Summary
Here, we describe a protocol to generate organoids from human thyroid cancer cells. Starting from the same patient-derived cells, we establish both organoids and primary lines. The organoid medium is supplemented with conditioned medium obtained from the primary cell line. This modification enables culture of the organoid lines for up to 10 months. Even after long-term culture, the organoids retain the genetic and phenotypic characteristics of their tissue of origin.
Subject areas: Cell Biology, Cancer, Organoids
Graphical abstract
Highlights
-
•
Generate organoids from human thyroid cancer cells
-
•
Use the conditioned medium obtained from the primary cell line for the growth
-
•
Generate models that maintain genetic and phenotypic characteristics of their tissue of origin
-
•
Use the models for translational research approaches
Publisher's note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Here, we describe a protocol to generate organoids from human thyroid cancer cells. Starting from the same patient-derived cells, we establish both organoids and primary lines. The organoid medium is supplemented with conditioned medium obtained from the primary cell line. This modification enables culture of the organoid lines for up to 10 months. Even after long-term culture, the organoids retain the genetic and phenotypic characteristics of their tissue of origin.
Before you begin
Antonica and colleagues in 2012 developed a method to reproduce thyroid follicles starting from embryonic stem cells using inducible constructs that expressed NKX2-1 and PAX8 in different stages of stem cells’ growth (Antonica et al., 2012).
Saito and colleagues in 2018 generated organoids from murine thyroid cells supporting proliferation of the adult stem cells using an hormone mix added to the 3D culture (Saito et al., 2018).
The use of both methods led to obtain thyroid follicles arising from murine tissues using a non-specific and commercially available hormone mix added with several growth factors, which is usually employed for the establishment of the organoids derived from different tissues.
In this work we propose an alternative method to grow organoids starting from human thyroid cancer cells and based on the use of a more affordable home-made culture medium. The conditioned medium enriched with the natural growth factors produced by the thyroid cancer cells was used to culture the organoids. Organoids can grow in this thyroid-specific culture medium up to 10 months.
Institutional permissions
The study design protocol was approved by the Ethics Committee of Azienda Universitaria Policlinico Umberto I of Rome. The study was conducted in compliance with the Declaration of Helsinki, and each subject signed an informed consent before participating in the study.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chrmical, peptides, and recombinant proteins | ||
| DMEM | Gibco-Thermo Fisher Scientific | #11965084 |
| DMEM/F12 | Gibco-Thermo Fisher Scientific | #11320033 |
| Fetal bovine serum (FBS) | Gibco-Thermo Fisher Scientific | #A4766801 |
| Penicillin/ Streptomycin | Gibco-Thermo Fisher Scientific | #15140122 |
| L-Glutamine | Gibco-Thermo Fisher Scientific | #25030081 |
| Conditioned Medium | Culture medium from primary culture (48H) | This study |
| Gly-His-Lys (GHL) | Sigma-Aldrich | #G-1887 |
| Hydrocortisone (HC) | Sigma-Aldrich | #H-0135 |
| Insulin from bovine pancreas (IBP) | Sigma-Aldrich | #I-5500 |
| Apo-Transferrin (ApoT) | Sigma-Aldrich | #T-2252 |
| Somatostatin (Som) | Sigma-Aldrich | #S-9129 |
| Tireotropin hormone (TSH) | Sigma-Aldrich | #T-3538 |
| Biological samples | ||
| Patient-derived thyroid cancer cells | Thyroid cancer patient tissues | This study |
| Other | ||
| Collagenase IV from Clostridium histolyticum | Gibco-Thermo Fisher Scientific | #17104019 |
| Basement Membrane Extract Reduced Growth Factor | Corning® Matrigel® | #CLS356231 |
Materials and equipment
Solutions preparations
Timing: 2 days
-
•
Thaw 3D Reduced Growth Factor Basement Membrane Extract (3D RGF-BME) at 2°C–8°C overnight and store one box of 1 mL tips at −80°C overnight.
-
•
The day after, prepare 1 mL aliquot of the RGF-BME in 1.5 mL tubes using the 1 mL tips stored at −80°C overnight.
-
•
Prepare the media and the solutions reported in UNDERLINED UPPERCASE letters in the text. The composition of each solution is reported follow:
| DMEM PEN/STREP 10× | ||
|---|---|---|
| Components | Stock concentration | Final concentration |
| DMEM | 1× | 1× |
| PENICILIN | 1,000× | 1,000 units/mL |
| STREPTOMYCIN | 1,000× | 1,000 μg/mL |
| DIGESTION MEDIUM | ||
|---|---|---|
| Components | Stock concentration | Final concentration |
| DMEM | 1× | 1× |
| Collagenase IV from Clostridium histolyticum | 10 mg/mL | 1 mg/mL |
| CULTURE MEDIUM | ||
|---|---|---|
| Components | Stock concentration | Final concentration |
| DMEM/F12 | 1:1 | 1:1 |
| Fetal bovine serum (FBS) | 100% | 10% |
| PENICILIN | 1,000× | 100 units/mL |
| STREPTOMYCIN | 1,000× | 100 μg/mL |
| L-Glutamine | 1,000× | 2 mM |
| ASSAY MEDIUM | ||
|---|---|---|
| Components | Stock concentration | Final concentration |
| DMEM/F12 | 1:1 | 1:1 |
| Basement Membrane Extract Reduced Growth Factor | 100% | 2% |
| Gly-His-Lys (GHL) | 1,000× | 20 μg/mL |
| Hydrocortisone (HC) | 1,000× | 10 nM (3.65 μg/mL) |
| Insulin from bovine pancreas (IBP) | 1,000× | 1 μg/mL |
| Apo-Transferrin (ApoT) | 1,000× | 5 μg/mL |
| Somatostatin (Som) | 1,000× | 10 ng/mL |
Step-by-step method details
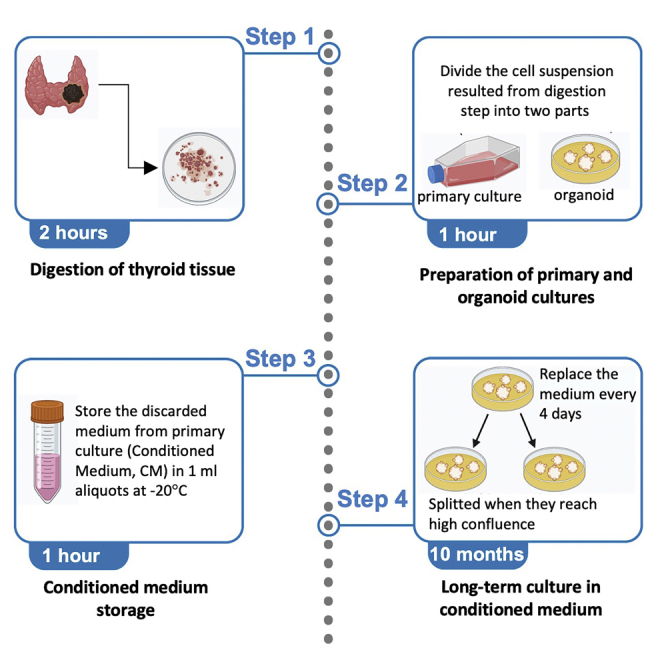
Digestion of thyroid tissues
Timing: 1–2 h
-
1.
Establish thyroid organoids starting from surgery specimens of thyroid cancer patients undergone to thyroidectomy.
-
2.
Take the surgical specimens (50–100 mg of tissue) in a 50 mL tube with 25 mL of DMEM PEN/STREP 10× on ice.
-
3.
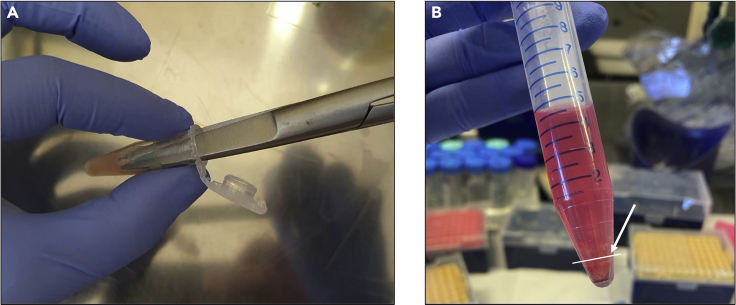
Put fragments of the specimens in a 1.5 mL tube with 500 μL of DMEM medium without supplements and mince using a sterile scissors inside the vial (Figure 1A).
-
4.
Digestion step: transfer the minced tissue in a 15 mL tube and add 5–10 mL of DIGESTION MEDIUM, incubate the mixture for 30 min at 37°C with rotation.
-
5.
In the meantime, working on ice, add 125 μL of 3D RGF-BME to each well in a sterile 96 well-plate with flat bottom, incubate the plate for 30 min at 37°C in the incubator (Debnath et al., 2003).
-
6.
After the digestion step, centrifuge cells in digestion medium at 1,000 rpm for 5′ and discard the supernatant (Figure 1B). Resuspend cells in 2 mL of DMEM medium without supplements.
Figure 1.
First steps of the digestion of thyroid tissues
(A) The mincing of the specimens using a sterile scissors inside the vial.
(B) Pellet after centrifugation of the specimens digested.
Preparation of primary and organoid cultures
Timing: 1 h
The cell suspension resulted in the step number 9 of the digestion phase is used to obtain both primary and organoid cultures.
-
7.
Divide the cell suspension resulted from digestion step into two parts, using two 15 mL tubes, transferring 1 mL of cells suspension in each tube. The cells will be used to obtain a primary culture and organoids respectively.
-
8.
Resuspend first half of the cells in 10 mL of CULTURE MEDIUM and plate it in 100 mm dish to obtain a primary culture. Incubate cells at 37°C in an atmosphere of 5% CO2. Replace the culture medium every 48 h.
Note: store the discarded medium from primary culture (CONDITIONED MEDIUM) in 1 mL aliquots at −20°C.
-
9.
Count cells with the methods usually used in your laboratory.
Note: Starting from a 50–100 mg specimen, we obtain usually among 1 × 105 and 1 × 106 cells/mL, we count cell with the hemocytometer.
-
10.
Working on ice, resuspend the second part of the cells in ASSAY MEDIUM in order to dilute cells to 1 × 104 cells/mL and swirl to mix.
Note: any unused 3D RGF-BME (both in the steps 8 and 13) can be stored at 2°C–8°C up to one week or stored in working aliquots at ≤ −20°C.
-
11.
Add 175 μL of cell suspension to each well of the 96-well plate containing 3D RGF-BME prepared in step 7 of digestion of thyroid tissues section.
-
12.
Incubate the plate at 37° in an atmosphere of 5% CO2.
-
13.
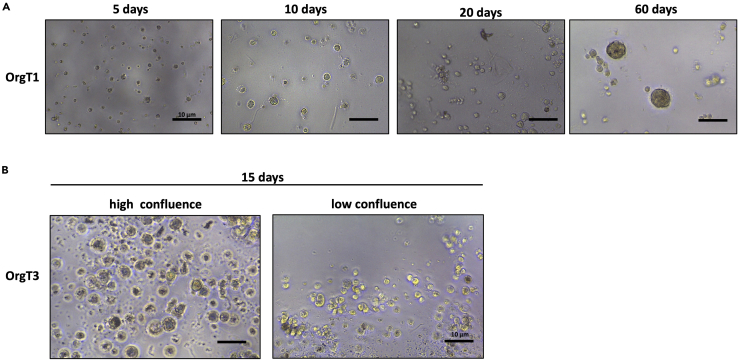
Each day, observe cell growth and structure formation using an inverted microscope and put the plate back into the incubator (Figure 2A).
Figure 2.
First step of organoid lines growth and confluence of particles
(A) Some representative images of the growth of OrgT1 line, at 5× of magnification. The time points represented are after 5 days, 10 days, 20 days, and 60 days of culture.
(B) Some representative images of the OrgT3 at low and high confluence of growth.
Long-term culture in conditioned medium
Timing: 1 weekto10 months
The organoids can be cultured for long time replacing the medium every 4 days. When they reach higher confluence as shown in Figure 2B, they need to be split into more than 1 well.
-
14.
Every 4 days, carefully pipette off old medium using a sterile serological pipette and replace the culture medium with 200 μL of CONDITIONED MEDIUM, the medium from primary culture stored at −20°C in step 11 of preparation of primary and organoid cultures section.
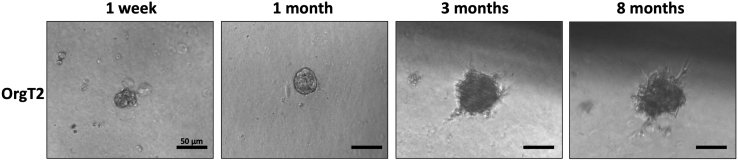
Note: There is no specific size that the organoids have to reach. It is mandatory that they grow for at least 10 days, when they show a more defined structure (as shown in Figure 3, after 1 month of growth). When the desired size and quantity of organoids have been reached, the organoids can be trypsinized and collected in pellets or fixed on slides for molecular and morphological analyses, respectively.
Figure 3.
Organoid line growth over the time
Some representative images of the growth of OrgT2 line, at 10× of magnification. The time points represented are after 1 week, 1 month, 3 months and 8 months. The reference bars correspond to 50 μm.
Split organoids
Timing: 1 h
When the organoid structures reach higher confluence (as shown in Figure 2B), they need to be split into more than 1 well.
-
15.
Prepare the 96-well plate with fresh 3D BME-RGF as described in step 8 of digestion of thyroid tissues section.
-
16.
Pipette off the medium from 96-well plate containing the organoids.
-
17.
Add 125 μL of 0,05% trypsin EDTA, incubate the plate at 37°C in the cell’s incubator for 5 min and mix the solution using a 1,000 μL tips until 3D RGF-BME reach the liquid state.
-
18.
Dilute the mixture in 125 μL CULTURE MEDIUM and divide it into 3 wells of the 96-well plate with fresh 3D BME-RGF prepared in step 16.
Expected outcomes
Using the method described in the methodology section, we established 4 lines of thyroid organoids, detailed in Table 1, one from healthy cells (N-line) and 3 of them derived from thyroid cancer cells (T-lines):
Table 1.
Organoid lines characteristics
| Hystotype | Tissue mutation | Organoid line mutation | Culture time | |
|---|---|---|---|---|
| OrgN1 | Normal Thyroid | – | – | 4 months |
| OrgT1 | PTC | BRAF p.V600E | BRAF p.V600E (30%) | 4 months |
| OrgT2 | Lymph node metastasis of PTC | BRAF p.V600E | BRAF p.V600E (3%) | 8 months |
| OrgT3 | ATC | PTEN p.R130X; TP53 p.R273C | PTEN p.R130X (100%); TP53 p.R273C (100%) | 10 months |
PTC: papillary thyroid cancer; ATC: anaplastic thyroid cancer.
OrgN1 arises from a normal thyroid tissue;
OrgT1 arises from papillary thyroid cancer (PTC);
OrgT2 arises from a lymph node metastasis of PTC;
OrgT3 arises from anaplastic thyroid cancer (ATC).
We also established a primary cell line for each organoid line starting from the same mixture of cells (with the method described in (Dima et al., 2015)), as described in the previous section. In addition, all the tumor specimens used have been characterized for the presence of hotspot mutations in the most important thyroid cancer driver genes, BRAF, N- K- H-RAS, PTEN, TP53 (Table 1) using Sanger Sequencing, as previously described in (Verrienti et al., 2016). For all organoid lines we used 4 wells of the 96-well plates after 4 months of culture. The 4 organoid lines were cultured from 4 to 10 months, a culture time longer than that of the primary cell lines established from the same cells. Indeed, primary cell lines were cultured from 1 to 2 months. Organoid lines were photographed one time for week for all the time they were cultured. In Figures 2 and 3 are reported some representative images of OrgT1 and T2 line.
In order to analyze and monitor the mutational status of the organoid lines over the culture time, using AllPrep DNA/RNA micro kit (QIAGEN), the DNA extracted from organoid lines of thyroid cancer were analyzed using next-generation sequencing (NGS), as described in (Sponziello et al., 2020; Verrienti et al., 2020). As summarized in Table 1, we confirmed the presence of the mutation found in the thyroid cancer tissues of origin in all 3 organoid cancer lines.
The OrgT1 line was grown up to 4 months when it was still positive for the p.V600E mutation of the BRAF gene. The OrgT1 life was shorter than those the other organoid lines. Maybe due to the origin of this line. Indeed, OrgT1 derived from a well differentiated thyroid cancer, that generates a lower rate of growth cells, Figure 2A.
After 8 months from the establishment, OrgT2 harbored a low-frequency p.V600E mutation in the BRAF gene (3%). This line arises from a lymph node metastasis; the stromal cells in this kind of tissue have a high growth rate when cultured. Therefore, stromal cells may have covered the mutated tumor cells after the establishment of this line, Figure 3.
On the contrary, the ATC organoid line (OrgT3) showed both the p.R130X mutation in the PTEN gene and p.R273C in the TP53 gene at 100% of allele frequency. The high rate of growth of ATC cells in culture and the optimized culture conditions led to select tumoral cells for more than 10 months of culture time, Figure 2B.
We found a method to establish thyroid cancer organoids and maintain the 3D structures in culture for a long time without using synthetic supplements in the culture medium. Instead, we exploited the natural release from the cancer cells of molecules necessary for cell-to-cell communication, establishing a primary culture arised from the same cell mixture. Then, the conditioned medium generated from the primary culture was used to supplement the growth organoid line. Moreover, this method results in being less expensive and more specific for thyroid cancer organoid growth than other methods that use commercial supplements composed of synthetic growth factors to grow organoids arised from different organs.
Limitations
Even if we found an alternative less expensive method to grow thyroid cancer organoids more experiments are mandatory to know the exact composition of the conditioned medium.
Moreover, the different characteristic of the human-derived cells, as number of cells, necrosis of the origin nodule, growth rate of the cells, etc., can influence the expected outcome of the protocols.
Troubleshooting
Problem 1
Small specimen (less than 50 mg).
Sometimes specimens might be very small due to pathology necessities, also in these cases it is possible to grow primary-cells thyroid organoids.
Potential solution
-
•
For small specimens, better results are obtained if smaller plates are used –20 mm dishes are recommended for specimens weighting 50 mg.
-
•
If specimens are less than 30 mg we recommend to use one well of a 12-well plate.
Problem 2
Primary cells may not attach to the dish.
Primary cells from some specimens will be more difficult to grow than others.
Potential solution
-
•
In this case we advise to use the conditioned medium of another human thyroid primary cell line, it is better if the primary cell line genotype is similar to the one of cancer cells in the specimen.
Problem 3
Primary cell culture is contaminated.
Primary cells culture contaminates very easily although the protocol is optimized to avoid contamination.
Potential solution
-
•
In case of frequent contamination, it is possible to rapidly wash the specimen in 100% Pen/Strep before passing in 1/10 Pen/Step and sample processing.
Problem 4
Lack of sterile scissors.
Some laboratories might lack sterile scissors or sterilization systems.
Potential solution
-
•
In case of lacking sterile scissors two sterile scalpel blades can be used to cut the specimen into small pieces. We recommend number 21 blades.
Problem 5
Matrigel is difficult to pipette and won’t polymerize well.
Matrigel is difficult to handle since its very close temperature use window (Liquid between +2 - +8°C).
Potential solution
-
•
In case of trouble in pipetting Matrigel it will solidify in the pipette tip and won’t be smooth for organoids growth. We recommend checking the liquid temperature that should always be between +2 and +8°C, it will be better to use refrigerator-cooled pipette tips.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Valeria Pecce (valeria.pecce@uniroma1.it).
Materials availability
There are restrictions to the availability of organoid lines used to set this protocol, they have finished their life cycle due to their human origin. However, we can provide under request different lines with similar characteristics.
Acknowledgments
Author contributions
All authors contributed to write the paper; V.P., M.S., and A.V. set the methodology and conducted the experiments; S.B. and G.G. recruited patients and collected clinic data; C.D. organized and coordinated the group.
Declaration of interests
The authors declare no competing interests.
Data and code availability
The organoid lines genetic data supporting the current study have not been deposited in a public repository but are available from the corresponding author on request.
References
- Antonica F., Kasprzyk D.F., Opitz R., Iacovino M., Liao X.H., Dumitrescu A.M., Refetoff S., Peremans K., Manto M., Kyba M., Costagliola S. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71. doi: 10.1038/nature11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J., Muthuswamy S.K., Brugge J.S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/S1046-2023(03)00032-X. [DOI] [PubMed] [Google Scholar]
- Dima M., Pecce V., Biffoni M., Di Gioia C.R.T., Tallini G., Biffoni M., Rosignolo F., Verrienti A., Sponziello M., Damante G., et al. Molecular profiles of cancer stem-like cell populations in aggressive thyroid cancers. Endocrine. 2015;53:145–156. doi: 10.1007/s12020-015-0739-y. [DOI] [PubMed] [Google Scholar]
- Saito Y., Onishi N., Takami H., Seishima R., Inoue H., Hirata Y., Kameyama K., Tsuchihashi K., Sugihara E., Uchino S., Ito K. Development of a functional thyroid model based on an organoid culture system. Biochem. Biophys. Res. Commun. 2018;497:783–789. doi: 10.1016/j.bbrc.2018.02.154. [DOI] [PubMed] [Google Scholar]
- Sponziello M., Brunelli C., Verrienti A., Grani G., Pecce V., Abballe L., Ramundo V., Damante G., Russo D., Lombardi C.P., et al. Performance of a dual-component molecular assay in cytologically indeterminate thyroid nodules. Endocrine. 2020;68:458–465. doi: 10.1007/s12020-020-02271-y. [DOI] [PubMed] [Google Scholar]
- Verrienti A., Tallini G., Colato C., Boichard A., Checquolo S., Pecce V., Sponziello M., Rosignolo F., de Biase D., Rhoden K., et al. RET mutation and increased angiogenesis in medullary thyroid carcinomas. Endocr. Relat. Cancer. 2016;23:665–676. doi: 10.1530/ERC-16-0132. [DOI] [PubMed] [Google Scholar]
- Verrienti A., Pecce V., Abballe L., Ramundo V., Falcone R., Inanloo Nigi Jak F., Brunelli C., Fadda G., Bosco D., Ascoli V., et al. Analytical validation of a novel targeted next-generation sequencing assay for mutation detection in thyroid nodule aspirates and tissue. Endocrine. 2020;69:451–455. doi: 10.1007/s12020-020-02372-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The organoid lines genetic data supporting the current study have not been deposited in a public repository but are available from the corresponding author on request.