Highlights
-
•
Patient-specific instruments (PSI) improve surgical orthopaedic interventions.
-
•
Resection margins are all safe for oncologic resections in our series.
-
•
All types of bone tumour were included.
-
•
Planification margins can be shortened to 5 mm thanks to their accuracy.
-
•
The correlation index between planned and obtained margins is excellent.
Keywords: Surgical reconstruction, Cutting guide accuracy, MRI evaluation, Bone tumour
Abstract
Background
Patient Specific Instruments (PSI) is currently a proven technique for bone tumor resection. In a previous publication, we analyzed the quality of margin resection of pelvic sarcoma resections with the use of PSI (by pathologic evaluation of the margins). In this new study, we compare preoperative resection planning and actual resection margins by MRI analysis of the resection specimens.
Methods
Between 2011 and 2020, 31 patients underwent bone tumor resection with the use of PSI. Preoperatively, the margins were planned with a software and PSI were made according to these margins. Postoperatively, the surgical resection specimens were analyzed with MRI. Resection margins were measured with the same software used in the preoperative planning.
Results
All margins were safe (free of tumor). The differences between preoperative planned margins and the obtained ones were within the range −5 to +5 mm. The correlation between planned margin and the obtained one was excellent (R2 = 0.841; p < 0.0001).
Conclusions
This study demonstrates the accuracy of PSI. In our series, all resection margins were safe. A minimal 5 mm-margin has to be planned but a larger sample is needed to give recommendations.
1. Introduction
Up until the 1970’s, amputation was the reference for surgical treatment of bone sarcoma and yet, survival remained poor [1]. The main objective of primary amputation was to achieve a safe surgical margin. Nowadays, this latter objective remains the primary endpoint [1], [2], [3], [4] but limb salvage surgery has become a standard of care. In this aspect, a secondary endpoint is to preserve the function of the affected limb by sparing as much of bone and soft tissues that are free of disease.
This change towards limb salvage surgery has been made possible thanks to the improvement in imaging, chemotherapy radiotherapy and surgical techniques [3]. With time and experience, surgeons have been increasingly confident in coming closer to the tumor while never transgressing a safe surgical margin. In this aspect, surgical accuracy is the crucial factor to remain at safe distance from the lesion while keeping as much normal tissues [4].
It has been shown by many teams that intra-operative assistance [2] such as navigation and Patient Specific Instrument (PSI) [5] improve accuracy in oncological surgery.
PSI were shown by many teams to be an excellent and reliable tool in the safe and accurate resection of bone sarcomas [6], [7], [8], especially in complex cases such as pelvic tumors. The technique requires an advanced pre-operative planning to define accurately the tumor localization and the appropriate section planes. Based on these planes, a cutting jig is designed that fits perfectly the anatomy of the bone surface. This device will then be 3D-printed and sterilized.
Intra-operatively the instrument provides control over the safe margins by accurately showing and guiding the oscillating blade in the predefined resection plane. Lastly, for biological reconstructions, PSIs can greatly help the surgeon by allowing a precise cut of the allograft selected to reconstruct the defect, using the same predefined section planes.
Several studies have already shown excellent accuracy results of the resections during in-vitro experiments [5] or in clinical situations [9]. However, at that time, no information was reported on the clinical impact of the PSIs.
Our hypothesis is that improving accuracy and safe margins has an impact on the local recurrence and overall survival rates, as demonstrated for bone tumor resection in general. Therefore, the aim of this study is to evaluate the margin quality in a consecutive series of 31 primary bone sarcoma resections with the use of PSI.
In a previous study, we demonstrated the effectiveness of these PSIs on the margin quality [7]. Indeed, the resection margins performed with these PSIs all revealed tumor-free histological images. The series studied included patients with pelvic bone tumors. PSI allowed in this technically highly demanding type of surgery a 100% success rate on resection margins. But what is the genuine difference between three-dimensional planning measures and the margin acquired with the surgical sawblade?
Many of the current studies aim to prove the clinical efficiency of these PSIs by demonstrating a superiority in the evolution of the operated patient [5], [7], [8], [9], [10], [11], [12], [13], [14].
In a previous article, we demonstrated the effectiveness of of MRI in the study of resection margins of bone sarcomas [15].
In this study, we propose to compare the preoperatively planned section planes with the postoperative resection margins using MRI of the specimen in a series of 31 patients.
2. Material and methods
2.1. Patient series
Between March 2011 and December 2020, 57 patients underwent surgery for bone tumor resection.
Only patients operated with the use of PSI for tumor resection and whose surgical specimen had been analyzed using MRI were included, leaving 31 patients for this study. 26 patients were excluded. Exclusion criteria were: no specimen MRI, no use of PSI, tumor not visible on specimen MRI. The study population, type of resected tumors and body locations are summarized in Table 1.
Table 1.
Database of type of tumor and body locations.
| Patients | n = 31 | n |
|---|---|---|
| Mean Age (years) | 29,3 (range, 7.8 to 83.0) | |
| Type of tumor | Fibrous dysplasia | 1 |
| Osteosarcoma | 10 | |
| Spindle cell sarcoma | 2 | |
| Ewing Sarcoma | 9 | |
| Giant Cell Tumor | 1 | |
| Malignant fibrous histiocytoma | 1 | |
| Chondrosarcoma | 4 | |
| adamantinoma | 1 | |
| High grade pleiomorphic sarcoma | 1 | |
| Leiomyosarcoma | 1 | |
| Location | Femur | 6 |
| Pelvis | 12 | |
| Tibia | 4 | |
| Forearm | 4 | |
| humerus | 4 | |
| Ankle | 1 | |
2.2. Method
2.2.1. PSI planning and conception
All PSIs were conceived with the help of the company 3D-Side using their dedicated online web interface. Each patient was given the same pre-operatory planning support software. On a selected MRI sequence, the tumor was delineated on each slide. The tumor surfaces were then merged in a 3D volume that was superposed to the CT-scan. The sequences with the highest contrast between the healthy tissue and the tumor were selected on MRI.
Once this process is completed, a cutting guide (PSI) is virtually created and validated by the surgeon. This PSI is then materialized by 3D printing as shown in Fig. 1. In selected cases of biological reconstruction, a PSI was created for the resection of the tumor lesion and also for the mirror cutting of selected bone allograft. In this case, the graft was selected in our tissue bank based on the best match of the CT-scans of the grafts. This technique allowed to obtain perfect junction between the graft and the host bone.
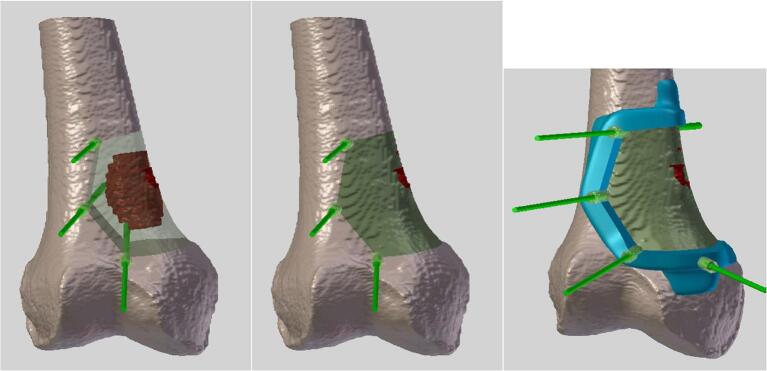
Fig. 1.
Planning images of a distal femur tumor resection. These images are acquired by a fusion of both CT-scanner and MRI images. Delineation of the tumor is in red. PSI is in blue. Kirschner wires are in light green. Bone resection is in dark green.
2.2.2. Tumor surgery and postoperative imaging
The PSI were sterilized and used in the surgical site. The design of the instrument allows only one position of the PSI in the patient (Fig. 2). The PSI is then stabilized on the bone surface with Kirschner wires. The PSI is then used to guide the depth and the direction of an oscillating saw blade. One important aspect of this technology is the fact that the PSI does not help for soft tissues dissection and resection margins.
Fig. 2.
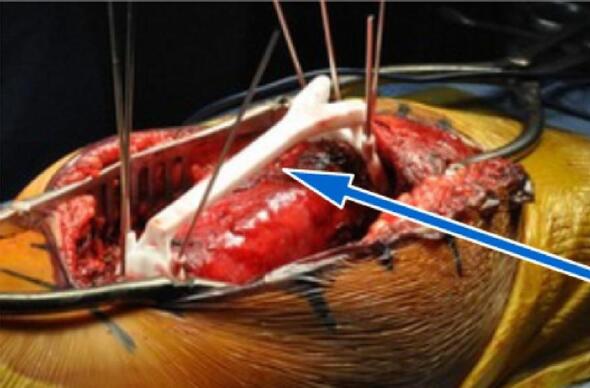
PSI with K-wires fixation. The PSI is fixed directly onto the bone intraoperatively. Flat surfaces help the sawblade to cut precisely around the tumor.
Once the tumor is resected “en bloc”, it is sent directly to the radiology department for an MRI scan prior being analyzed in the pathology department.
The conception of those PSIs can take between 7 and 10 days. However, the tumor growth can be controlled by chemotherapy and/or radiotherapy if indicated. Only chondrosarcomas are unresponsive to chemotherapy and radiotherapy, therefore we performed surgery alone for these entities.
2.2.3. Resection margins delineation on “ITK-SNAP” software using MRI acquisitions
Resected tumors were assessed by MRI right after the surgery. The most discriminant sequences were selected to ensure a correct delineation of the tumor. We then compared the planned resection margins with the margins observed on the resected specimen. The resection margin delineations were realized using a “single-blinded-like” protocol: the post-operative margin measurements were executed by a blinded operator (the author) without any knowledge of planned resection margins.
The PSI of three different patients were used to make two guided cutting planes. One PSI of a last patient was used to make three guided cutting planes. Giving, finally 36 cutting planes and margins for measures.
2.2.4. Comparison method
For each resected bone tumor, we looked for the shortest distance between the cutting plane and the tumor lesion. On MRI, the latter distance was measured three times and these measurements were averaged. These measurements were then compared to the preoperative planned measures.
3. Results
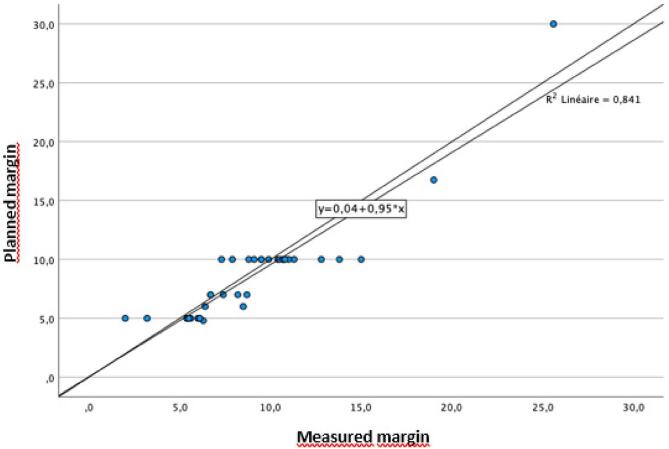
The mean difference measured between the planned margin and the obtained margin was 0.4 ± 1.8 mm (range, −4.4. to +5 mm). Negative value was given when the margin was less and positive value when the margin was more than the planned one (Table 2). There were 12 negative differences and 24 positive ones. The mean difference of 0.4 mm was not significantly different from 0 (p = 0,196). The correlation between planned margin and the obtained one was excellent (R2 = 0.841; p < 0.0001) (Fig. 3).
Table 2.
Comparison between planned margin and measured margin with MRI.
| Patient (P) | Minimal planned margin (mm) | Minimal margin measured on MRI (mm) | Difference (mm) |
|---|---|---|---|
| P1 | 5 | 3,2 | −1,8 |
| P2 | 10 | 11,3 | 1,3 |
| 16,75 | 19,0 | 2,3 | |
| P3 | 5 | 5,4 | 0,4 |
| P4 | 10 | 10,7 | 0,7 |
| 4,8 | 6,3 | 1,5 | |
| P5 | 5 | 6,0 | 1,0 |
| P6 | 10 | 15,0 | 5,0 |
| P7 | 10 | 7,3 | −2,7 |
| P8 | 10 | 13,8 | 3,8 |
| P9 | 10 | 9,5 | −0,5 |
| P10 | 30 | 25,6 | −4,4 |
| P11 | 10 | 11,0 | 1,0 |
| P12 | 10 | 9,1 | −0,9 |
| P13 | 6 | 8,5 | 2,5 |
| P14 | 6 | 6,4 | 0,4 |
| P15 | 5 | 5,4 | 0,4 |
| P16 | 10 | 10,4 | 0,4 |
| 10 | 9,9 | −0,1 | |
| P17 | 10 | 12,8 | 2,9 |
| P18 | 7 | 8,2 | 1,2 |
| P19 | 7 | 7,4 | 0,4 |
| P20 | 10 | 7,9 | −2,1 |
| P21 | 7 | 6,7 | −0,3 |
| P22 | 5 | 6,1 | 1,1 |
| P23 | 7 | 6,7 | −0,3 |
| P24 | 5 | 5,6 | 0,6 |
| P25 | 7 | 8,7 | 1,7 |
| 5 | 2,0 | −3,0 | |
| 5 | 5,5 | 0,5 | |
| P26 | 10 | 8,8 | −1,2 |
| P27 | 10 | 10,5 | 0,5 |
| P28 | 10 | 10,7 | 0,7 |
| P29 | 5 | 6,1 | 1,1 |
| P30 | 10 | 9,5 | −0,5 |
| P31 | 10 | 10,8 | 0,8 |
| Mean | 0,4 | ||
| Median | 0,5 | ||
| Range | 9,4 |
Bold typography: measures where cutting was less than planned.
Italic typography: closest and furthest measure to planning.
Fig. 3.
Correlation between planned margin and measured margin.
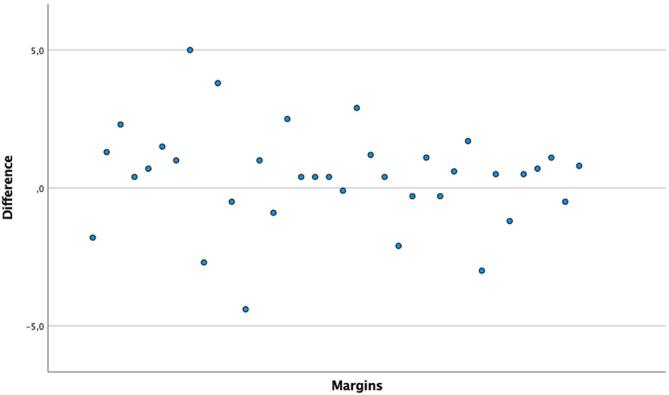
There was no negative difference more than 5 mm. 100% of differences were within the range −5 to +5 mm. 94% of the differences were within the range −4 to +4 mm; 93% within −3 and +3 mm; 75% within −2 and +2 mm and 53% within −1 and +1 mm (See Fig 4).
Fig. 4.
Margin differences (mm) between preoperative planning and postoperative measure.
As shown in Table 3, all resections in bone tissue were performed in safe margins (only one was noted R1, as planned for nerve root salvage). PSI does not impact resection margins in soft tissue. Despite the latter, good results were achieved in soft tissue resection margins. These results were assessed through a histopathological evaluation.
Table 3.
Histopathological margin assessment.
| Type of tissue | Margin classification | N = 31 |
|---|---|---|
| R soft tissues | R0 | 26 |
| R1 | 4 | |
| R2 | 0 | |
| Not visualized | 1 | |
| R bone tissues | R0 | 28 |
| R1 | 2 (planned) | |
| R2 | 0 | |
| Not visualized | 1 | |
The adamantinoma’s margins were impossible to visualize histopathologically.
4. Discussion
Patient Specific Instruments (PSI) are increasingly used in modern orthopedic surgery. Their field of use includes oncology, pediatric, arthroplastic and reconstructive surgery. In oncological resection, their objective is to improve the quality of surgical margins.
Cancer local control is the clinical endpoint of the PSI use. In complex bone tumor surgery, freehand cutting has become more and more outdated as several publications demonstrated that the procedure is exposed to major risk of positive resection margins [11], [16], [17]. When using this method, the clinical outcome therefore results in high rates of local recurrence. These tumor relapses are correlated to the poorer survival rates of the patients [18]. In the specific field of bone tumor surgery, navigation and PSIs have been demonstrated to be revolutionary in avoiding those positive margins and thus local recurrences. Furthermore, the operating time for complex surgeries have been greatly improved as reported in our previous studies [6], [7], [9], [10], [18], [19]. In the latter, we demonstrated the effectiveness of PSIs in pelvic bone tumor surgeries in terms of resection margins and several clinical parameters such as local recurrence, complication rate, age at the operation, operating time, type of tumor, etc.
This work provides additional data on the effectiveness and accuracy of the PSIs. Amongst 36 cutting planes, the mean difference measurement is not significantly different from 0 (0.4 mm). This demonstrate that the PSI is highly effective in both positioning and guiding the saw blade.
Out of 36, 19 cutting planes were planned with safety margins of 10 mm. Given the accuracy of the cutting guide within 5 mm, we would be able to reduce our planned margins at 5 mm when is needed and still remain safe.
To the best of our knowledge, only two other studies have evaluated the accuracy of PSI in bone tumor resections [8], [13]. Park et al. performed their study on a sample of 12 patients and compared the planned margins with the measures of the final pathology report. The mean cutting deviation was measured at 1.2 mm for the shortest margin and 1.4 mm for the greatest margin with a range comprised between 0 and 3 mm. The limitation of this study is that the pre- and postoperative measurement methods were very different. More recently, Müller et al. made a comparison using 3D modelling of 11 resected surgical specimens. They used a CT-scan of the resected tumors and analyzed the margins in the same software used for planning the resection. The mean cutting deviation was measured at 3.60 ± 2.46 mm with a range comprised between −6.4 mm to 7.7 mm. This study yielded results more inaccurate than reported in the study by Park et al. This may be attributed to the complexity of the osteotomies in their series.
Our study reports more accurate results with in a significantly larger sample size. A major difference with previous studies is also that our postoperative measurements are based on MRI images, making the contrast and image definition more accurate. In addition, the postoperative measurements were performed with the same software used for the preoperative tumor delineation.
The author who performed the postoperative measurements did so in a single-blind fashion to reduce information bias.
Chondrosarcoma is a particular tumoral entity due to its resistance to chemotherapy and radiotherapy. An interesting question would have been whether the delay between tumor delineation and resection (on average 3 weeks, depending on the complexity of the implant) allowed enough time for the chondrosarcoma to progress and expand a few millimeters and thus unbalance the accuracy of our measurements and statistical results. Amongst 36 measured cutting planes, 6 involved a chondrosarcoma. For 3 planes, the difference in measurement between postoperative and preoperative was positive (0.5 mm; 1.7 mm; 1.2 mm). For the other 3 planes, the difference was negative (-0.5 mm; −3mm; −0.3 mm). No conclusion can therefore be drawn as to whether patients suffering from chondrosarcomas should benefit from a faster conception for PSIs. A larger sample size is needed to confirm or refute this hypothesis.
However, our results must be interpreted in the light of the limitations of this study. Our study design is retrospective and monocentric. To our best knowledge, our sample is currently the largest in the literature. The rarity of bone tumors is responsible for the considerable heterogeneity of our series in terms of tumor entity, size and location. For the same reason, no control group was defined.
Nowadays, PSIs are in constant improvement in the orthopedic field. We believe that our results can help in achieving better results in terms of margins leading to less tumor relapses and better patient survival but also preserving healthy bone tissue whenever possible. Contemporarily, planning safe margins with PSIs have still some limits. We define a new statement about the accuracy limit of these instruments.
5. Conclusion
The quality of resection margins in bone tumor resection is improving day by day using technology such as PSIs. This study demonstrated that the accuracy of these cutting guides can be trusted. Still, an unwavering caution needs to be held due to some overestimation of planned resection margins. A larger sample size and more studies on this topic should allow us to achieve a gold standard in planning resection margin related to the accuracy of PSI.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Pierre-Louis Docquier reports financial support was provided by Foundation Against Cancer. Laurent Paul reports a relationship with 3D-Side that includes: employment.
Acknowledgement
Funding was received from the Foundation against cancer to pay the price of the PSI for all patients.
References
- 1.Abed R., Grimer R. Surgical modalities in the treatment of bone sarcoma in children. Cancer Treat. Rev. 2010;36:342–347. doi: 10.1016/j.ctrv.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Cartiaux O., et al. Computer-assisted and robot-assisted technologies to improve bone-cutting accuracy when integrated with a freehand process using an oscillating saw. J. Bone Joint Surg. Am. 2010;92:2076–2082. doi: 10.2106/JBJS.I.00457. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi N., Matumoto S., Manabe J. New method of evaluating the surgical margin and safety margin for musculoskeletal sarcoma, analysed on the basis of 457 surgical cases. J. Cancer Res. Clin. Oncol. 1995;121:555–563. doi: 10.1007/BF01197769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritacco L.E., Milano F.E., Farfalli G.L., Ayerza M.A., Muscolo D.L., Aponte-Tinao L.A. Accuracy of 3-D planning and navigation in bone tumor resection. Orthopedics. 2013;36(7) doi: 10.3928/01477447-20130624-27. [DOI] [PubMed] [Google Scholar]
- 5.Cartiaux O., Paul L., Francq B.G., Banse X., Docquier P.-L. Improved accuracy with 3D planning and patient-specific instruments during simulated pelvic bone tumor surgery. Ann. Biomed. Eng. 2014;42:205–213. doi: 10.1007/s10439-013-0890-7. [DOI] [PubMed] [Google Scholar]
- 6.Evrard R., Schubert T., Paul L., Docquier P.-L. Resection margins obtained with patient-specific instruments for resecting primary pelvic bone sarcomas: A case-control study. Orthop. Traumatol. Surg. Res. OTSR. 2019;105:781–787. doi: 10.1016/j.otsr.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Wong K.-C., Sze K.-Y., Wong I.-O.-L., Wong C.-M., Kumta S.-M. Patient-specific instrument can achieve same accuracy with less resection time than navigation assistance in periacetabular pelvic tumor surgery: a cadaveric study. Int. J. Comput. Assist. Radiol. Surg. 2016;11:307–316. doi: 10.1007/s11548-015-1250-x. [DOI] [PubMed] [Google Scholar]
- 8.Müller D.A., Stutz Y., Vlachopoulos L., Farshad M., Fürnstahl P. The Accuracy of Three-Dimensional Planned Bone Tumor Resection Using Patient-Specific Instrument. Cancer Manag. Res. 2020;12:6533–6540. doi: 10.2147/CMAR.S228038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouin F., Paul L., Odri G.A., Cartiaux O. Computer-Assisted Planning and Patient-Specific Instruments for Bone Tumor Resection within the Pelvis: A Series of 11 Patients. Sarcoma. 2014;2014:1–9. doi: 10.1155/2014/842709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deloin X., et al. Pelvic chondrosarcomas: Surgical treatment options. Orthop. Traumatol. Surg. Res. 2009;95:393–401. doi: 10.1016/j.otsr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Jentzsch T., Vlachopoulos L., Fürnstahl P., Müller D.A., Fuchs B. Tumor resection at the pelvis using three-dimensional planning and patient-specific instruments: a case series. World J. Surg. Oncol. 2016;14:249. doi: 10.1186/s12957-016-1006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan F.A., Lipman J.D., Pearle A.D., Boland P.J., Healey J.H. Surgical technique: Computer-generated custom jigs improve accuracy of wide resection of bone tumors. Clin. Orthop. 2013;471:2007–2016. doi: 10.1007/s11999-012-2769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J.W., et al. Bone tumor resection guide using three-dimensional printing for limb salvage surgery. J. Surg. Oncol. 2018;118:898–905. doi: 10.1002/jso.25236. [DOI] [PubMed] [Google Scholar]
- 14.Wong K.C., Kumta S.M., Geel N.V., Demol J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput. Aided Surg. 2015;20:14–23. doi: 10.3109/10929088.2015.1076039. [DOI] [PubMed] [Google Scholar]
- 15.Bellanova L., Schubert T., Cartiaux O., Lecouvet F., Galant C., Banse X., Docquier P.-L. MRI-Based Assessment of Safe Margins in Tumor Surgery. Sarcoma. 2014;2014:1–5. doi: 10.1155/2014/686790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delloye C., Banse X., Brichard B., Docquier P.-L., Cornu O. Pelvic reconstruction with a structural pelvic allograft after resection of a malignant bone tumor. J. Bone Joint Surg. Am. 2007;89:579–587. doi: 10.2106/JBJS.E.00943. [DOI] [PubMed] [Google Scholar]
- 17.S.E. Bosma, K.C. Wong, L. Paul, J.G. Gerbers, P.C. Jutte, A Cadaveric Comparative Study on the Surgical Accuracy of Freehand, Computer Navigation, and Patient-Specific Instruments in Joint-Preserving Bone Tumor Resections, Sarcoma (2018) e4065846. [DOI] [PMC free article] [PubMed]
- 18.Sabourin M., et al. Surgical management of pelvic primary bone tumors involving the sacroiliac joint. Orthop. Traumatol. Surg. Res. OTSR. 2009;95:284–292. doi: 10.1016/j.otsr.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Young P.S., Bell S.W., Mahendra A. The evolving role of computer-assisted navigation in musculoskeletal oncology. Bone Jt. J. 2015;97-B(2):258–264. doi: 10.1302/0301-620X.97B2.34461. [DOI] [PubMed] [Google Scholar]