1.
Dear Editors,
Herpes zoster is a relatively common disease caused by the reactivation of latent varicella zoster virus (VZV), human herpes type 3, in compromised patients. 1 Visceral disseminated VZV infection, a type of VZV disease without typical skin lesions, sometimes occurs in transplant recipients. 2 The presence of graft‐versus‐host disease, ongoing use of immunosuppressants, and lymphoid malignancies have been reported as risk factors for the reactivation of VZV after allogeneic stem cell transplantation (allo‐SCT). 3 , 4 However, there are extremely limited reports of cases of disseminated VZV infection following coronavirus disease 2019 (COVID‐19) vaccination. It is possible the shift to specific immunity mediated by messenger RNA (mRNA)‐based vaccines against severe acute respiratory syndrome coronavirus 2 (SARS‐COV‐2) weakens non‐specific immunity against non‐targeted viruses in an immunocompromised host. 5 , 6 , 7 We herein describe a case of visceral disseminated VZV infection following COVID‐19 vaccination that occurred concurrently with a decrease in the non‐specific CD8+ T‐cell count in an allo‐SCT recipient.
A 72‐year‐old man with acute myelomonocytic leukemia received cord blood transplantation with reduced intensity conditioning, consisting of fludarabine (180 mg/m2), melphalan (80 mg/m2), and antithymocyte globulin (2.5 mg/kg). Tacrolimus and short‐term methotrexate were administered as prophylaxis against graft‐versus‐host disease. Oral acyclovir (200 mg, daily) was given as prophylaxis against herpes zoster. After achieving engraftment, tacrolimus was tapered and discontinued on day 190. Glucocorticosteroids for cryptogenic organizing pneumonia were started on day 218 and tapered and stopped by day 333. Hemorrhage cystitis due to complex infection with adenovirus and BK virus occurred on day 328 and resolved with hydration. Since the recovery of CD4+ T cells reached almost 200/μl, oral daily acyclovir was ceased on day 521.
He received his first dose of Pfizer‐BNT 162b2 mRNA vaccine (COMIRNATY) on day 589 and did not experience early‐onset adverse effects. Fourteen days later, he developed serious, intolerable stomachache and back pain. A physical examination on admission, revealed no significant manifestations. The following differential diagnoses were all denied by contrast‐enhanced computed tomography of the chest–abdomen–pelvis, electrocardiography, echocardiography, and upper gastrointestinal fiberscopy: aortic dissection, pulmonary embolism, pneumothorax, pleuritis, cholangitis, gastrointestinal perforation, acute myocarditis, coronary syndromes, and ulcers in the esophagus, stomach, and duodenum. Medications taken at that time included a DPP‐4 inhibitor and metformin for type 2 diabetes mellitus, bisoprolol and apixaban for paroxysmal atrial fibrillation, and sulfamethoxazole trimethoprim, as prophylaxis against pneumocystis pneumonia, which appeared to be unrelated to the pain attack. Polymerase chain reaction (PCR) revealed his serum viral load of VZV was highly elevated (1.4 × 103 copies/1 × 106 cells), suggesting a diagnosis of visceral disseminated VZV infection. He had never received vaccination against VZV. At 4 days after his initial pain attack, scattered, small raised bumps appeared on the skin of his face, abdomen, and extremities, irrespective of the dermatomes. The exudate fluid of these bumps was positive for VZV antigen. Intravenous acyclovir (7.5 mg/kg, three times a day) was started. At 14 days after the initiation of antiviral treatment, his skin lesions and pain disappeared and PCR revealed his serum viral load had decreased to 4.7 × 101 copies/1 × 106 cells. His CD8+ T‐cell count dropped after COVID‐19 vaccination, which may have resulted in the reactivation of VZV (Figure 1). He received his second dose of the same vaccine after an interval of 8 weeks without any adverse effects. At the time of writing, he has achieved sustained complete remission of leukemia without any post‐infectious sequelae.
FIGURE 1.
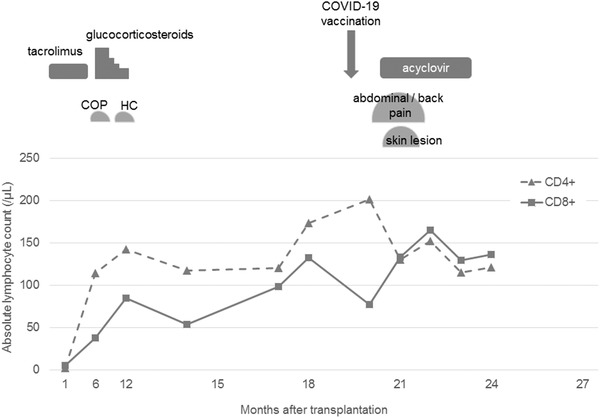
The clinical course and alteration of the absolute lymphocyte count before and after coronavirus disease 2019 (COVID‐19) vaccination. COP, cryptogenic organizing pneumonia; HC, hemorrhage cystitis
We reported a case of allo‐SCT with visceral disseminated VZV infection following COVID‐19 vaccination. The immunomodulation induced by mRNA‐based vaccines against SARS‐COV‐2 may trigger disturbance of immunity against VZV, leading to viral reactivation and dissemination. The CD8+ T‐cell count could be a surrogate marker of the magnitude of immunomodulation by mRNA‐based vaccines in allo‐SCT recipients.
While visceral disseminated VZV infection following allo‐SCT has been reported, 2 little is known about its relevance to COVID‐19 vaccination. There is only one reported case of disseminated VZV infection, which occurred 3 days after the second dose of an mRNA‐based vaccine in a patient who had received cord blood transplantation. 8 In line with the previous case, disseminated VZV infection occurred despite the discontinuation of tacrolimus and glucocorticosteroids, suggesting COVID‐19 vaccination may have triggered the reactivation of VZV in the current case. In addition, the cessation of prophylactic acyclovir 2 months before vaccination may have failed to prevent the reactivation of VZV, 9 and the patient's old age and comorbidity of type 2 diabetes mellitus may have also been associated with reactivation.
Although the reactivation of VZV following COVID‐19 vaccination was not reported in initial clinical trials, 10 an observational study in Israel showed an increased risk of herpes zoster in vaccinated patients. 11 Causality between VZV infection and COVID‐19 vaccination was recently suggested in a case series. In a systemic review of case reports describing 91 cases of VZV following COVID‐19 vaccination with three types of vaccines (Pfizer‐BNT162b2, AstraZeneca‐ChAdOx1‐nCoV19, and Moderna‐mRNA‐1273), the mean day of onset was 3.6–9 days after vaccination. 7 In the setting of other vaccines against inactivated hepatitis A, influenza virus, rabies, and Japanese encephalitis virus, the reactivation of herpesvirus infection was observed; however, this was uncommon. 12 Furthermore, in a study of school‐aged children, the administration of an inactivated influenza vaccine was suggested to be associated with an increased risk of non‐influenza respiratory virus infection. 13
The mechanisms of reactivation of VZV after COVID‐19 vaccination has yet to be determined. One possible hypothesis is that the temporal massive shift of naïve CD8+ T cells mediated by mRNA‐based vaccines contributes to the disturbance of VZV‐specific CD8+ T‐cell immunity, resulting in the failure to control VZV activation. 5 , 6 , 7 Patients with severe COVID‐19 have been reported to experience an immunosuppressive state with a decrease of T lymphocytes within 2 weeks after infection. 14 Similar to the disease, mRNA‐based vaccines can temporarily cause T‐cell immune dysfunction after initial rapid hyperactivation followed by exhaustion. Moreover, lower CD8+ T‐cell counts after vaccination seems to be related to higher seroconversion of anti‐SARS‐COV‐2 immunoglobin G (IgG) antibodies in allo‐SCT recipients. 15 Although we could not analyze the subsets of CD8+ T cells (e.g., naïve, effector, or memory T cells) in detail, the transient decrease of CD8+ T cells after vaccination reflects the alteration of immunity by mRNA‐based vaccines. Further studies should be conducted to validate this hypothesis.
The outcome of visceral disseminated VZV infection is dismal after allo‐SCT. 2 On the other hand, in the current case, a relatively rapid response to acyclovir was obtained and the symptoms resolved without any sequelae, including post‐herpetic neuralgia. The transient, but not profound, immune disturbance induced by mRNA‐based vaccines along with immediate treatment with antiviral agents may prevent progression to fulminant VZV infection.
In conclusion, we encountered a case of visceral disseminated VZV infection following COVID‐19 vaccination in an allo‐SCT recipient. We should bear in mind that reactivation of VZV infection may occur after COVID‐19 vaccination, particularly in transplant recipients. The decrease of CD8+ T cells after vaccination can be a surrogate marker of viral reactivation after allo‐SCT. Causality between COVID‐19 vaccination and reactivation of VZV has not yet been confirmed and further investigations are warranted to elucidate the precise mechanism and relevance of VZV reactivation after the administration of an mRNA‐based vaccine.
CONFLICT OF INTEREST
Masayuki Hino received research funding from Pfizer, speaker's honoraria from Pfizer and Sanofi. The other authors have no conflicts of interest to declare.
ETHICS STATEMENT
Written informed consent was obtained from the patient for publication of this article.
AUTHOR CONTRIBUTIONS
Mitsutaka Nishimoto took care of the patient and wrote the manuscript. Nobuhiro Sogabe and Masayuki Hino took care of the patient and made important recommendations regarding the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. Masatomo Kuno, Yosuke Makuuchi, Hiroshi Okamura, Yasuhiro Nakashima, Hideo Koh, and Hirohisa Nakamae for taking care of the patient.
Nishimoto M, Sogabe N, Hino M. Visceral disseminated varicella zoster virus infection following COVID‐19 vaccination in an allogeneic stem cell transplant recipient. Transpl Infect Dis. 2022;24:e13810. 10.1111/tid.13810
REFERENCES
- 1. Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42(2):325‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Doki N, Miyawaki S, Tanaka M, et al. Visceral varicella zoster virus infection after allogeneic stem cell transplantation. Transpl Infect Dis. 2013;15(3):314‐318. [DOI] [PubMed] [Google Scholar]
- 3. Kim DH, Messner H, Minden M, et al. Factors influencing varicella zoster virus infection after allogeneic peripheral blood stem cell transplantation: low‐dose acyclovir prophylaxis and pre‐transplant diagnosis of lymphoproliferative disorders. Transpl Infect Dis. 2008;10(2):90‐98. [DOI] [PubMed] [Google Scholar]
- 4. Arvin AM. Varicella‐zoster virus: pathogenesis, immunity, and clinical management in hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2000;6(3):219‐230. [DOI] [PubMed] [Google Scholar]
- 5. Furer V, Zisman D, Kibari A, Rimar D, Paran Y, Elkayam O. Herpes zoster following BNT162b2 mRNA COVID‐19 vaccination in patients with autoimmune inflammatory rheumatic diseases: a case series. Rheumatology. 2021;60:SI90‐SI95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Psichogiou M, Samarkos M, Mikos N, Hatzakis A. Reactivation of varicella zoster virus after vaccination for SARS‐CoV‐2. Vaccines. 2021;9(6):572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Triantafyllidis KK, Giannos P, Mian IT, Kyrtsonis G, Kechagias KS. Varicella zoster virus reactivation following COVID‐19 vaccination: a systematic review of case reports. Vaccines. 2021;9(9):1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Said J, Virgen C, Lian C, Cutler C, Merola J, LeBoeuf N. Disseminated varicella‐zoster virus infections following messenger RNA‐based COVID‐19 vaccination. JAAD Case Rep. 2021;17:126‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomson KJ, Hart DP, Banerjee L, Ward KN, Peggs KS, Mackinnon S. The effect of low‐dose aciclovir on reactivation of varicella zoster virus after allogeneic haemopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35(11):1065‐1069. [DOI] [PubMed] [Google Scholar]
- 10. Pormohammad A, Zarei M, Ghorbani S, et al. Efficacy and safety of COVID‐19 vaccines: a systematic review and meta‐analysis of randomized clinical trials. Vaccines. 2021;9(5):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barda N, Dagan N, Ben‐Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid‐19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walter R, Hartmann K, Fleisch F, Reinhart WH, Kuhn M. Reactivation of herpesvirus infections after vaccinations? Lancet. 1999;353(9155):810. [DOI] [PubMed] [Google Scholar]
- 13. Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis. 2012;54(12):1778‐1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diez‐Domingo J, Parikh R, Bhavsar AB, Cisneros E, McCormick N, Lecrenier N. Can COVID‐19 increase the risk of herpes zoster? A narrative review. Dermatol Ther. 2021;11(4):1119‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chiarucci M, Paolasini S, Isidori A, et al. Immunological response against SARS‐COV‐2 after BNT162b2 vaccine administration is impaired in allogeneic but not in autologous stem cell transplant recipients. Front Oncol. 2021;11:737300. [DOI] [PMC free article] [PubMed] [Google Scholar]


