Abstract
Background
Previous studies on post-infection fatigue in dengue are few but suggest that up to 25% of dengue patients may suffer from fatigue. This study aimed to evaluate the prevalence and associations of post-infection fatigue in dengue patients compared with non-dengue fever patients.
Methods
Post-infection fatigue and its demographic and clinical associations were assessed in adult dengue and non-dengue fever patients 2 months after the acute infection in a prospective cohort study in Sri Lanka. Fatigue at 2 months (primary endpoint) was assessed with the fatigue questionnaire as a dichotomous outcome based on a pre-recommended cut-off (score ≥4) and as the total score from the questionnaire (higher score indicates more fatigue).
Results
Of 260 patients, 158 had dengue and, of these, 51 (32%) had fatigue at 2 months. Risk was higher in dengue patients (vs non-dengue; relative risk [RR] 4.93 [95% confidence interval {CI} 2.3 to 10.4]) and more so in female dengue patients (vs male dengue patients; RR 2.45 [95% CI 1.24 to 4.86]). Severe dengue patients had a higher mean fatigue score (p=0.024).
Conclusions
Post-infection fatigue is an underappreciated burden of this widely prevalent infection. Our findings are useful to triage patients at risk of fatigue for follow-up.
Keywords: Colombo Dengue Study, dengue, fatigue, post-infection fatigue, Sri Lanka
Introduction
Post-viral fatigue is a frequently described clinical phenotype in the medical literature, but the number of prospective studies that have attempted to characterize it using a validated self-report tool are limited.1–3 Dengue is now arguably the most significant RNA virus infection in humans in terms of annual case incidence (an estimated 390 million cases per year) and the population at risk (an estimated 3.9 billion people), with active disease transmission occurring in 129 countries.4,5 There is evidence for post-infection fatigue in dengue from a few studies that systematically assessed it3,6 and from several other studies that use a variety of terms such as asthenia, malaise and weakness to refer to a similar phenomenon months after the acute infection.7,8 Evaluation of quality of life scores in dengue patients demonstrate that the deterioration of these scores outlasts the duration of fever9 and may impose a significant burden in terms of lost productivity.10
In a prospective study from Singapore,3 127 patients hospitalized for dengue fever were interviewed by telephone 2 months after the acute infection with a validated questionnaire to assess fatigue, the Fatigue Questionnaire (FQ).11 Post-infectious fatigue was found in 31 (25%) patients, but it is uncertain whether the findings are specific to dengue, since the study did not include patients hospitalised with other febrile illnesses. There are several other studies from Brazil, Peru, Cuba, India and Singapore that assessed the persistence of symptoms after recovering from acute dengue infection.7,8,12–17 However, these either do not specifically assess post-infection fatigue or simply assess it as a single question (e.g. Do you have fatigue?) in the medical interview without using a validated tool.
This study aims to systematically evaluate symptoms of post-infection fatigue persisting at 2 months post-infection in dengue patients while comparing the same in non-dengue fever patients. We also aim to identify clinical and demographic associations for post-infection fatigue in dengue.
Materials and methods
The Colombo Dengue Study (CDS) is a prospective observational cohort study conducted at the National Hospital of Sri Lanka (NHSL) by University of Colombo in collaboration with University of New South Wales, Sydney, Australia. A cohort description and an interim analysis of CDS has been published previously.18 In brief, CDS recruits adult patients with an acute febrile illness for <3 d when clinically suspected as dengue fever (by independent assessment of two medical officers). Patients are recruited from the NHSL, which is the largest public hospital in Sri Lanka. This hospital is in the Colombo district, which has the highest dengue incidence in Sri Lanka.19 This article refers to patients enrolled into the study from January 2018 to January 2020. Following admission, diagnosis of dengue is confirmed by both an NS1 antigen test at bedside (SD BIOLINE Dengue NS 1 antigen test, Alere San Diego, San Diego, CA, USA) and reverse transcription quantitative polymerase chain reaction (RT-qPCR).20 The RT-qPCR was carried out as previously described using oligonucleotide primers, dual-labelled probe for dengue virus (DENV) to four serotypes (Life Technologies, Pitam Pura, India) and TaqMan Multiplex Master Mix (catalogue no. 4461881; Applied Biosystems, Waltham, MA, USA) were used for the multiplex RT-qPCR in an ABI 7500 PCR system (Applied Biosystems).
All patients who were admitted on suspicion of dengue fever were managed as such, unless confirmed to have an alternative diagnosis (e.g. typhus, malaria, influenza, leptospirosis) during the hospital stay. After excluding those with an alternative diagnosis, two groups of patients were available to be interviewed at 2 months: confirmed dengue patients (either NS1 test or RT-qPCR was positive) and fever patients with an illness similar to dengue fever but remained undiagnosed (both NS1 test and RT-qPCR were negative for dengue). Children (<18 y of age) and patients with a past psychiatric history were excluded. During the acute infection, all consenting suspected dengue patients were interviewed on admission to hospital and followed up daily from admission to discharge and demographic characteristics, symptoms, laboratory parameters and outcomes (mortality, development of plasma leakage and severe dengue) were recorded. The severity of dengue infection and plasma leakage was documented based on World Health Organization 2009 guidelines on the classification of dengue fever.21
Post-infection fatigue was assessed by the FQ,11 as used by Seet et al.3 in the previous study from Singapore, 2 months after discharge via a telephone interview of consenting patients (conducted by the same investigator who interviewed the patient during acute infection). This tool has been validated previously for dengue patients in Sri Lanka.6 The questionnaire consists of 11 items related to physical and mental fatigue. Each item of the questionnaire has four responses (0, none; 1, mild; 2, moderate; 3, severe). A higher score indicates more fatigue and a cut-off ≥4 has been recommended by previous authors22 when a dichotomous outcome (presence or absence of fatigue) needs to be recorded. We report the results in two subsections corresponding to these two ways of interpreting the FQ (the absolute score and the presence or absence of fatigue). This is because recording a dichotomous outcome alone may not capture the different intensities of symptoms experienced by people.
For an effect size (relative risk [RR]) of 3 between the two groups at a confidence of 0.95 and a power of 80%, the required sample size was 138 per group (in absence of an estimate from previous literature, the incidence of fatigue in non-dengue patients was considered to be at 5%). The presence of fatigue and mean scores of the FQ were compared as dependent variables (in both dengue and non-dengue patients) against patient demographics, symptoms and laboratory investigation results. Furthermore, for dengue patients, the same dependent variables were assessed against the presence of plasma leakage, severe dengue, viral load and infecting serotype. The χ2 test, independent t test and Mann–Whitney U test were used to explore statistical significances of dichotomous and continuous variables depending on the normality of distributions. Statistical significance was set at p<0.05 and was adjusted for multiple comparisons using the Bonferroni correction. When laboratory investigations were compared (as multiple measurements were available for each test), we compared the following parameters: first test value within 3 d of fever (early disease) and highest, lowest and mean value per test throughout the entire hospital stay. All analyses were performed using Stata version 14.0 (StataCorp, College Station, TX, USA).
Results
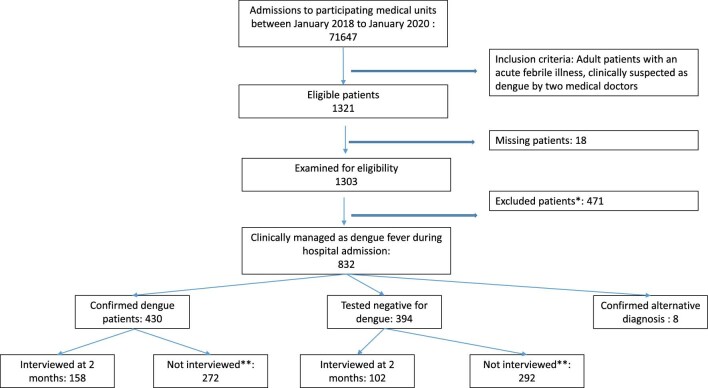
A total of 260 patients (dengue 158, non-dengue 102) were interviewed at 2 months. Their mean age was 34.3 y (range 14–83, standard deviation 15.3) and 164 (63.3%) were males. A breakdown of patient numbers that were followed up during hospital admission, confirmed to have dengue infection (or not) and then subsequently interviewed is given in Figure 1. The dengue group had more males, a younger age distribution and more clinical symptoms during acute illness compared with the non-dengue patients, as shown in Table 1. A detailed breakdown of patient responses to each component of the FQ is given in Supplementary Table 1.
Figure 1.
Flow chart of patient recruitment for follow-up interviews at 2 months. *Excluded due to either no consent, age <18 y, self-discharge, admitted with fever for >3 d or already in plasma leakage on admission. **Not interviewed due to no consent (for interviews), past psychiatric illness or not providing contactable phone numbers (two attempts made per person). All interviewed patients were included in the analysis.
Table 1.
Descriptive statistics of demographic variables and illness-related characteristics of dengue and non-dengue patients
| Dengue patients | Non-dengue patients | ||
|---|---|---|---|
| Characteristics | (n=158) | (n=102) | p-Value |
| Demographics | |||
| Male, n (%) | 88 (56.1) | 76 (74.%) | 0.003** |
| Age (years), median | 28 | 36 | 0.000** |
| Pre-existing metabolic comorbidity,a n (%) | 16 (10.1) | 19 (18.6) | NS |
| Smoker, n (%) | 12 (7.6) | 10 (9.8) | NS |
| Alcohol use, n (%) | 11 (7.0) | 12 (11.8) | NS |
| Average monthly income (US$), median | 188.50 | 215.42 | NS |
| Clinical symptoms, n (%) | |||
| Headache | 110 (69.6) | 48 (47.1) | 0.000** |
| Myalgia | 106 (67.1) | 56 (54.9) | 0.048* |
| Arthralgia | 87 (55.1) | 41 (40.2) | 0.019* |
| Diarrhoea | 29 (18.4) | 9 (8.8) | 0.034* |
| Abdominal pain | 51 (32.3) | 15 (14.7) | 0.001** |
| Dyspnoea | 4 (2.5) | 1 (1.0) | NS |
| Vomiting | 61 (38.6) | 24 (23.5) | 0.011* |
| Retro-orbital pain | 20 (12.7) | 10 (9.8) | NS |
| Bleeding | 24 (15.2) | 5 (5.0) | 0.010* |
| Laboratory investigations, median | |||
| Haemoglobin (g/dL) | 13.72 | 13.9 | NS |
| Haematocrit (%) | 40.45 | 40.65 | NS |
| Platelet count (×103/μL) | 97.50 | 129.00 | 0.0001** |
| Total leukocyte count (×109/L) | 3.68 | 5.65 | 0.0000** |
| Neutrophil count (×109/L) | 1.93 | 2.59 | 0.0002** |
| Lymphocyte count (×109/L) | 1.22 | 1.63 | 0.0003** |
| Aspartate aminotransferase (U/L) | 61.0 | 39.00 | 0.0013** |
| Alanine aminotransferase (U/L) | 48.67 | 36.00 | 0.0353* |
| Serum sodium (mmol/L) | 136.00 | 137.00 | 0.0056* |
| Serum potassium (mmol/L) | 3.70 | 3.90 | 0.0323* |
| Serum creatinine (µmol/L) | 67.75 | 1.30 | 0.0159* |
| C-reactive protein (mg/dL) | 14.00 | 14.50 | NS |
| Serum total bilirubin (mg/dL) | 8.39 | 1.20 | NS |
*Statistically significant with a p-value <0.05.
**Statistically significant with a Bonferroni adjusted p-value <0.013 (demographics), <0.006 (clinical symptoms) or <0.004 (for laboratory investigations).
Includes diabetes mellitus, hypertension, hyperlipidaemia and ischaemic heart disease.
NS: not significant.
Associations for the presence or absence of fatigue (as a dichotomous outcome)
When defining post-infection fatigue as a dichotomous outcome based on the cut-off (score ≥4), 60 patients had fatigue. This included 51 (32.3%) dengue patients and 9 (8.8%) non-dengue patients. Dengue patients had a significantly higher risk of fatigue at 2 months after the acute infection (RR 4.93 [95% confidence interval {CI} 2.3 to 10.4], p<0.001). Females with dengue infection were more likely to have fatigue than males (RR 2.45 [95% CI 1.24 to 4.86], p<0.05). A similar gender-based association for post-infection fatigue was not seen for non-dengue patients (Table 2). None of the clinical features and laboratory parameters assessed had a significant association with the presence of post-infection fatigue in both dengue and non-dengue patients (Tables 3 and 4). There were no associations between minimum, maximum, median and mean values of laboratory parameters assessed throughout the hospital stay and the presence of post-infection fatigue in dengue patients (Supplementary Table 2). In dengue patients, the infecting serotype was known for 132 patients (DENV2: 86; DENV1, 3 and 4: 46), viral load was available for 116 patients, plasma leakage was observed in 45 patients and severe dengue was seen in 10 patients. None of these characteristics were associated with the presence of fatigue 2 months post-infection (Table 5).
Table 2.
Associations between patient sociodemographic characteristics and presence of fatigue
| Dengue (n=158) | Non-dengue (n=102) | |||||
|---|---|---|---|---|---|---|
| Fatigue | Fatigue | |||||
| Characteristics | Yes | No | RR (95% CI) | Yes | No | RR (95% CI) |
| Age (years), median | 30.0 | 27.0 | 1.02 (1.00 to 1.05) | 28.0 | 36.0 | 1.00 (0.96 to 1.04) |
| Gender | ||||||
| Female | 30 | 39 | 2.45* (1.24 to 4.86) | 3 | 23 | 1.52 (0.35 to 6.58) |
| Male | 21 | 67 | 6 | 70 | ||
| Pre-existing comorbidity | ||||||
| Yes | 7 | 9 | 1.73 (0.61 to 4.95) | 4 | 15 | 4.16 (1.00 to 17.32) |
| No | 44 | 98 | 5 | 78 | ||
| Smoking | ||||||
| Yes | 4 | 8 | 1.05 (0.30 to 3.67) | 1 | 9 | 1.17 (0.13 to 10.42) |
| No | 47 | 99 | 8 | 84 | ||
| Alcohol consumption | ||||||
| Yes | 2 | 9 | 0.44 (0.09 to 2.14) | 1 | 11 | 0.93 (0.11 to 8.18) |
| No | 49 | 98 | 8 | 82 | ||
*Statistically significant with a p-value <0.05.
Table 3.
Comparison of clinical features of dengue and non-dengue patients with the presence of fatigue
| Dengue | Non-dengue | ||||||
|---|---|---|---|---|---|---|---|
| Clinical features | Fatigue | No fatigue | OR (95% CI) | Fatigue | No fatigue | OR (95% CI) | |
| Headache | Yes | 34 | 76 | 0.82 (0.40 to 1.26) | 4 | 44 | 0.89 (0.22 to 3.53) |
| No | 17 | 31 | 5 | 49 | |||
| Myalgia | Yes | 33 | 73 | 0.85 (0.42 to 1.73) | 3 | 53 | 0.38 (0.09 to 1.60) |
| No | 18 | 34 | 6 | 40 | |||
| Arthralgia | Yes | 31 | 56 | 1.41 (0.72 to 2.78) | 3 | 38 | 0.72 (0.17 to 3.07) |
| No | 20 | 51 | 6 | 55 | |||
| Diarrhoea | Yes | 13 | 16 | 1.95 (0.85 to 4.44) | 0 | 9 | NA |
| No | 38 | 91 | 9 | 84 | |||
| Abdominal pain | Yes | 19 | 32 | 1.39 (0.69 to 2.81) | 2 | 13 | 1.76 (0.33 to 9.41) |
| No | 49 | 75 | 7 | 80 | |||
| Dyspnoea | Yes | 2 | 2 | 2.14 (0.29 to 15.66) | 0 | 1 | NA |
| No | 49 | 105 | 9 | 92 | |||
| Vomiting | Yes | 14 | 47 | 0.48 (0.23 to 1.00) | 3 | 21 | 1.71 (0.39 to 7.45) |
| No | 37 | 60 | 6 | 72 | |||
| Retro-orbital pain | Yes | 5 | 15 | 0.67 (0.23 to 1.95) | 1 | 9 | 1.17 (0.13 to 10.42) |
| No | 46 | 92 | 8 | 84 | |||
| Bleeding | Yes | 10 | 14 | 1.62 (0.66 to 3.95) | 0 | 5 | NA |
| No | 41 | 93 | 9 | 88 | |||
NA: not applicable.
Table 4.
Comparison of laboratory investigations (within the first 3 d of fever) of dengue and non-dengue patients with the presence of fatigue
| Dengue | Non-dengue | |||
|---|---|---|---|---|
| Laboratory parameter | Fatigue (n=51) | No fatigue (n=107) | Fatigue (n=9) | No fatigue (n=93) |
| Haemoglobin (g/dL), median | 13.3 | 13.3 | 13.6 | 13.6 |
| Haematocrit (%), median | 39.4 | 39.7 | 41.4 | 41.5 |
| Platelet count (×103/μL), median | 126.0 | 135.0 | 130.0 | 137.5 |
| Total leukocyte count (×109/L), median | 4.2 | 4.1 | 5.2 | 5.3 |
| Neutrophil count (×109/L), median | 2.3 | 2.9 | 2.5 | 3.1 |
| Lymphocyte count (×109/L), median | 0.6 | 0.7 | 1.1 | 1.2 |
| Aspartate aminotransferase (U/L), median | 50.0 | 51.5 | 80.0 | 45.0 |
| Alanine aminotransferase (U/L), median | 34.0 | 41.5 | 89.5 | 36.5 |
| Serum sodium (mmol/L), median | 135.0 | 136.0 | 141.0 | 137.0 |
| Serum potassium (mmol/L), median | 3.7 | 3.7 | 4.4 | 3.8 |
| Serum creatinine (µmol/L), median | 59.0 | 75.0 | 41.6 | 1.3 |
| C-reactive protein (mg/dL), median | 13.0 | 15.5 | 8.5 | 14.0 |
| Serum total bilirubin (mg/dL), median | 4.9 | 8.5 | 7.1 | 1.1 |
Table 5.
Serotype, plasma leakage and severity of dengue with the presence of fatigue among dengue patients
| Variable | Fatigue | No fatigue | RR (95% CI) | |
|---|---|---|---|---|
| Serotype | DENV2 | 25 | 61 | 0.85 (0.39 to 1.83) |
| DENV1, 3 and 4 | 15 | 31 | ||
| Plasma leakage | Yes | 19 | 26 | 1.85 (0.90 to 3.80) |
| No | 32 | 81 | ||
| Severe dengue | Yes | 6 | 4 | 3.43 (0.92 to 12.7) |
| No | 45 | 103 | ||
Comparison of fatigue scores (as a continuous variable) across different groups
Dengue patients had a significantly higher mean fatigue score compared with non-dengue patients (3.5 vs 0.7; p<0.001). The mean difference in fatigue scores for female dengue patients was significantly higher compared with male patients (mean 5.34 vs 2.00; p<0.001). A similar difference between genders was not seen between non-dengue patients (mean 1.38 vs 0.43; p>0.05). Both dengue and non-dengue patients with diabetes had significantly higher fatigue scores compared with those without diabetes (Supplementary Table 3). There were no significant associations for fatigue scores and the presence or absence of clinical symptoms in dengue and non-dengue patients (Supplementary Table 4). There were also no significant differences in fatigue scores between groups infected with different serotypes (DENV2 vs others) or between those who had plasma leakage and others. However, those who had severe dengue had a significantly higher mean fatigue score than other dengue patients (7.4 vs 3.18; p=0.024) (Supplementary Table 5). There was no correlation between viral load and fatigue score (data not shown).
Discussion
This study shows that dengue fever is associated with post-infection fatigue up to 2 months post-infection compared with non-dengue patients with an acute febrile illness. Post-infection fatigue was seen in approximately one-third of dengue patients. Female dengue patients were more likely to meet the definition of fatigue according to the FQ, while those with diabetes or severe dengue were also likely to have a higher score on the FQ compared with others.
Studies on post-infection fatigue in dengue are rare. A recent systematic review that collated evidence from prospective studies assessing fatigue after any acute infection (even when a specific diagnosis was not made) using a validated self-report tool found only 17 eligible studies published by March 2016 across multiple databases (MEDLINE, PsychINFO, Embase).23 Almost half of these studies (n=9) were on glandular fever, while 3 included any acute infection non-specifically.23 One study each was on Ross River virus24 and dengue virus3 patients. These individual studies attribute various characteristics, such as illness severity, pre-existing psychiatric problems, clinical examination findings (e.g. lymphadenopathy) and perceived levels of stress, to persistent fatigue (assessed 2–6 months after acute infection).1,3,23–25 The authors of the meta-analysis conclude that risk factors for post–acute infection fatigue can be classified under five recurring themes: biological, social, behavioural, emotional and cognitive.23
The prevalence of post-dengue fatigue (32.3%) in our study is the highest reported in the literature, but the estimate of 25% by Seet et al.3 in Singapore using the same questionnaire and cut-off is not far behind. Similarly, a smaller, single-centre study in Sri Lanka (which also used the FQ) found 17% of patients (9/52) to have fatigue 1 month after infection.6 In combination, these results suggest that nearly one-fifth to one-third of dengue patients may suffer from symptoms of post-infection fatigue 1–2 months after infection. This is significant considering that an estimated 96 million symptomatic dengue cases occur every year.
It is not clear how dengue leads to the persistent fatigue. It may be that the complex immunological response (especially in secondary infection) initiated by infection leads to excess cytokine production during the acute phase26 and the interaction of these cytokines with the neuroendocrine, musculoskeletal and immunological systems might contribute to persistent fatigue.27 These interactions may also be modulated by host factors such as genetics, sickness, personality traits and vulnerability to psychological stress. However, both our observations and those by Seet et al.3 indicate that most symptoms and laboratory markers (during acute infection) and having plasma leakage did not predict post-infection fatigue. Notably though, in our study, those with severe dengue had on average a significantly higher score on the FQ compared with other dengue patients, but the size of this subgroup was too small for a definite conclusion. We did not
measure cytokine levels during acute admission to correlate with persistent fatigue.
As for associations of post-infection fatigue in dengue, Seet et al.3 concluded that female gender and older age were significant associations. Other studies on post-dengue fatigue are mostly descriptive and do not explore associations for fatigue.6,17 Several other prospective studies explore associations for persistent symptoms (not necessarily fatigue) in dengue and if fatigue was assessed, it was only recorded as part of a medical interview without using a dedicated self-report tool.8,13,16 Yet the association of female gender with persistent symptoms is a recurring finding in several of these studies.7,8,14,15 The largest of these was a multicentre prospective study from Peru that enrolled 3659 dengue patients and 5408 non-dengue febrile controls to record the persistence of six types of symptoms (respiratory, gastrointestinal, rash, fatigue, headache and body pain) up to 2 months after acute dengue infection. Fatigue in this study was defined as the presence of ‘malaise/asthenia’ and not by a detailed questionnaire. Interestingly, this study found a higher prevalence of fatigue in non-dengue patients (3.8% vs 2.7%; p<0.01), but since a validated tool was not used to assess fatigue, these results are not directly comparable to ours. However, in this study also, females in the dengue group were significantly more likely to report at least one persistent symptom, but not in the control group. The recurrent observation of vulnerability to fatigue in females in both dengue and other conditions (e.g. higher prevalence of chronic fatigue syndrome) have been attributed to hormonal imbalances, reproductive function,28 genetics29 and psychosomatic handling of stress30 or a combination of these. Still, other authors suggest that this discrepancy may be due to males being more reluctant to report symptoms due to cultural or gender norms in some societies. Past psychiatric history (e.g. depression or anxiety disorders) may also influence in self-reporting of fatigue, but in our study anyone with a past psychiatric history was excluded. However, we acknowledge that some people may have undiagnosed psychiatric comorbidities and it is also difficult to adjust for personality traits influencing the role of sickness. While the exact reason for the higher incidence of post-dengue fatigue in females cannot be resolved by an epidemiological cohort study such as ours, the recurrent association of females having either fatigue or persistent symptoms after an acute illness should alert clinicians to arrange a follow-up visit. It should also alert researchers and policy planners to consider evaluating the economic impact of post-dengue fatigue (days lost to work or school) to minimize a neglected part of the dengue disease burden. A recent modelling of economic burden due to persistent dengue symptoms in Mexico estimated an additional cost of approximately US$22.6 million per year,10 which is not trivial.
This study has several limitations. The most notable is that a definite diagnosis was not made for patients in the non-dengue group. However, making a diagnosis is not considered cost effective for patients who often recover from a viral flu-like illness and they were symptomatically managed as for dengue until discharge. Making a diagnosis in this context is for academic interest only, as it usually does not alter patient management. However, if patients were clinically suspected of having an alternative diagnosis (e.g. malaria, leptospirosis), specific diagnostic tests were carried out and those with a confirmed alternative diagnosis were excluded from this study. As for other limitations, the definition of fatigue in this study as determined by the FQ was entirely subjective and based on self-reports, which may be biased by pre-existing sickness and personality traits. As highlighted in Table 1, there were some significant differences at baseline between the dengue group and non-dengue group and hence caution must be exercised in interpretations when these two groups are compared against each other. For example, although we can confirm that females with dengue were at higher risk of having fatigue compared with males with dengue, we cannot confirm the results of the same analysis for the non-dengue group (which showed no association between genders), given the small number of females in that group. We also cannot confirm that the higher risk of females having fatigue is unique to dengue fever, as the non-dengue ‘control’ group had fewer females. The control group was smaller than the expected sample size because some patients had to be excluded after an alternative diagnosis was confirmed and more in this group refused consent to be interviewed at 2 months. Similarly, in the serotype analysis, most infections were of DENV2, so all other serotypes had to be combined into a single group to compare with DENV2. Finally, this cohort is from a single centre in Sri Lanka that excluded patients <18 y of age. The lack of heterogeneity in some confounders (sociodemographic variables, cultural variables, younger age groups), limits the generalizability of our results. Yet it highlights the fact that this phenomenon needs to be explored globally to understand its true impact.
In conclusion, up to one-third of dengue patients may be have post-infection fatigue, with a higher risk in females. From a clinical perspective, these patients may benefit from a follow-up visit to discuss their adjustment to activities of daily living, study and work. However, arranging follow-up for all patients during epidemics may be impractical and therefore developing a triaging system to recall patients based on risk associations as identified in this and other similar studies would be a more feasible approach. Alternatively, patient education at discharge on post-infection fatigue in dengue will help those needing further assistance to seek medical advice. From a research perspective, more studies in different countries are required to systematically evaluate post-infection fatigue in dengue to identify the role of confounders and associations that may influence its impact.
Supplementary Material
Contributor Information
Ponsuge C Sigera, Department of Parasitology, Faculty of Medicine, University of Colombo, 00800, Colombo 08, Sri Lanka.
Senaka Rajapakse, Department of Clinical Medicine, Faculty of Medicine, University of Colombo, 00800, Colombo 08, Sri Lanka.
Praveen Weeratunga, Department of Clinical Medicine, Faculty of Medicine, University of Colombo, 00800, Colombo 08, Sri Lanka.
Nipun L De Silva, Department of Clinical Medicine, Faculty of Medicine, General Sir John Kotelawala Defence University, 10390, Ratmalana, Sri Lanka.
Laksiri Gomes, Centre for Dengue Research, Faculty of Medical Sciences, University of Sri Jayewardenepura, 10250, Nugegoda, Sri Lanka.
Gathsaurie N Malavige, Centre for Dengue Research, Faculty of Medical Sciences, University of Sri Jayewardenepura, 10250, Nugegoda, Sri Lanka.
Chaturaka Rodrigo, Department of Pathology, School of Medical Sciences, University of New South Wales, Sydney, NSW 2052, Australia.
Sumadhya D Fernando, Department of Parasitology, Faculty of Medicine, University of Colombo, 00800, Colombo 08, Sri Lanka.
Authors’ contributions
PCS, SR, PW, CR and SDF conceptualized the study. PCS did the patient interviews, data analysis (with contributions from PW and NLdS) and writing of the first draft under the supervision of SDF and CR. LG and GNM provided laboratory supervision and support for dengue serotyping. CR and SDF shared equal supervisory responsibilities for the project. All authors revised and approved the final version of the manuscript. CR and SDF contributed equally to this work.
Funding
The work was supported by the University of Colombo, Sri Lanka (grant no, AP/3/2/2017/CG/25) and a National Health and Medical Research Council, Australia (Investigator grant no. 1173666).
Competing interests
Authors have no conflicts of interest regarding the content of this manuscript.
Ethics approval
Ethics approval for the study was obtained from the Ethics Review Committee, Faculty of Medicine, University of Colombo (EC/17/080) and the Ethics Review Committee, NHSL (ETH/COM/2017/12). Written consent was obtained from the copyright holders to use the Fatigue Questionnaire. Written informed consent was obtained from all the participants before their recruitment for the study.
Data availability
The data presented in this article can be shared upon reasonable request to the corresponding author. Details that may lead to identification of individual patients will not be made available.
References
- 1. White PD, Thomas JM, Kangro HO et al. Predictions and associations of fatigue syndromes and mood disorders that occur after infectious mononucleosis. Lancet. 2001;358(9297):1946–54. [DOI] [PubMed] [Google Scholar]
- 2. Löwe B, Andresen V, Fraedrich K et al. Psychological outcome, fatigue, and quality of life after infection with shiga toxin–producing E scherichia coli O104. Clin Gastroenterol Hepatol. 2014;12(11):1848–55. [DOI] [PubMed] [Google Scholar]
- 3. Seet RCS, Quek AML, Lim ECH. Post-infectious fatigue syndrome in dengue infection. J Clin Virol. 2007;38(1):1–6. [DOI] [PubMed] [Google Scholar]
- 4. Bhatt S, Gething PW, Brady OJ et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brady OJ, Gething PW, Bhatt S et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6(8):e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Umakanth M. Post dengue fatigue syndrome (PDFS) among dengue IgM-antibody positive patients at Batticaloa Teaching Hospital, Sri Lanka. Open Access Lib J. 2018;5:e4798. [Google Scholar]
- 7. Halsey ES, Williams M, Laguna-Torres VA et al. Occurrence and correlates of symptom persistence following acute dengue fever in Peru. Am J Trop Med Hyg. 2014;90(3):449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. García G, González N, Pérez AB et al. Long-term persistence of clinical symptoms in dengue-infected persons and its association with immunological disorders. Int J Infect Dis. 2011;15(1):e38–43. [DOI] [PubMed] [Google Scholar]
- 9. Lum LC, Suaya JA, Tan LH et al. Quality of life of dengue patients. Am J Trop Med Hyg. 2008;78(6):862–7. [PubMed] [Google Scholar]
- 10. Tiga DC, Undurraga EA, Ramos-Castañeda J et al. Persistent symptoms of dengue: estimates of the incremental disease and economic burden in Mexico. Am J Trop Med Hyg. 2016;94(5):1085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res. 2004;56(2):157–70. [DOI] [PubMed] [Google Scholar]
- 12. Gonzalez D, Martinez R, Castro O et al. Evaluation of some clinical, humoral and imagenological parameters in patients of dengue haemorrhagic fever six months after acute illness. Dengue Bull. 2005;29:79–84. [Google Scholar]
- 13. Low JG, Ooi EE, Tolfvenstam T et al. Early Dengue infection and outcome study (EDEN) – study design and preliminary findings. Ann Acad Med Singapore. 2006;35(11):783–9. [PubMed] [Google Scholar]
- 14. Dettogni RS, Tristão-Sá R, Dos Santos M et al. Single nucleotide polymorphisms in immune system genes and their association with clinical symptoms persistence in dengue-infected persons. Hum Immunol. 2015;76(10):717–23. [DOI] [PubMed] [Google Scholar]
- 15. Teixeira LdAS, Lopes JSM, Martins AGdC et al. [Persistence of dengue symptoms in patients in Uberaba, Minas Gerais State, Brazil]. Cad Saude Publica. 2010;26(3):624–30. [DOI] [PubMed] [Google Scholar]
- 16. Tristão-Sá R, Kubelka CF, Zandonade E et al. Clinical and hepatic evaluation in adult dengue patients: a prospective two-month cohort study. Rev Soc Bras Med Trop. 2012;45(6):675–81. [DOI] [PubMed] [Google Scholar]
- 17. Laul A, Laul P, Merugumala V et al. Clinical profiles of dengue infection during an outbreak in northern India. J Trop Med. 2016;2016:5917934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sigera PC, Amarasekara R, Rodrigo C et al. Risk prediction for severe disease and better diagnostic accuracy in early dengue infection; the Colombo dengue study. BMC Infect Dis. 2019;19:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tissera HA, Jayamanne BDW, Raut R et al. Severe dengue epidemic, Sri Lanka, 2017. Emerg Infect Dis. 2020;26(4):682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernando S, Wijewickrama A, Gomes L et al. Patterns and causes of liver involvement in acute dengue infection. BMC Infect Dis. 2016;16:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization . Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 22. Fukuda K, Straus SE, Hickie I et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–9. [DOI] [PubMed] [Google Scholar]
- 23. Hulme K, Hudson JL, Rojczyk P et al. Biopsychosocial risk factors of persistent fatigue after acute infection: a systematic review to inform interventions. J Psychosom Res. 2017;99:120–9. [DOI] [PubMed] [Google Scholar]
- 24. Hickie I, Davenport T, Wakefield D et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333(7568):575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Candy B, Chalder T, Cleare AJ et al. Predictors of fatigue following the onset of infectious mononucleosis. Psychol Med. 2003;33(5):847–55. [DOI] [PubMed] [Google Scholar]
- 26. Rathakrishnan A, Wang SM, Hu Y et al. Cytokine expression profile of dengue patients at different phases of illness. PLoS One. 2012;7(12):e52215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kavelaars A, Kuis W, Knook L et al. Disturbed neuroendocrine-immune interactions in chronic fatigue syndrome. J Clin Endocrinol Metab. 2000;85(2):692–6. [DOI] [PubMed] [Google Scholar]
- 28. Harlow BL, Signorello LB, Hall JE et al. Reproductive correlates of chronic fatigue syndrome. Am J Med. 1998;105(3 Suppl 1):94S–9S. [DOI] [PubMed] [Google Scholar]
- 29. Prins JB, van der Meer JW, Bleijenberg G. Chronic fatigue syndrome. Lancet. 2006;367(9507):346–55. [DOI] [PubMed] [Google Scholar]
- 30. Deale A, Chalder T, Wessely S. Illness beliefs and treatment outcome in chronic fatigue syndrome. J Psychosom Res. 1998;45(1):77–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this article can be shared upon reasonable request to the corresponding author. Details that may lead to identification of individual patients will not be made available.