most exciting feature of the MIKES methodology is the ability to identify components of complex mixtures… without any prior chemical separation whatsoever.”
Needless to say, not every mass spectrometrist embraced these new ideas. For instance, Dai Games from University College Cardiff pointed out [8]:
“In the euphoria of discovering a new way of putting a mass spectrometer together, the advantages are typically highlighted, and drawbacks… tend to be overlooked or deemphasized.”
And champions of these new tandem mass spectrometry instruments and methods, including Graham Cooks, responded [9]:
“When people are given something they manifestly did not ask for and certainly do not appreciate, they tend to act unkindly. Especially so if they are experts whose own work is about to be encompassed within the emerging work. An early criticism of tandem mass spectrometry was that, because mass spectroscopists do not understand simple mass spectra, they are ill-advised to add to their difficulties.”
These early practitioners of MS/MS clearly demonstrated the analytical potential of these new instruments and methods, both for mixture analysis [7], [9] and for structure elucidation [5], [6]. But for these techniques to enter the analytical mainstream, new MS/MS instruments were needed, and they had to become widely available.
3rd age – instrumental developments/availability
Although the earliest instruments for MS/MS were sector mass spectrometers (by far the most common kind of mass spectrometer up through the 1970s), the development of MS/MS, and its interface with chromatography and the potential for computer control, offered new opportunities for instrument development. And this is the point in the seven ages where I entered the picture. I like to think of these as the days of “do it yourself mass spec.”
My interest in mass spectrometry was piqued while an undergraduate Chemistry major at the University of Arizona in the early 1970s. Back then, most mass spectrometers were large, clunky instruments, without any sign of computer control or data acquisition. They featured big electromagnets and were designed to make measurements in physics or physical chemistry, or to elucidate the structure of a purified organic compound, but were not widely used for analytical chemistry. Although our department didn’t have a functioning mass spectrometer, the atmospheric analysis lab on campus where I worked one summer did have a mass spec, with a gas chromatograph - Hewlett Packard’s first quadrupole GC/MS system, a 5930A. It even had a data system that used audio cassette tapes for data storage. Unfortunately, neither the mass spec nor the data system was working, and we were not successful getting any data on that system over the summer. But my undergraduate studies (including a course in computer interfacing plus research with Mike Burke in digital simulation of gas chromatography) established my interest in computers and electronics to enable new analytical instruments!
In 1975 I started graduate studies in Analytical Chemistry at Michigan State University. Because I was interested in the role of computers and electronics in advancing analytical chemistry, I chose to work with Chris Enke, who had a remarkable “big picture” view of the field. But he was an electrochemist, and I thought electrochemistry was black magic. Nevertheless, Chris said I could join his group. I told Chris that I wanted to develop the “ultimate computerized mass spectrometer,” and that it should have a quadrupole in it! Where did that come from? In my undergraduate instrumental analysis lecture, Bonner Denton had passed around a quadrupole, a new kind of mass analyzer that was far more attractive for computer control than a huge electromagnet! Bonner also told us about his double quadrupole instrument for GC/MS, using an RF-only quadrupole as a notch filter in front of the quadrupole mass filter to remove He+ ions formed in the electron ionization source [10].
Driving home late at night from the 1975 FACSS meeting in Indianapolis, Chris and I dreamt up the idea of a computer-controlled tandem quadrupole MS/MS system. Chris suggested that I write a proposal that we could submit to the National Science Foundation (NSF). Being a fearless first-year graduate student, I did just that. In the proposal, we discussed two major applications for the computer-controlled MS/MS instrument, structure elucidation and mixture analysis. Boldly, I wrote “The ability to control the acquisition of tandem mass spectral data in real time in order to answer a chemical question rapidly and with confidence will be a big step toward the goal of the ultimate system for chemical analysis” [11].
The proposal reviews were not great…

“The proposal indicates a serious lack of familiarity with mass spectrometry, and there is little chance that the instrument will produce useful data.”
“It is doubtful that the proposed instrument offers any real advantages over sector instruments.”
“Experience with tandem mass spectrometers indicates that computer control is impractical.”
“The author of this proposal has no real experience in mass spectrometry, and I am skeptical that he can really develop the proposed instrument. It is my opinion that the studies proposed here could be better pursued by researchers already active in the field.”
“The quadrupole isn’t a real mass spectrometer – it’s just a toy.”
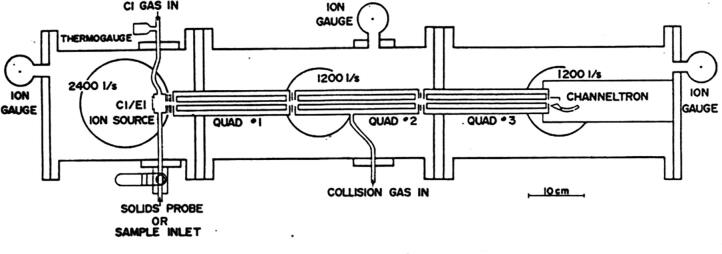
Not dissuaded, Chris and I sent a copy of the proposal to the Office of Naval Research (ONR). The Navy had mass spectrometers onboard their nuclear submarines to monitor the atmosphere, and they could imagine how the triple quadrupole instrument could improve those analyses. Furthermore, ONR had a lot of interest in funding the development of computer-controlled analytical instruments, and so they funded the proposal. I bought 2,000 lb of stainless steel and a lot of electronics and some mass spec components, and we started constructing the instrument we had envisioned, as shown in Fig. 2.
Fig. 2.
Scale drawing (top view) of the triple quadrupole MS/MS instrument proposed to the NSF and ONR. [11].
Chris and I attended the 1977 Conference of the American Society for Mass Spectrometry, where we discussed our idea with a number of well-known mass spectrometrists. None of them thought the instrument would work, particularly our proposal to fragment ions in the center (RF-only) quadrupole at low (∼10 eV) collision energies in contrast to the high (keV) collision energies used in sector MS/MS instruments. But we ran into Jim Morrison of LaTrobe University in Australia, whom Chris had met many years earlier when Jim was on sabbatical at Princeton. Jim was the first mass spectrometrist to think that the instrument would work! Why? Jim and his student, Don McGilvery, had built (but not yet published) a triple quadrupole instrument for optical spectroscopy of mass-selected ions using a tunable dye laser (not for mass spectrometry – indeed, they had never obtained a mass spectrum with the instrument). They would mass-select an ion in the first quadrupole, then pass it into the second (RF-only) quadrupole where it would interact with a laser beam. The absorbance of the light was much too small to measure, but the internal energy from absorbing a photon could be enough to induce photodissociation, and the resulting fragment ion could be detected with the third quadrupole and ion detector. They would scan the laser wavelength (over several hours) and deduce the absorption spectrum of the mass-selected ion by plotting the fragment ion intensity vs. time (and therefore vs. laser wavelength). Even at 10−7 Torr, the lowest pressure they could achieve in the center quadrupole, they observed far more collision-induced than photo-induced fragmentation, and that’s just what we wanted for our MS/MS experiments. Jim invited me to visit his lab to perform some preliminary experiments, since our instrument at MSU was still under construction. I contacted our ONR program officer to see if I could hop a ride on a Navy ship heading across the Pacific, but he agreed to add travel funds to cover the visit, and it was a significant expense in 1977 (my airfare was equivalent to nine months of my graduate student stipend!).
When I first arrived at La Trobe, Jim showed me their triple quadrupole instrument. It had a simple inlet for gases, an EI source, two home-built quadrupole mass filters with a mass range to m/z 100, a center quadrupole with a cold trap to reduce the pressure to ∼10−7 Torr, and a lot of manual electronics (with vacuum tubes!). A tunable dye laser was used to photodissociate ions in the center quadrupole. They had implemented computer control of the laser wavelength and data acquisition, but not of the quadrupoles. I asked Jim how to obtain a mass spectrum, and he replied, “We’ve never done that - all our experiments have monitored a single photodissociation fragment ion from a single parent ion”. Jim guessed that I should simply “turn this knob” (the one he’s turning in Fig. 3) at ¼ rpm for ∼ 20 min to get a mass spectrum from m/z 10 to 100. “And if I wanted to scan the first quadrupole?” He pointed to another knob. I turned that knob for 20 min and obtained the first mass spectrum on that instrument, using an XY recorder to record the mass spectrum (ion signal vs. time). I then visited the machine shop, where I scrounged a ¼ rpm synchronous motor and a ring stand and lab stool. I connected the motor to the knob with a piece of Tygon tubing, started the motor, and went for a cup of tea! To perform CID, I introduced gas into the center quadrupole up to a pressure of ∼ 2 × 10−4 Torr. An example of one of those MS/MS spectra is shown in Fig. 4.
Fig. 3.
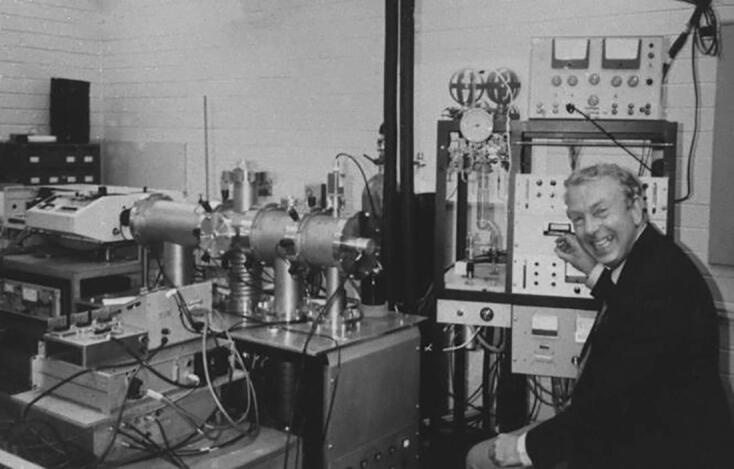
Jim Morrison at the controls of the LaTrobe triple quadrupole instrument in 1977. [12].
Fig. 4.
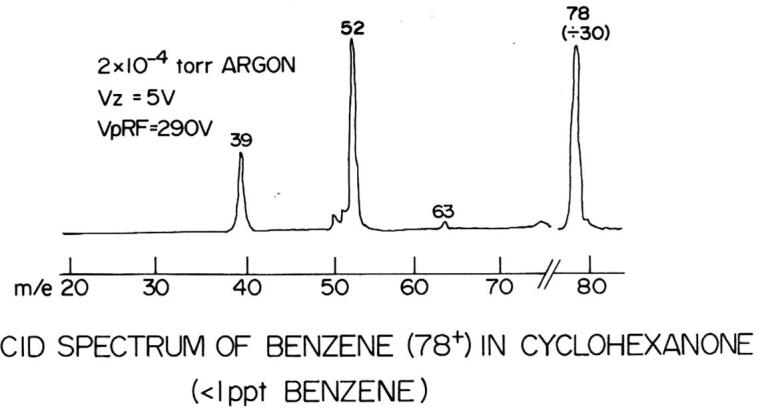
MS/MS spectrum of m/z 78 of a trace contaminant (benzene) in a sample of cyclohexanone, obtained on the LaTrobe triple quadrupole instrument.
A couple of months as a guest in Jim’s lab led to the first triple quadrupole MS/MS publications - a brief communication on “selected ion fragmentation with a tandem quadrupole mass spectrometer” by myself and Chris that appeared in JACS in 1978 [13] and a more detailed paper on “high efficiency collision-induced dissociation in an RF-only quadrupole” in the International Journal on Mass Spectrometry and Ion Physics in 1979, including as coauthors Jim and his graduate students Don McGilvery and Dianne Smith [14]. The first paper from LaTrobe on photodissociation with the triple quadrupole instrument by Jim and Don was also published in 1978 [15]. The ONR also insisted that we file for a patent, and we did so, including Jim as an equal inventor [16]. Although we didn’t know it at the time, Marvin Vestal and Jean Futrell at the University of Utah had published a paper in 1974 describing a triple quadrupole instrument for photodissociation studies [17], based on discussions with Jim during his sabbatical there in 1971.
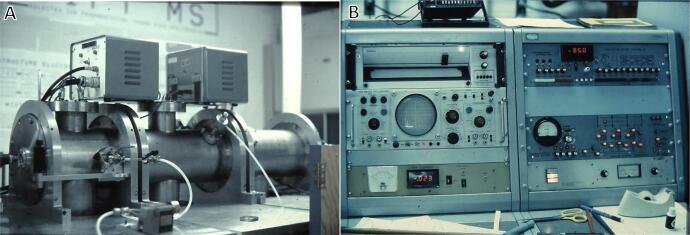
After that very productive stay in Australia, I returned to MSU to complete construction of our instrument, shown in Fig. 5. The vacuum chambers (Fig. 5A), table, and ion optics were all crafted in the MSU Chemistry machine shop; the quadrupole mass filters and power supplies were purchased from Extranuclear Labs, but we built our own quadrupole collision cell and its power supply. I also designed and assembled all the electronics for control of the vacuum system, ion optics, and data acquisition (Fig. 5B). The instrument was controlled and data were acquired by a Digital Equipment Corporation PDP 11/40 with 32kB of ferrite core memory. Quite the computer in 1978 (the $70 k price tag would be $0.5 M in today’s dollars), but far less powerful than the cell phone in your pocket today! Despite our plans to computerize the instrument, there were still lots of knobs, buttons, and dials, plus an oscilloscope and strip chart recorder, just in case!
Fig. 5.
Vacuum chambers (A) and electronics (B) of the triple quadrupole MS/MS instrument constructed at MSU.
There were of course many intriguing questions along the way for this new instrument concept. For instance, how to get an MS spectrum on an MS/MS system? Although others suggested that we add an extra ion detector after quadrupole 1, it was far simpler to use the standard detector after the third quadrupole by simply putting quadrupole 3 into RF-only mode. The resultant MS/MS and MS scan modes are illustrated in Fig. 6.
Fig. 6.
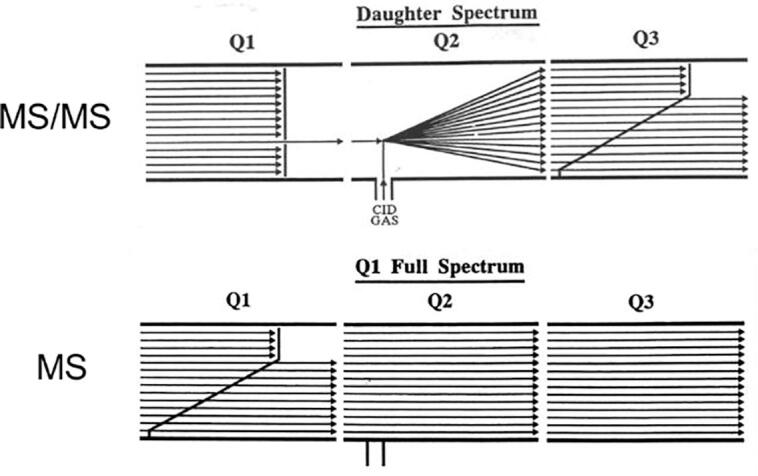
Scan modes for MS/MS (a daughter or product ion scan) and for MS on a triple quadrupole instrument.
I accepted a faculty position at the University of Florida in 1979, planning to build another triple quad. But my proposal to NSF was successful this time, and I was able to work with Finnigan Instruments (now Thermo) to get the first commercial triple quadrupole mass spectrometer. Although Finnigan estimated that the “worldwide market would be perhaps 10 instruments”, forty years later the triple quad is perhaps the world’s most widely used mass spectrometer, with over $1billion worth of instruments sold each year! The transition from the days of “do it yourself mass spec” to commercial availability of computer-controlled triple quadrupole MS/MS instruments (first by Finnigan, and subsequently by Sciex, Agilent, Waters, and others) opened the door for the remaining ages of MS/MS.
4th age – establishment of a solid fundamental foundation:
The development of new instruments and methods, as well as the widespread acceptance of these instruments and methods, is dependent upon the establishment of a fundamental foundation. Many labs have contributed to laying that foundation. Indeed, a lot of my experiments at LaTrobe addressed these issues of fundamentals for the triple quadrupole.
One of the major criticisms from reviewers of the triple quadrupole proposal was that collision-induced dissociation would be very inefficient at low ion kinetic energies, based on extrapolating the efficiency of CID at high kinetic energies on sector MS/MS instruments. Hence, one of the major questions we wanted to address in my LaTrobe experiments was whether we needed high collision energy to fragment ions for MS/MS. The maximum kinetic energy on the LaTrobe system was 20 eV, far less than the keV ion energies used in sector MS/MS. We wondered whether we could add enough kinetic energy to the ions by increasing the RF amplitude or frequency on the quadrupole collision cell, but neither increased the CID efficiency [14]. To help understand that observation, Jim suggested that we might use SIMION, a not-yet-published computer simulation program that he had conceived and Don McGilvery in his group had programmed. The insight we gained from those simulations [14] was invaluable in our research, presaging the remarkable role that SIMION has had in design and understanding of mass spectrometers and ion optics for the 40 years since.
The sector MS/MS community had found that lighter gases were more efficient for CID, but helium was “quite dear” in Australia, to use Jim’s terminology, so I tried hydrogen gas. But our experiments showed that more massive gases such as argon led to substantially higher CID efficiencies, up to 65%, in part because of the strong focusing of the CID product ions in the RF-only quadrupole [14]. And indeed, every modern tandem mass spectrometer, whether triple quad, time-of-flight, or FTMS, employs low-energy collision-induced dissociation. And needless to say, many other researchers contributed to establishing that fundamental foundation.
5th age – Widened scope of application
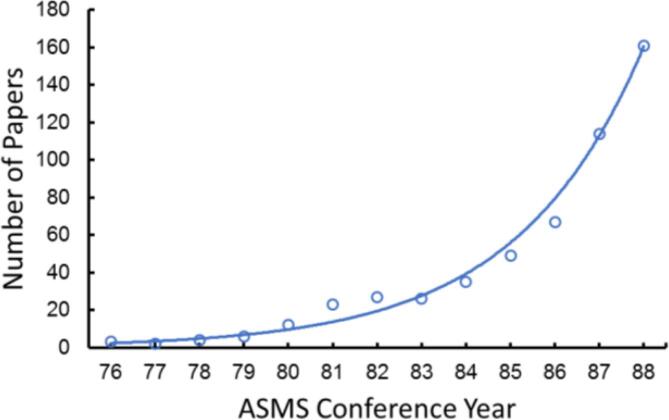
As Herb Laitinen had pointed out, an important step in the evolution of an analytical method is an increased range of applications, and that is well illustrated with MS/MS. In the early days, the growth of analytical MS/MS could be tracked by counting the number of conference presentations and published papers. Plotting the number of presentations at the annual ASMS conference from 1976 to 1988 shows an exponential growth, doubling every two years, as shown in Fig. 7. As a technique grows, however, it becomes harder to track, since the technique or instrument may no longer be reflected in the title.
Fig. 7.
Growth of analytical MS/MS papers at the ASMS Annual Conference from 1976 through 1988.
Our earliest publications explored two application areas, direct mixture analysis, as discussed above, and structure elucidation [18]. The dominant ionization method in the 1970s was electron ionization (EI), which already provided a wealth of information for structure elucidation; indeed, many textbooks were written on the subject, including Fred McLafferty’s classic text on mass spectral interpretation [19]. Nevertheless, adding a second stage of mass analysis provided additional insight to aid in elucidating the structure of an organic compound. The ultimate approach would be to select every ion in the EI spectrum of a compound, one at a time, and to obtain its daughter or product ion mass spectrum, as shown in Fig. 8.
Fig. 8.
Complete MS/MS data set for i-propanol [18].
However, MS/MS is most valuable for structure elucidation when the ionization method yields little or no structural info. The growth of soft ionization methods over the last thirty years, including electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), and matrix-assisted laser desorption ionization (MALDI), has been a huge driver of the interest in MS/MS. The widespread acceptance of LC/MS using ESI and APCI has been particularly important in the rapid growth of MS/MS, since these ionization sources generally do not provide any fragmentation information for compound identification.
The biggest scope of applications of MS/MS has been in the area of mixture analysis. Recall that the earliest interest in MS/MS by analytical chemists was for direct mixture analysis, “without any prior chemical separation whatsoever” [7]. But as we said a few years later [20]:

“Recent applications of MS/MS … have shown the wisdom of not giving up everything we have learned about sample extractions and chromatography, but rather making sensible trade-offs between the selectivity of MS/MS and the selectivity of sample preparation and separation.”
And indeed, the most common use of MS/MS today is in conjunction with chromatographic separation, both GC/MS/MS and LC/MS/MS, for trace mixture analysis. To understand the power of these tandem analytical methods, it is helpful to review what Fred McLafferty called the 4 S’s of trace analysis [21], as shown in Table 2.
Table 2.
The 4 S’s of trace analysis.
| Sensitivity | Change in instrument response for a change in analyte concentration |
| Selectivity | Freedom from interferences – “chemical noise” |
| Speed | Analysis time including sample preparation and separation |
| $ | Total cost of analysis |
High sensitivity is clearly important to be able to detect analytes at trace levels - if you want a detectable signal from an analyte at trace levels (small amounts or small concentrations), you clearly need high sensitivity. But in real samples (typically complex mixtures), high selectivity is just as important so that you can distinguish the analyte’s signal from that arising from interferences. When I was a grad student, extensive sample clean-up (extractions and column chromatography, all offline) were the most common ways of achieving that selectivity. But in modern trace analysis, tandem analytical methods such as GC/MS and LC/MS/MS have become the dominant analytical methods for trace mixture analysis. The role of such tandem methods for mixture analysis was explored in a great article by Graham Cooks and Ken Busch [22], as illustrated in Fig. 9. Assuming that “chemical noise” (the varying signal arising from chemical components in the sample matrix other than the target analyte, i.e., interferents) exceeds the instrumental noise, which is common in mass spectrometry, then additional stages of analysis (say two stages in LC/MS or three stages in LC/MS/MS) improve the signal-to-noise ratio and thereby improve the limits of detection.
Fig. 9.
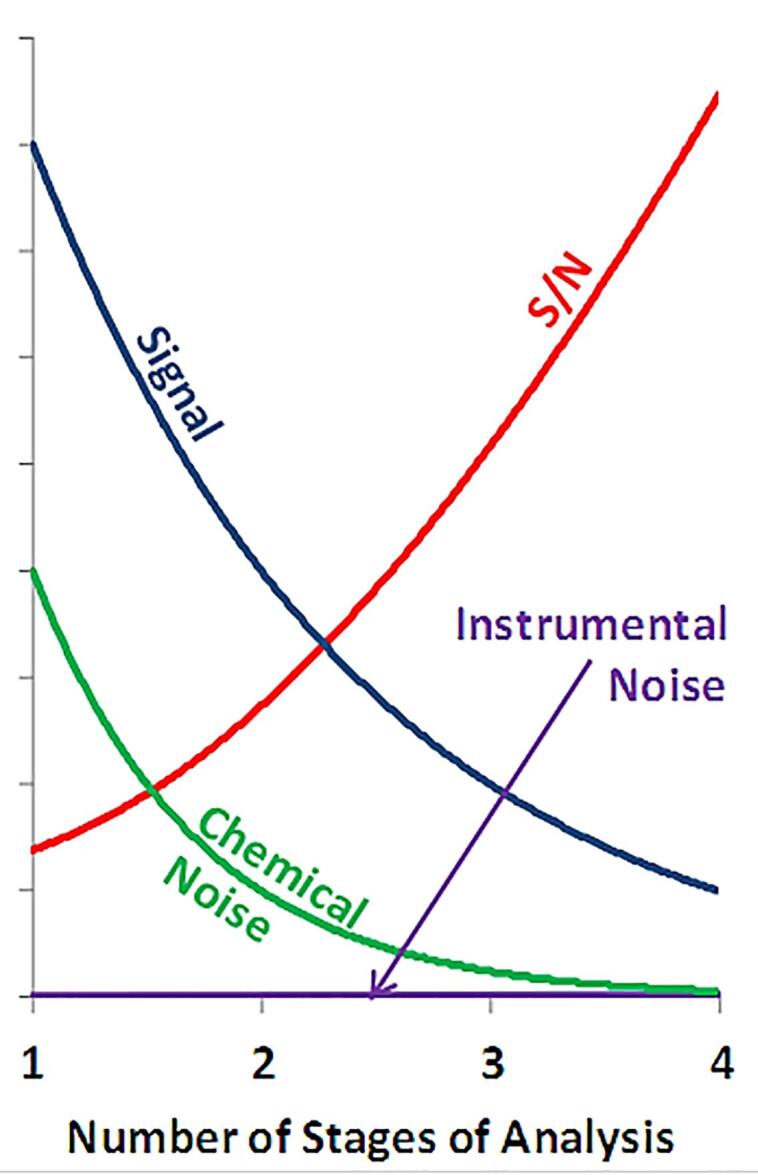
The effect of increasing numbers of stages of analysis on signal, chemical noise, and signal-to-noise ratio. Adapted from [22].
We can even think about adding more stages of analysis, for instance ion mobility separation. For more insights into the role of tandem analytical methods for trace analysis, see a recent tutorial [23]. The tremendous range of applications of tandem mass spectrometry has led to the widespread acceptance of MS/MS as a standard, routine analytical technique.
6th age – acceptance as a routine, standard method
Tandem mass spectrometry is being deployed today in almost every conceivable chemical and biological enterprise, from environmental analyses to drug discovery and development, to proteomics, metabolomics and clinical analysis, and beyond (food and personal care product development, petrochemical assays, forensic analysis, …). And the triple quadrupole mass spectrometer has become the “gold standard” for quantitative analysis in such applications. We can consider just a few of those application areas here.
For environmental analysis, the triple quadrupole provides the selectivity necessary to efficiently separate toxic substances from background chemical noise, and the sensitivity to detect them at the low concentrations present after being diluted with enormous volumes of water in lakes and rivers. The USEPA determined that triple quadrupole mass spectrometry is the most efficient and least costly technology available to monitor many of these compounds, including perfluorinated compounds (PFAS), in drinking water and in the environment [24], [25].
The triple quadrupole has even found widespread use in elemental analysis, in inductively coupled plasma (ICP/MS) [26]. Although an elemental (atomic) ion can’t be fragmented by CID in the collision quadrupole, polyatomic ions that fall at the same m/z can be fragmented or reacted to change their m/z [26]. One can also take advantage of the fact that interfering polyatomic ions will lose more kinetic energy in the collision cell than will atomic ions [27].
The triple quadrupole has streamlined drug discovery assays from months down to weeks, reducing drug development timelines by years, enabling improved therapies to reach patients at an unprecedented rate. Consider for instance the drug Rituximab, a remarkably effective treatment for non-Hodgkin’s lymphoma. Ron Levy, the co-discover of this drug at Stanford Medical School, used triple quadrupole LC/MS/MS systems in his lab in the discovery of Rituxan, leading to its development by Genentech Corp (which uses triple quadrupoles for quality control) [28], [29].
And finally, one simply has to attend the annual MSACL Conference to appreciate the central role of triple quadrupole MS/MS for clinical analysis. One reason for the increased interest in tandem mass spectrometry for such analyses is the lower limits of detection and quantitation that can be achieved compared to other clinical methods such as those based on immunoassays. Consider for example analyses for low levels testosterone. In a 2003 editorial highlighting the power of new LC/MS/MS methods for low levels of testosterone entitled “Immunoassays for Testosterone in Women: Better than a Guess?”, Dave Herold and Rob Fitzgerald famously observed about the immunoassays at low levels [30]:
“Are assays that miss target values by 200 –500% meaningful? Guessing would be more accurate and additionally could provide cheaper and faster testosterone results for females—without even having to draw the patient’s blood… Laboratory professionals should not be associated with a test where an educated guess would provide an equivalent or better result.”
That’s a pretty remarkable perspective on the contrast between MS/MS assays and immunoassays when the analyte is at low levels. And that perspective has helped lead to the development of clinical assays based on MS/MS for many analytes including testosterone [31].
Another example of an important clinical assay based upon LC/MS/MS is thyroglobulin, which can be measured as a marker for the recurrence of certain forms of thyroid cancer [32]. Some patients develop autoantibodies against thyroglobulin that may interfere with the analysis by immunoassays. For those patients LC/MS/MS is the method of choice. Although most clinical MS/MS methods are for small molecules such as metabolites, steroids, and drugs, the thyroglobulin assay was one of the first MS/MS assays for a protein in the routine clinical lab.
One of the best examples of the impact of the triple quadrupole is that it enabled the wide-scale screening of newborns in the US and throughout the world using samples collected by a simple heel-prick within 24 h of birth [33]. More than 10 million babies are screened annually worldwide, identifying serious or life-threatening inherited diseases in more than 10,000 newborns each year. Essentially every child born in the US, as well as in many other countries, has been tested in the first hours of their life with this instrument!
Clearly, the utility of MS/MS and the triple quadrupole extends far beyond clinical sciences, with widespread application in environmental analysis, proteomics, metabolomics, and even elemental analysis. And new and innovative applications are still being developed!
7th age – Senescence, overtaken by newer methods
Needless to say, the inventor of an analytical instrument or technique hopes that it never reaches age 7, as it is overtaken by newer instruments and methods. Although sector tandem mass spectrometers (and indeed sector mass spectrometers more broadly) were the dominant mass spectrometers 40 years ago, they have been almost totally replaced by triple quadrupoles and other mass spectrometers, most of them based on concepts and technologies developed in triple quadrupole instruments and techniques. It is fascinating that triple quadrupole instruments have dominated trace analysis by mass spectrometry for forty years.
Furthermore, the high-performance mass spectrometers of the 21st century are based upon the triple quadrupole platform, with the 3rd quadrupole replaced by a high-resolution mass analyzer such as a time-of-flight (TOF) or a Fourier-transform ion trap (either an Orbitrap or an ion cyclotron resonance analyzer). These hybrid instruments trade off the accurate mass capabilities of these high-resolution mass analyzers for the simplicity and quantitative performance of the third quadrupole of a triple quadrupole system.
Although the triple quadrupole remains the accepted standard for targeted quantitation in GC/MS/MS and LC/MS/MS, for untargeted (global or exploratory) analysis, where you want to identify new and unexpected compounds, tandem mass spectrometers that employ high resolution mass analyzers for the second stage of MS/MS can provide exact mass for unknowns, and that can be incredibly helpful. These new high resolution mass spectrometers have almost completely replaced the sector mass spectrometers that dominated mass spectrometry 40 years ago. And they incorporate the multipole collision cell and low-energy CID that are the heart of the triple quadrupole instrument.
Conclusions and perspectives
Innovation, serendipity, and persistence all play a key role in new science and new instruments, as is well illustrated in the development of the triple quadrupole MS/MS instrument. That development included many examples of innovation, including the use of quadrupoles for the mass analyzers in MS/MS, low-energy CID in an RF-only multipole collision cell, and the importance of computer control of a tandem mass spectrometer. And as is often the case, innovation builds upon the work of others that may not be immediately appreciated (as in my own case, learning about the quadrupole in an undergraduate lecture, including its use in RF only mode, and interest in computer control of analytical instruments). And young minds are often the most innovative, since they haven’t yet learned all the reasons why their innovative ideas “won’t work.” I think that there can be a big advantage of not being an expert in the field, steeped in all that knowledge, including some that is not correct (aka folklore, such as the belief that low-energy CID would never work).
We can explore innovation in the case of the triple quadrupole mass spectrometer. As noted above, experts in the field of mass spectrometry were convinced that the triple quadrupole mass spectrometer would never work. In their reviews of the NSF proposal, they pointed out three fundamental flaws in the proposed instrument: (1) First, that “it is doubtful that the proposed instrument offers any real advantages over magnetic sector instruments” (which at that time dominated the field of mass spectrometry). In particular, reviewers were critical of the new quadrupole mass analyzer, with some pointing out that it “wasn’t a real mass spectrometer, it was just a toy”. (2) Second, the experts in the field pointed out that “experience with tandem mass spectrometers indicates that computer control is impractical”, negating the central theme of that proposal to build a computer-controlled instrument. (3) Finally, reviewers were convinced that the proposal to fragment ions by low-energy collision-induced dissociation, with ion kinetic energies of tens of electron volts, would never work, since the other tandem mass spectrometers used high-energy CID, with ion kinetic energies of thousands of eV.
Let’s consider each of these innovations retrospectively, from today’s point of view. (1) We proposed to use the quadrupole mass filter rather than the common magnetic sector design because of its ease of computer control, its rapid scan speed, its compact size for inclusion in a tandem instrument, and its use as a focusing cell for efficient collisional-induced dissociation. Today, magnetic sector instruments are exceedingly rare, and the vast majority of commercial mass spectrometers are based on the quadrupole! (2) Although mass spectrometers in the 1970s were almost all manual instruments, all modern mass spectrometers are computer controlled, including advances such as autotuning and data-dependent scanning. Indeed, the projection that we wrote in that NSF proposal that “The ability to control the acquisition of tandem mass spectral data in real time to answer a chemical question rapidly and with confidence will be a big step toward the goal of the ultimate system for chemical analysis” was prophetic. (3) Key to the triple quadrupole invention was the development of low-energy CID in an RF-only multipole collision cell. Although experts in the field were convinced that it would never work, it turned out to be far more efficient than high-energy CID, and it was a key claim in the patent that we filed. Indeed, low-energy CID is the basis of every modern tandem mass spectrometer, whether triple quad, time-of-flight, or FTMS.
Ah, and the role of serendipity… First, my teaming up with Chris Enke for my PhD research. Chris brought a vision of a computer-controlled two-stage analytical instrument that would separate a sample mixture in one stage (but not by chromatography, since it would not be possible to randomly select analytes for the second stage) and then identify the components in the second stage. And I brought an interest in designing the “ultimate computerized analytical instrument” (actually I thought of it as “instrumentalizing” a computer) and wanted to incorporate the quadrupole mass filter that I had learned about as an undergrad. What a perfect opportunity for innovation! Even more serendipitous was our running into Jim Morrison at ASMS in 1977, whom Chris had met years before. Without any idea that Jim had already built a triple quadrupole instrument for optical spectroscopy, we described the triple quadrupole instrument we were building. Many readers will be able to identify similar moments of serendipity in their own scientific lives.
And finally, the importance of persistence. As noted above, persisting even when experts in the field were convinced our idea wouldn’t work. And persisting even when those experts, as proposal reviewers, meant that NSF didn’t fund our proposal. And there was certainly some serendipity as well in ONR deciding to fund the proposal.
There are still a lot of opportunities for innovation… embrace serendipity and be persistent!
If you’d like to read more about the triple quadrupole’s history and current status, you should check out Celia Henry Arnaud’s perspective article on the 40th birthday of the instrument [34]. And there’s also a video, commissioned by Agilent, in which Chris and I share our reminiscences on the roles of innovation, serendipity, and persistence in the development of the triple quadrupole [35].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Many individuals have made invaluable contributions to the work described here. First, Chris Enke at Michigan State, who welcomed a bold young graduate student into his research group, in spite of his perhaps crazy research ideas. And of course, the faculty at Arizona, including Mike Burke and Bonner Denton, who had helped plant some of those crazy ideas. Jim Morrison at LaTrobe extended a warm welcome to me and shared invaluable insights about mass spectrometry; I have to include graduate students at LaTrobe, including Don McGilvery and Dianne Smith. Many collaborators have helped drive that research over the past 40 years, most importantly the 100+ PhD students who have been part of my research group at the University of Florida. And none of this research would have been possible without resources provide by ONR, NIH, NSF, and other funding sources. I also acknowledge my family, especially my wife, Katie, who has supported my research and my life. Finally, I need to recognize MSACL for this award, and for helping spread the word about mass spectrometry for clinical analysis! I should also acknowledge the cartoons I’ve used to illustrate “the seven ages”; they were drawn by Leo Hershfield and appeared in books written 60 years ago by Richard Armour, including “Going Around in Academic Circles: A Low View of Higher Education” and “The Medical Muse Or, What to Do Until the Patient Comes”. I appreciate permission from Leo’s family to reproduce those figures here.
References
- 1.Laitinen H.A. The seven ages of an analytical method. Anal. Chem. 1973;45:2305. doi: 10.1021/ac60336a600. [DOI] [Google Scholar]
- 2.R.G. Cooks, J.H. Beynon, R.M. Caprioli, G.R. Lecter, Metastable Ions, Elsevier, Amsterdam, Figure 15 (p. 39), 1973.
- 3.Budzikiewicz H., Grigsby R.D. Half protons or doubly charged protons? The history of metastable ions. J. Am. Soc. Mass Spectrom. 2004;15:1261–1265. doi: 10.1016/j.jasms.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Cooks R.G., Beynon J.H. Metastable ions and ion kinetic energy spectrometry: the development of a new research area. J. Chem. Ed. 1974;51:437–443. doi: 10.1021/ed051p437. [DOI] [Google Scholar]
- 5.Jennings K.R. The changing impact of the collision-induced decomposition of ions on mass spectrometry. Int. J. Mass Spectrom. 2000;200:479–493. doi: 10.1016/S1387-3806(00)00325-0. [DOI] [Google Scholar]
- 6.Shukla A.K., Futrell J.H. Tandem mass spectrometry: dissociation of ions by collisional activation. J. Mass Spectrom. 2000;35(9):1069–1090. doi: 10.1002/1096-9888(200009)35:9<1069::AID-JMS54>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Kondrat R.W., Cooks R.G. Direct analysis of mixtures by mass spectrometry. Anal. Chem. 1978;50:81A–92A. doi: 10.1021/ac50023a781. [DOI] [Google Scholar]
- 8.Games D.E. Combined liquid chromatography/mass spectrometry (LC/MS) Finnigan Spectra. 1983;9(1):2. [Google Scholar]
- 9.Cooks R.G. Creativity through instrumentation. Anal. Chem. 1985;57:823A–843A. doi: 10.1021/ac00284a789. [DOI] [Google Scholar]
- 10.R.E. Reinsfelder, PhD Dissertation, The Development of New Quadrupolar Mass Spectrometric Techniques and Their Application to Chemical Analysis, University of Arizona, 1977.
- 11.C.G. Enke, Selected Ion Fragmentation with a Tandem Quadrupole Mass Spectrometer (NSF proposal) 1976.
- 12.Enke C.G., Yost R.A. Jim morrison, friend and colleague. J. Am. Soc. Mass Spectrom. 2013;24:1319–1323. doi: 10.1007/s13361-013-0692-z. [DOI] [PubMed] [Google Scholar]
- 13.Yost R.A., Enke C.G. Selected ion fragmentation with a tandem quadrupole mass spectrometer. J. Am. Chem. Soc. 1978;100:2274–2275. doi: 10.1021/ja00475a072. [DOI] [Google Scholar]
- 14.Yost R.A., Enke C.G., McGilvery D.C., Smith D., Morrison J.D. High efficiency collision induced dissociation in an RF only quadrupole. Int. J. Mass Spectrom. Ion Phys. 1979;30:127–136. doi: 10.1016/0020-7381(79)80090-X. [DOI] [Google Scholar]
- 15.McGilvery D.C., Morrison J.D. A mass spectrometer for the study of laser-induced photodissociation of ions. Int. J. Mass Spectrom. Ion Phys. 1978;28:81–92. doi: 10.1016/0020-7381(78)80071-0. [DOI] [Google Scholar]
- 16.C.G. Enke, R.A. Yost, J.D. Morrison, U.S. Patent No. 4,234,791, Tandem Quadrupole Mass Spectrometer for Selected Ion Fragmentation Studies and Low–Energy Collision–Induced Dissociation Therefor, November 18, 1980.
- 17.Vestal M.L., Futrell J.H. Photodissociation of CH3Cl+ and CH3Br+ in a tandem quadrupole mass spectrometer. Chem. Phys. Let. 1974;28:559–561. doi: 10.1016/0009-2614(74)80104-1. [DOI] [Google Scholar]
- 18.Yost R.A., Enke C.G. Triple quadrupole mass spectrometry. Anal. Chem. 1979;51(12):1251A–1264A. doi: 10.1021/ac50048a002. [DOI] [PubMed] [Google Scholar]
- 19.McLafferty F.W. 2nd Ed. W. A. Benjamin; Reading, Mass: 1973. Interpretation of Mass Spectra. [Google Scholar]
- 20.Johnson J.V., Yost R.A. Tandem mass spectrometry for trace analysis. Anal. Chem. 1985;57(7):758A–768A. [Google Scholar]
- 21.McLafferty F.W. Tandem mass spectrometry in trace toxicant analysis. Biomed. Mass Spectrom. 1981;8:446–448. doi: 10.1002/bms.1200080917. [DOI] [PubMed] [Google Scholar]
- 22.Cook R.G., Busch K.L. Counting molecules by desorption ionization and mass spectrometry/mass spectrometry. J. Chem. Educ. 1982;59:926–933. doi: 10.1021/ed059p926. [DOI] [Google Scholar]
- 23.Yost R.A. Why tandem mass spectrometry for trace analysis: Concepts of tandem analytical techniques. Rapid Commun Mass Spectrom. 2022;36(13) doi: 10.1002/rcm.9310. [DOI] [PubMed] [Google Scholar]
- 24.W.L Budde, personal communication, January 22, 2019.
- 25.J. Shoemaker, D. Tettenhorst, Method 537.1 Determination of Selected Per- and Polyflourinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC/MS/MS), U.S. Environmental Protection Agency, Washington, DC, 2020.
- 26.Jackson B., Liba A., Nelson J. Advantages of reaction cell ICP-MS on doubly charged interferences for arsenic and selenium analysis in foods. J. Anal. At. Spectrom. 2015;30:1179–1183. doi: 10.1039/C4JA00310A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walkner C., Gratzer R., Meisel T., Bokhari S.H.N. Multi-element analysis of crude oils using ICP-QQQ-MS. Org. Geochem. 2017;103:22–30. doi: 10.1016/j.orggeochem.2016.10.009. [DOI] [Google Scholar]
- 28.R.E. Finnigan, personal communication, February 4, 2018.
- 29.Beck A., Diemer H., Ayoub D., Debaene F., Wagner-Rousset E., Carapito C., Van Dorsselaer A., Sanglier-Cianférani S. Analytical characterization of biosimilar antibodies and Fc-fusion proteins. Trends Anal. Chem. 2013;48:81–95. doi: 10.1016/j.trac.2013.02.014. [DOI] [Google Scholar]
- 30.Herold D.A., Fitzgerald R.L. Immunoassays for testosterone in women: better than a guess? Clin Chem. 2003;49:1250–1251. doi: 10.1373/49.8.1250. [DOI] [PubMed] [Google Scholar]
- 31.Moal V., Mathieu E., Reynier P., Malthièry Y., Gallois Y. Low serum testosterone assayed by liquid chromatography-tandem mass spectrometry. Comparison with five immunoassay techniques. Clin. Chim. Acta. 2007;386:12–19. doi: 10.1016/j.cca.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Kushnir M.M., Rockwood A.L., Roberts W.L., Abraham D., Hoofnagle A.N., Meikle A.W. Measurement of Thyroglobulin by LC-MS/MS in Serum and Plasma in Presence of Anti-Thyroglobulin Autoantibodies. Clin. Chem. 2013;59:982–990. doi: 10.1373/clinchem.2012.195594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.la Marca G. Mass spectrometry in clinical chemistry: the case of newborn screening. J. Pharm. Biomed. Anal. 2014;101:174–182. doi: 10.1016/j.jpba.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 34.Henry Arnaud C. As the triple quadrupole turns 40, mass spec gurus look back on what it’s meant to chemistry. Chem. Eng. News. 2018:96:10. https://cen.acs.org/articles/96/i10/triple-quadrupole-turns-40-mass.html [Google Scholar]
- 35.R.A. Yost, C.G. Enke, A look back at the birth of the triple quadrupole mass spectrometer https://www.youtube.com/watch?v=whEO8kspM_g.