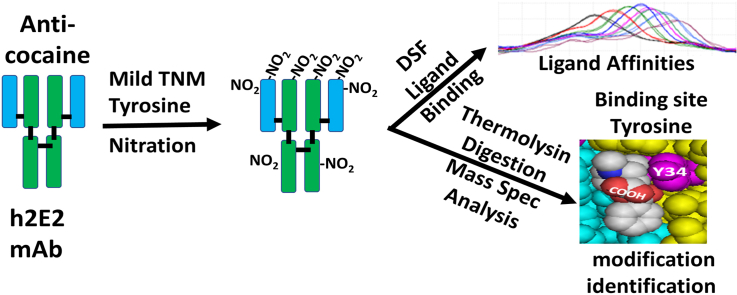
Abstract
Tetranitromethane was used to selectively modify tyrosine residues of a humanized anti-cocaine mAb (h2E2), under development for the treatment of cocaine use disorders. The effect of mild tyrosine nitration on the affinity of cocaine and two high affinity cocaine metabolites, cocaethylene and benzoylecgonine, was assessed using differential scanning fluorimetry to measure ligand affinities via ligand-induced thermal stabilization of the mAb antigen binding region. Nitrated tyrosine residues were identified by mass spectral analysis of thermolysin peptides. One objective was to understand the binding affinity differences observed for these three ligands, which are not explained by the published crystal structure of the h2E2 mAb Fab fragment co-crystalized with benzoylecgonine, since the carboxylic acid of benzoylecgonine that is esterified to form cocaine and cocaethylene is not in contact with the mAb. Importantly, the binding affinity of the cocaine metabolite benzoylecgonine was not decreased by mild nitration, whereas the binding affinities of cocaine and cocaethylene were decreased about two-fold. These ligands differ only in the substituent attached to the carboxylate moiety of the compound, with benzoylecgonine having an unesterified carboxylate, and cocaine and cocaethylene having methyl and ethyl esters, respectively, at this position. The results are consistent with nitration of light chain tyrosine residue 34, resulting in a less favorable interaction with cocaine and cocaethylene carboxylate esters, while not affecting binding of benzoylecgonine. Thus, light chain Tyr34 residue may have molecular interactions with cocaine and cocaethylene not present for benzoylecgonine, leading to the observed affinity differences for these three ligands.
Keywords: Monoclonal antibody, Cocaine binding, Tyrosine nitration, DASPMI rotor dye, Differential scanning fluorimetry, Ligand affinity
Abbreviations: mAb, monoclonal antibody; h2E2, humanized anti-cocaine monoclonal antibody; TNM, tetranitromethane; DSF, differential scanning fluorimetry; DASPMI, (4-(4-(dimethylamino)styryl)-N-methylpyridinium iodide; PBS, phosphate buffered saline; CE, cocaethylene; Coc, cocaine; BE, benzoylecgonine; TmB, Boltzmann fit derived melting temperature; TmD, temperature of the maximum of the first derivative of the melting curve; ABC, 50 mM ammonium bicarbonate buffer
Graphical abstract
Highlights
-
•
Highlights for Kirley, Greis, and Norman BBReports manuscript January 13, 2022.
-
•
Tetranitromethane modification of h2E2 mAb decreases cocaine binding affinity.
-
•
Tyrosine nitration differentially effects h2E2 mAb cocaine and metabolite affinities.
-
•
Mild nitration does not decrease the binding affinity of benzoylecgonine.
-
•
Light chain Tyr34 is nitrated by TNM and may interact with cocaine's methyl ester.
1. Introduction
Cocaine use disorder remains a significant health concern in the United States. In 2020 alone, more than 19,000 deaths were attributed to cocaine use (Center for Disease Control, National Center for Health Statistics). Despite the pharmacology of cocaine being well-understood, there are no FDA approved pharmacotherapies for the treatment of cocaine use disorder. Therefore, as a therapy for cocaine use disorders, we have developed a high affinity anti-cocaine mAb, named h2E2, which also binds the active metabolite of cocaine, cocaethylene (CE), and the inactive metabolite, benzoylecgonine (BE) with high (nM) affinity [[1], [2], [3]].
We developed several methodologies to structurally characterize this antibody [[4], [5], [6]]. Ligand binding assays were developed to analyze this mAb and its Fab fragment, including intrinsic tyrosine and tryptophan mAb fluorescence quenching [6], the use of extrinsic fluorescent dyes and differential scanning fluorimetry (DSF) [7], and both absorbance [8] and fluorescence [9] methods using the DASPMI dye to quantitate high affinity cocaine binding sites. We also showed that non-reducing SDS-PAGE can be used to qualitatively assess ligand-induced stabilization of the protein domains of the intact mAb and its ligand-binding fragments [10] and to monitor mAb Fab fragment sub-domain stabilization by cocaine and its metabolites [11].
We recently published the structure of the Fab fragment of this h2E2 mAb, both with and without co-crystallized benzoylecgonine (BE) ligand [12]. However, cocaine, and not BE, was experimentally added to co-crystallize with the Fab fragment, but the long crystal formation time and the crystallization conditions resulted in non-enzymatic ester hydrolysis, converting cocaine to BE during crystallization [12]. Although the crystal structure obtained was consistent with all previous data concerning the importance of both tyrosine and tryptophan residues for cocaine binding, the well-established differences in the affinities (Kds) for the three high affinity ligands were not explained by the crystal structure containing the BE ligand, since the carboxylate group on the BE molecule, which is esterified to form cocaine and cocaethylene, is protruding straight outwards from the binding site, having no contacts with the amino acids comprising the binding site.
However, the crystal structure demonstrated that there is a tyrosine residue in the light chain of the Fab binding site, which is in contact with the BE ligand, and nearby the carboxylate portion of the bound BE ligand (i.e., light chain Tyr34). Thus, we hypothesized that this residue may have some interaction with the methyl (cocaine) and ethyl (cocaethylene) groups comprising the ester extensions of the BE carboxylate on cocaine and cocaethylene, resulting in the observed increases in affinity relative to the BE ligand. Therefore, we employed the tyrosine selective chemical modification reagent, tetranitromethane (TNM), under mild and limited reaction conditions, to modify exposed and reactive tyrosine residues. We assessed differences caused by TNM modification on the affinities of these three ligands, as measured by differential scanning fluorimetry, using the DASPMI dye previously shown to only bind to the Fab portion of this mAb and report on ligand binding via changes in its fluorescence [7]. The results demonstrated that under mild nitration conditions with TNM, the affinity of binding for both cocaine and cocaethylene are reduced, while the affinity for the BE ligand is not. Mass spectral analysis of thermolysin peptides of the nitrated mAb revealed that light chain Tyr34 was one of only a few tyrosine residues nitrated under these conditions, suggesting that this residue is important for the observed differences in ligand binding affinities.
2. Materials
The generation, production, and purification of the h2E2 anti-cocaine monoclonal antibody by the manufacturer, Catalent, was previously described [3]. Tetranitromethane (TNM) was obtained from Sigma-Aldrich (catalog number T25003). 10 mM ligand stock solutions of ligands were made in distilled water from solids as described [1]. Solid 4-(4-(dimethylamino)styryl)-N-methylpyridinium iodide dye (4-Di-ASP iodide, DASPMI) was purchased from ThermoFisher Scientific (Invitrogen/Life Technologies catalog # D-288), dissolved in dry DMSO, and stored light protected at −20°C. Applied Biosystems 48 well RT PCR plates (cat # 4375816) and the sealing optical adhesive transparent film (cat # 4375928) used in the StepOne RT PCR used to perform the DSF analyses were from ThermoFisher Scientific. Acrylamide, bisacrylamide, and all reagents used to pour, run, and stain SDS-PAGE gels were from BioRad.
3. Methods
3.1. UV/Vis spectroscopy
Visible and ultraviolet absorbance wavelength scans and measurements were performed at 22 °C using a micro 1.0 cm path length quartz cell (100 μL sample volume) in a Beckman DU 800 spectrophotometer.
3.2. Tetranitromethane (TNM) treatment of the mAb
All samples treated with TNM contained final concentrations of 1.0 mg/ml (i.e., 6.92 μM mAb, equivalent to 0.35 mM tyrosine residues) h2E2 mAb in 50 mM Tris-Cl buffer, pH = 8.0, with the addition of a final concentration of 0.35 mM TNM (i.e., a 1:1molar ratio of TNM to the concentration of mAb tyrosine residues). The reaction was carried out at 22 °C for various times, and, for samples to be analyzed by DSF, terminated and buffer exchanged by application of the sample to a Sephadex G-50 column equilibrated in PBS buffer. The void volume fractions, containing the modified mAb, were identified by absorbance at 280 nm, and pooled. Total protein concentration was determined in the PBS buffer, and total nitration of tyrosine residues was determined after alkalinization of sample aliquots by adding NaOH to a final concentration of 0.15 M (nitro-tyrosine molar extinction coefficient of 4100 M−1cm−1 at 428 nm).
3.3. Differential scanning fluorimetry (DSF) analysis, mAb ligand binding, and data analysis
The Real Time PCR (RT PCR) machine used to perform the differential scanning fluorimetry (DSF) protein thermal melt experiments was a ThermoFisher Scientific/Life Technologies StepOne RT PCR instrument, using the standard ROX dye calibration as the detector, setting the passive reference to none (excitation at 485 nm, emission at 610 nm). Samples were analyzed in PBS buffer (20 μL per well, in duplicate) containing final concentrations of 2.5 μM h2E2 mAb, 50 μM of DASPMI dye, and various concentrations of ligands. The DSF heating rate was 0.45 °C/min, recording from 35 to 95°C. DSF data were analyzed, and the ligand affinities (Kd values) were determined at the TmB temperature of the apo (unliganded) mAb, as previously described [7].
3.4. Non-reducing SDS-PAGE analysis of the mAb
For non-reducing SDS-PAGE analyses, 7% acrylamide gels were poured, run, and stained with Coomassie Blue, as described by Laemmli [13]. Prior to running on the gels, antibody samples were diluted to a final protein concentration of 0.2 mg/mL in non-reducing Laemmli sample buffer (containing 5% SDS and 25% glycerol). Before electrophoresis, samples were either not heated, or boiled for 5 min to totally denature all of the mAb protein domains [10,11]. 15 μL of each mAb sample (3 μg) were loaded into each gel well.
3.5. Proteolysis and mass spectral analysis of nitrated mAb peptides
Identification of nitrated tyrosine residues in TNM treated h2E2 mAb was accomplished by thermolysin digestion followed by mass spectrometry analyses. 25 μg of each sample (either TNM treated or control mAb) were dried and reconstituted into 50 mM ammonium bicarbonate buffer (ABC), pH = 8.0, reduced with dithiothreitol, and alkylated with iodoacetamide [14]. Each sample was then digested with 1.0 μg of thermolysin at 70 °C for 2.5 h in 100 μL of 50 mM ABC. The reaction was quenched by acidification with 0.1% formic acid, dried in a SpeedVac, redissolved in 50 μL of 0.1% formic acid, and 5 μL (0.5 μg) of each sample was used for nanoLC-MS/MS analysis. Mass spectrometry data were collected on an Orbitrap Eclipse mass spectrometer coupled to a Dionex Ultimate 3000 RSLCnano system (ThermoFisher Scientific). Samples were injected onto a 5 mm nanoviper μ-Precolumn at 5 μl/min in formic acid/H2O 0.1/99.9 (v/v) for 5 min to desalt and concentrate prior to chromatographic separation of peptides using the EASY-Spray column PepMap RSLC C18 150 mm column. Peptides were eluted using a gradient from 98% phase A (formic acid/H2O 0.1/99.9, v/v) to 32% phase B (formic acid/acetonitrile 0.1/99.9, v/v) for 30 min at 300 nL/min. Mass spectral data were collected, and charge states between 2 and 6 were required for MS2 analysis, using a 20s dynamic exclusion window. MS2 scans were performed in the ion trap with HCD fragmentation, and the data was recorded using Xcalibur 4.3 software (ThermoScientific). Data from each sample was processed with up to 6 missed cleavages to optimize the sequence coverage. Protein identification was done using Proteome discoverer version 2.4.1.15 and the Sequest HT search algorithm (Thermo Scientific) searched against the Uniprot Human [9606] database (November 12, 2020 version, containing 50,540 entries) which was modified by adding the sequences of the h2E2 mAb heavy and light chains, to allow them to be detected.
3.6. Previously published h2E2 Fab crystal structure and visualization of the nitration results in the vicinity of the drug binding site of the mAb
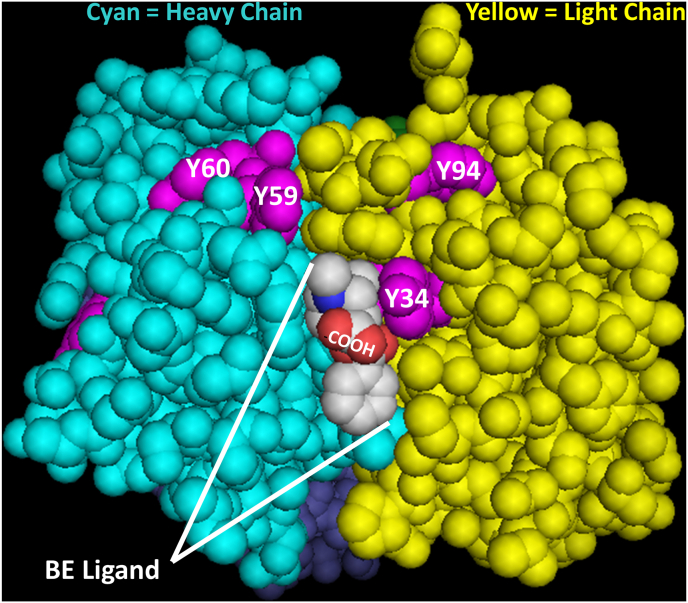
The published X-ray crystal structure of benzoylecgonine co-crystalized with the Fab fragment of the h2E2 anti-cocaine mAb (PDB 6NFN, see Ref. [12]) was visualized using PyMol software (see Fig. 4).
Fig. 4.
Mapping of the nitrated tyrosine residues on the crystal structure (PDB 6NFN) of the Fab fragment of the h2E2 anti-cocaine mAb co-crystallized with benzoylecgonine (BE). The structure is shown in space filling mode, with the ligand binding site pointed upwards towards the observer. Note the proximity of the light chain Y34 residue (all nitrated tyrosine residues are shown in purple color) to the bound BE ligand, and particularly to the carboxylate moiety (COOH) of the BE molecule, which protrudes up and out of the binding site, resulting in no mAb Fab protein-BE ligand carboxylate contacts in the published crystal structure. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Results
4.1. Time course and the extent of the reaction of TNM with the h2E2 mAb
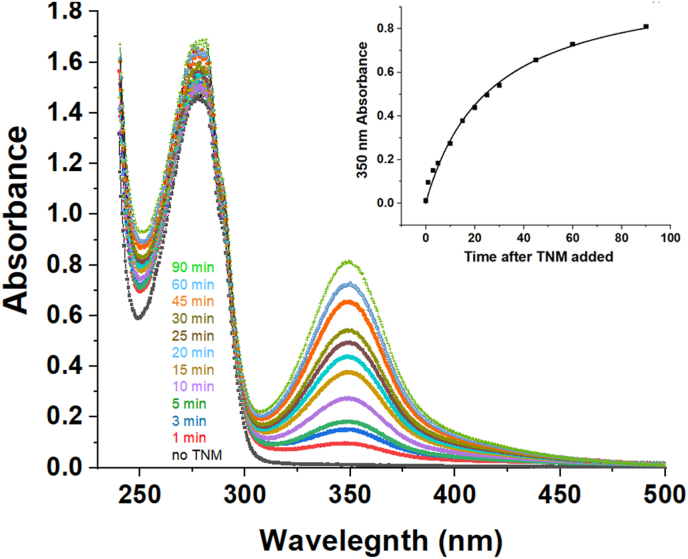
Fig. 1 is the time course for the generation of the nitroformate ion (ε at 350 nm = 14,400 M−1cm−1) formed when tetranitromethane reacts with tyrosine residues, using 1.0 mg/ml h2E2 mAb in 50 mM Tris-Cl, pH = 8.0 at 22 °C. The molar ratio of TNM reagent to mAb protein tyrosine residues employed in all the experiments in this study was 1:1. The inset plot is a graph of absorbance at 350 nm due to the nitroformate ion as a function of time. Under these same conditions, in independent experiments in which the excess TNM reagent was subsequently separated from the modified mAb by size exclusion chromatography using a Sephadex G-50 column equilibrated in PBS, quantitation of the amount of nitro-tyrosine formed per mol of h2E2 mAb resulted in averages of 3.9, 6.1, and 14.0 nitro-tyrosine residues formed per molecule of mAb (each h2E2 mAb has 50 total tyrosine residues) after 30 min, 60 min, and 26 h of reaction with TNM, respectively (using 4100 M−1cm−1 as the extinction coefficient for nitro-tyrosine at 428 nm in basic solutions).
Fig. 1.
Time course of 0.35 mM tetranitromethane (TNM) modification of 1 mg/ml h2E2 mAb (0.35 mM tyrosine residues) at 22°C in 50 mM Tris-Cl pH=8.0 buffer. The UV/Vis absorbance spectra were recorded 0–90 min after addition of TNM. The absorbance increase at 350 nm is due to nitroformate ion formation resulting from TNM reacting with mAb tyrosine residues. Inset - The increase in absorbance at 350 nm due to the nitroformate ion is plotted as a function of time after addition of TNM to the mAb.
4.2. DSF analysis of nitrated h2E2 mAb and evaluation of ligand affinities
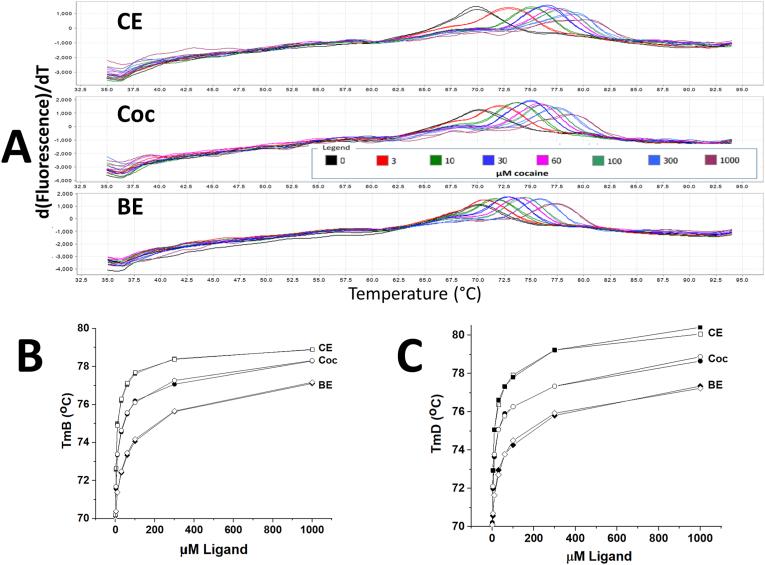
Fig. 2A shows the ligand titrations in PBS buffer (from 0 to 1000 μM for each ligand, in duplicate) for monoclonal antibody previously treated for 30 min with TNM. The first derivative of the fluorescence due to the DASPMI dye as a function of temperature are shown for all three ligands that bind to this mAb with nM affinities. Fitting of the raw DSF fluorescence data with the Boltzmann function (not shown) yields the Boltzmann melting temperature (TmB). Fig. 2B shows the increase of the TmB values for these samples as a function of concentration for each of the 3 ligands. Fig. 2C shows the increases of the TmD values for these same samples as a function of ligand concentration. TmD is the temperature of the maximum of the first derivative plot of the fluorescence (as shown in Fig. 2A), at each ligand concentration utilized. From the Boltzmann fitting of the DSF data, the free energy change upon binding for each ligand concentration used is quantitated, which is then used to calculate the binding affinity (Kd) at the mAb antigen binding region TmB temperature (approximately 70 °C in this case) for each ligand [7].
Fig. 2.
Differential scanning fluorescence (DSF) analysis in pH=7.4 PBS buffer of 2.5 μM h2E2 mAb previously treated for 30 min with TNM. Panel A – The first derivative of the fluorescence of the DASPMI reporter dye as a function of temperature, using 0–1000 μM of the three mAb ligands, cocaethylene (CE), cocaine (Coc), and benzoylecgonine (BE). Panel B – Plots of the Boltzmann melting temperature (TmB) from the raw fluorescence data used to generate the first derivative data in Panel A, as a function of ligand concentration. Panel C – The peak of the first derivative of the fluorescence data (TmD) shown in Panel A as a function of ligand concentration. Duplicate samples at each concentration of ligand were analyzed and shown in the figure.
Data from 3 such experiments as is shown in Fig. 2, all performed on mAb treated with TNM for 30 min, are summarized in Table 1. Published data for the control h2E2 mAb using the same DASPMI dye, DSF methodology, and calculations (see Ref. [7]) are given in Table 1 for comparative purposes. Each of the three independent experiments performed are included in the Table, as well as the statistics (mean ± standard deviation) for this group of experiments. Note the 1.90-fold and 2.56-fold increases in Kd (decreases in affinity) caused by TNM treatment for cocaine and cocaethylene, respectively, and the lack of change of the Kd for benzoylecgonine, resulting in a decrease in the relative binding affinity for benzoylecgonine from 5.98 to 3.37.
Table 1.
Differential scanning fluorimetry (DSF) analysis of 2.5 μM h2E2 mAb in PBS buffer after nitration with tetranitromethane for 30 min. The sample used for these 3 independent experiments was generated less than 1 week before analyzed. RBA = relative binding affinity (relative to the binding affinity of cocaine).
| Ligand | Kd (nM) (at apo TmB) | Avg Kd (nM) ± Std. Dev. (fold increase vs unmodified h2E2 mAb) | Expt. RBA | Avg RBA (Relative Binding Affinity) mean ± Std. Dev. | Literature RBA of unmodified mAb [7] | Literature Kd of unmodified mAb (nM) [7] |
|---|---|---|---|---|---|---|
| CE | 16.2 | 0.27 | ||||
| CE | 24.2 | 20.7 ± 4.1 (2.56) | 0.52 | 0.4 ± 0.13 | 0.286 | 8.1 |
| CE | 21.7 | 0.41 | ||||
| Coc | 59.9 | 1 | ||||
| Coc | 46.3 | 53.0 ± 6.8 (1.90) | 1 | 1.0 | 1 | 28.3 |
| Coc | 52.8 | 1 | ||||
| BE | 176 | 2.94 | ||||
| BE | 163 | 177 ± 14 (1.05) | 3.52 | 3.37 ± 0.37 | 5.98 | 169.1 |
| BE | 192 | 3.64 |
4.3. Non-reducing SDS-PAGE analyses of the control and TNM treated mAb, and correlation of the nitration effects with the mAb DSF profile in the absence of ligands
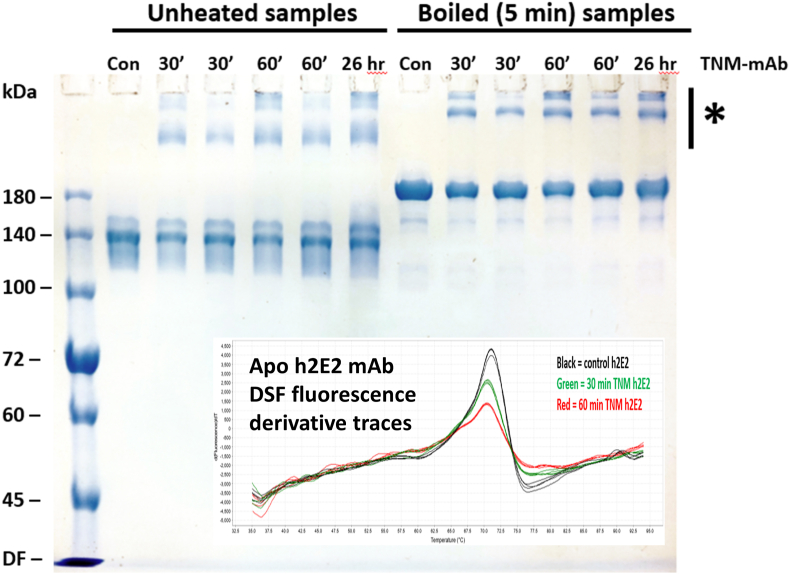
Fig. 3 is the non-reducing SDS-PAGE analysis of several TNM treated mAb preparations, treated with TNM for varying times, after no heating, and after the samples were boiled 5 min in the SDS sample buffer prior to electrophoresis. Shown as an inset is the first derivative DSF plot comparing control, 30 min TNM treated, and 60 min TNM treated mAb (in the absence of any ligands), all containing 2.5 μM mAb in PBS buffer. Note the time-dependent slight decrease in the TmD of the apo (unliganded) mAb with increasing TNM concentration, and the large decrease in the magnitude of the fluorescence derivative peak. For this non-reducing SDS-PAGE analysis, two samples of two independently treated TNM samples are analyzed for both the 30 and 60 min TNM treated samples. In each case, the first of the two identically treated samples was stored for approximately 4 months at 4 °C in PBS buffer before this analysis, while the second sample was stored for 1–2 weeks at 4 °C in PBS buffer. The 26 h TNM treated sample was run on this gel 4 weeks after labeling. There are high molecular weight aggregates present in all TNM treated mAb samples that are not evident in the control mAb (indicated by a bar and asterisk in the figure), and the highest apparent molecular weight aggregate that barely enters the gel increases with extended time of storage at 4 °C in PBS buffer.
Fig. 3.
Non-reducing 7% acrylamide SDS-PAGE analysis of TNM treated mAb preparations, both unheated, and boiled 5 min in the SDS sample buffer prior to electrophoresis. Two samples from two independently treated TNM samples were analyzed for both the 30 and 60 min TNM treated mAb samples. In each case, the first of the two identically TNM treated samples was stored for approximately 4 months at 4 °C in PBS buffer before this analysis, while the second sample was stored for 1–2 weeks at 4 °C in PBS buffer prior to running on this gel. Note that high molecular weight bands are present in all TNM treated mAb samples (indicated by a bar and asterisk), and the highest apparent molecular weight aggregate in the TNM treated samples that barely enters the gel increases with storage time at 4 °C in PBS buffer.
4.4. Thermolysin digestion and mass spectral identification and mapping of nitrated tyrosine residues
Control and 30 min TNM treated h2E2 samples were reduced and alkylated, digested with thermolysin, and the resultant peptides analyzed by mass spectral analysis. The no treatment control mAb produced no evidence of tyrosine nitration on either the heavy or light chains, as expected. The 30 min TNM treated mAb showed evidence of significant nitration at both Y34 (ITTSNY34ANW; 54% compared to unmodified ITTSNYANW) and Y94 (LWY94NTHY; 57% compared to unmodified LWYNTHY) on the light chain, as well as Y59 or Y60 (INQDGSEKY59Y60) nitration on the heavy chain (this analysis could not distinguish which of these two adjacent tyrosine residues on the heavy chain was nitrated). Y176 and Y292 on the heavy chain were also partially nitrated, but these residues are very far removed from the ligand binding site, and thus not likely candidates to effect ligand binding selectivity. Note that the residue numbering used above and throughout this study is the residue number in the amino acid sequence and PDB structural file, and not the Kabat numbering for the variable domain used in the crystal structure paper [12]. Thus, the Y34 nitrated tyrosine is equivalent to the Y32 in the structure paper. The relevant nitrated tyrosine residues which are near the ligand binding site were mapped onto the crystal structure (displayed with the ligand binding site pointed towards the observer), along with the co-crystallized benzoylecgonine (BE) ligand, as shown in Fig. 4. Note the proximity of the light chain Y34 to the BE ligand, specifically to the carboxylate moiety of the BE molecule.
5. Discussion
It is important to thoroughly characterize both the structure and function of mAbs intended for therapeutic purposes. The h2E2 humanized anti-cocaine mAb was developed for treatment of cocaine use disorders, and is in late stage pre-clinical development. The therapeutic function of this mAb is to bind cocaine and its active metabolites with high affinity, thereby sequestering the cocaine in the plasma and keeping it out of the brain [3], where it would inhibit the reuptake of dopamine and other neurotransmitters, which is responsible for its addictive nature. Thus, the differential affinity of the mAb for cocaine and its metabolites is important for its therapeutic use and possible further development.
It was anticipated that the crystal structure of the h2E2 Fab fragment co-crystallized with cocaine would explain the differential affinities of the ligands. However, the cocaine (methyl ester) was non-enzymatically hydrolyzed during the extended time of crystal formation, and the resultant BE co-crystal did not answer the differential affinity question, since there are no interactions of the mAb binding site residues with the carboxylate residue on the BE ligand (which is esterified to form Coc and CE). Nonetheless, the crystal structure did reveal one light chain tyrosine residue (Tyr 34) that was close to the BE carboxylate. Therefore, we investigated the effect of tyrosine modification with tetranitromethane on the affinities of the three ligands which bind with high affinity to this mAb, namely, CE, Coc, and BE (with Kd values of 1, 4, and 20 nM, respectively [6] at 22 °C). We used differential scanning fluorimetry (DSF) assays that employed the DASPMI rotor dye to measure the affinity of these three ligands after reaction of the mAb with TNM under mild conditions. We also attempted to analyze data from mAb which was reacted with TNM at longer times, but found some general destabilization of the ligand binding region and a loss of DSF signal, making the data unsuitable for quantitative ligand affinity analyses (data not shown). This destabilization was not evident from the non-denaturing SDS-PAGE of unheated TNM treated samples, although we noted an increase in aggregate formation as a function of both time of TNM reaction and time of storage at 4 °C of the nitrated mAb (see Fig. 3). Thus, we limited the quantitative affinity analyses by DSF and the mass spectral analysis of nitrated peptides to this short 30 min TNM treatment time. This data was reproducible and nicely fit by the Boltzmann function used to analyze the DSF data, and gave the expected rank order of affinities of the three ligands, i.e., Kd of BE > Coc > CE (see Fig. 2 and Table 1). Note that the Kd values calculated by DSF are at the TmB temperature of the mAb with no ligand, which is about 70 °C, explaining why the DSF Kds affinities reported earlier [1] and in Table 1 are higher (i.e., lower affinities) than the Kds calculated by intrinsic tyrosine and tryptophan fluorescence quenching [6], which were measured at 20 °C. The increase in cocaine and CE Kds after mild TNM treatment contrasted with the unchanged Kd for BE, resulting in a decrease in the relative binding affinity of BE to cocaine for the nitrated mAb (Table 1).
Analysis of nitrated tyrosine residues via mass spectra of thermolytic peptides revealed several tyrosine residues to be partially modified, as expected for a mAb containing 50 tyrosine residues. However, limiting the TNM reaction time to 30 min yielded an average of only 3.9 nitro-tyrosine residues per mAb, and mass spectral analysis revealed only 5 sites of nitration on the thermolytic peptides. Of these 5, only 3 were close to the BE ligand (heavy chain tyr59/tyr60 accounts for only 1 nitration of indeterminant localization on one of these two residues) as depicted in Fig. 4. However, it seems likely that nitration of the Y34 of the light chain is mainly responsible for the changes of cocaine and cocaethylene affinities, since this residue is the only tyrosine in direct contact with BE in the crystal structure. In addition, this residue is also the closest part of the mAb protein to the carboxylate residue moiety on BE, which is esterified to form both cocaine (a methyl ester) and cocaethylene (an ethyl ester). In conclusion, it seems probable that nitration of this Tyr34 residue on the light chain of the h2E2 mAb is responsible for the decreases in affinity for cocaine and cocaethylene. In addition, we hypothesize that this tyrosine residue would contact bound cocaine and cocaethylene, thereby explaining the increased affinity of the h2E2 mAb for the pharmacologically active cocaine and cocaethylene ligands relative to benzoylecgonine.
Declaration of competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Dr. Norman is named as a co-inventor on a portfolio of patents for the matter and use of the h2E2 humanized anti-cocaine monoclonal antibody.
Acknowledgments
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse Grant U01DA039550. We are grateful to Catalent PharmaSolutions, Inc. (Madison, WI) for providing the recombinant humanized h2E2 anti-cocaine mAb protein expressed using their GPex® technology. We acknowledge Dr. Guochang Fan in this Department for the use of the StepOne RT PCR instrument for DSF measurements. Mass spectrometry data were collected on an Orbitrap system that was obtained in part through an NIH high end instrumentation grant (S10OD026717-01) to KDG.
References
- 1.Wetzel H.N., Webster R.P., Saeed F.O., Kirley T.L., Ball W.J., Norman A.B. Characterization of a recombinant humanized anti-cocaine monoclonal antibody produced from multiple clones for the selection of a master cell bank candidate. Biochem. Biophys. Res. Commun. 2017;487:690–694. doi: 10.1016/j.bbrc.2017.04.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wetzel H.N., Tabet M.R., Ball W.J., Norman A.B. The effects of a humanized recombinant anti-cocaine monoclonal antibody on the disposition of cocaethylene in mice. Int. Immunopharm. 2014;23:387–390. doi: 10.1016/j.intimp.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norman A.B., Gooden F.C., Tabet M.R., Ball W.J. A recombinant humanized anti-cocaine monoclonal antibody inhibits the distribution of cocaine to the brain in rats. Drug Metabol. Dispos.: Biol. Fate Chem. 2014;42:1125–1131. doi: 10.1124/dmd.114.057034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirley T.L., Norman A.B. Unfolding of IgG domains detected by non-reducing SDS-PAGE. Biochem. Biophys. Res. Commun. 2018;503:944–949. doi: 10.1016/j.bbrc.2018.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirley T.L., Greis K.D., Norman A.B. Structural characterization of expressed monoclonal antibodies by single sample mass spectral analysis after IdeS proteolysis. Biochem. Biophys. Res. Commun. 2016;477:363–368. doi: 10.1016/j.bbrc.2016.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirley T.L., Norman A.B. Characterization of a recombinant humanized anti-cocaine monoclonal antibody and its Fab fragment. Hum. Vaccines Immunother. 2015;11:458–467. doi: 10.4161/21645515.2014.990856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirley T.L., Norman A.B., Wetzel H.N. A novel differential scanning fluorimetry analysis of a humanized anti-cocaine mAb and its ligand binding characteristics. J. Immunol. Methods. 2020;476:112676. doi: 10.1016/j.jim.2019.112676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirley T.L., Norman A.B. Ligand binding to a humanized anti-cocaine mAb measured by dye absorption spectroscopy. Biochem. Biophys. Res. Commun. 2021;535:93–98. doi: 10.1016/j.bbrc.2020.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirley T.L., Norman A.B. Cocaine binding to the Fab fragment of a humanized anti-cocaine mAb quantitated by dye absorption and fluorescence spectroscopy. J. Immunol. Methods. 2021;496:113103. doi: 10.1016/j.jim.2021.113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirley T.L., Norman A.B. Ligand binding to a humanized anti-cocaine mAb detected by non-reducing SDS-PAGE. Biochem. Biophys. Rep. 2020;23 doi: 10.1016/j.bbrep.2020.100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirley T.L., Norman A.B. Multi-domain unfolding of the Fab fragment of a humanized anti-cocaine mAb characterized by non-reducing SDS-PAGE. Biochem. Biophys. Res. Commun. 2020;533:580–585. doi: 10.1016/j.bbrc.2020.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan K., Zhou M., Ahrendt A.J., Duke N.E.C., Tabaja N., Ball W.J., Kirley T.L., Norman A.B., Joachimiak A., Schiffer M., Wilton R., Pokkuluri P.R. Structural analysis of free and liganded forms of the Fab fragment of a high-affinity anti-cocaine antibody, h2E2 Acta crystallographica. Acta Crystallogr. Struct. Biol. Commun. 2019;75:697–706. doi: 10.1107/S2053230X19013608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Dwivedi P., Muench D.E., Wagner M., Azam M., Grimes H.L., Greis K.D. Phospho serine and threonine analysis of normal and mutated granulocyte colony stimulating factor receptors. Sci. Data. 2019;6:21. doi: 10.1038/s41597-019-0015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]