Abstract
Purpose
Although studies have suggested that the coronavirus disease 2019 (COVID‐19) outbreak increased myopia progression, they had different settings and analysis methods. This study compared myopia progression before and during the COVID‐19 outbreak using meta‐analysis.
Methods
Relevant literature was searched on EMBASE, PubMed, ClinEpiDB and Web of Science and reviewed until 8 October 2021. The Newcastle–Ottawa Scale was used to evaluate the quality of the original studies. The mean difference of change in spherical equivalent refraction (SER) was used for evaluation before and during the COVID‐19 pandemic.
Results
The meta‐analysis included eight studies with 773, 797 individuals aged 5–18 years. Pooled analysis indicated that the mean difference of annual myopia progression during the pandemic was 0.41 D higher (95% confidence interval [CI]: 0.35–0.48, p < 0.01) than before the pandemic. Subgroup analysis using cycloplegic (mean difference, 0.30 D; 95% CI, 0.22–0.38; p < 0.01) or noncycloplegic refraction (mean difference, 0.60 D; 95% CI, 0.27–0.93; p < 0.01) indicated that the mean difference of annual myopia progression during COVID‐19 significantly increased in both refractive measurements.
Conclusion
Our findings demonstrated that the COVID‐19 pandemic accelerated myopic progression compared to the past. Government policies are urgently required to prevent and control myopia progression.
Keywords: COVID‐19, meta‐analysis, myopia pandemic, optometrist, public health
Key points.
This is the first meta‐analysis to report that the COVID‐19 pandemic significantly increased myopic progression among children and adolescents.
Optometrists must provide myopia control education to patients as the standard of care. Building a myopia control practice can help provide the highest quality of care to patients.
Parents should find ways to reduce the time spent performing near‐work and promote outdoor activities for children.
INTRODUCTION
Myopia is a serious global health issue, causing complications such as myopic macular degeneration, retinal detachment and open‐angle glaucoma, which can lead to irreversible visual impairment later in life. 1 , 2 Myopia prevalence continues to rise, predisposing affected individuals to a variety of ocular diseases, 1 , 3 affecting study performance 4 and activities of daily living. 5 Therefore, preventing and controlling myopia during its early onset is important to reduce sight‐threatening complications.
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) that led to coronavirus disease 2019 (COVID‐19), had a global impact and affected almost every aspect of people's lives. COVID‐19 is an emerging contagious infectious disease for which governments throughout the world have established various strategies to limit the spread of the virus. School‐aged children and adolescents in many counties have been particularly affected because of severe interruption to their school lives for months, as outside activities became forbidden and daily routines were restricted to indoor activities. 6 Children have been increasingly exposed to smartphones, tablets, computers and televisions at home. 7 Importantly, the light intensity of visual display terminals and duration of near‐work activity are also associated with myopia, 8 and spending more time in outdoor activities could delay myopia progression. 9 Therefore, intensive use of digital devices, home quarantine and home education during the COVID‐19 pandemic could increase myopia prevalence and lead to further myopia development and progression. 10
Currently, there is an association between COVID‐19 prevention policies, such as home confinement, home education, etc. and myopia progression. 11 , 12 Studies on this topic vary in design, sample size and method of analysis, showing that home quarantine during the COVID‐19 pandemic influenced myopia progression; however, no meta‐analysis has been conducted, warranting further research. To the best of our knowledge, this is the first meta‐analysis investigating whether the COVID‐19 pandemic significantly increased myopia compared with the pre‐pandemic situation, suggesting that the pandemic accelerated myopia progression among children and adolescents. This study aimed to compare myopia progression before and during the COVID‐19 pandemic, and provide evidence‐based findings to the community.
METHODS
This study followed the recommended guidelines of Preferred Reporting Item for Systematic Review and Meta‐Analyses (PRISMA). 13 The study was approved by the Ramkhamhaeng Research Ethics Committee (RU‐HRE64/0163). The study has not been registered in other databases. Before the study started, all authors discussed inclusion and exclusion criteria, including keywords for searching the literature, which were applied in this study.
Inclusion and exclusion criteria
The publications selected for analysis in this study had the following characteristics: (1) case–control, cross‐sectional, retrospective cohort and prospective cohort studies; (2) studies with mean spherical equivalent refraction (SER) in 2018, 2019 and 2020 or mean change in SER before and during the COVID‐19 pandemic as primary or secondary outcomes; (3) studies with participants younger than 18 years; (4) articles published before the start search (8 October 2021) and (5) studies that provided informative data for calculating mean differences.
The exclusion criteria were as follows: (1) reviews, case reports, case series, conference abstracts and letters to the editor; (2) in vitro studies and animal experiments; (3) duplication and (4) non‐English articles.
Searching strategies
A literature search was conducted in EMBASE, PubMed, ClinEpiDB and Web of Science databases to collect potentially eligible and relevant studies published until 8 October 2021. The search terms included the following: [(COVID‐19) OR (COVID‐19 pandemic) OR (coronavirus disease 2019) OR (novel coronavirus 2019) OR (2019‐nCoV) OR (SARS‐CoV 2) OR (COVID‐19 outbreak)] AND [(Home Confinement) OR (Lockdown) OR (Home‐isolation) OR (Social distancing) OR (Curfew) OR (home education) OR (Quarantine)] AND [(Myopia progression) OR (Refraction) OR (spherical equivalent) OR (refractive error) OR (refractive status)] AND [(children) OR (child) OR (young adult) OR (preschool)]. Two independent investigators (TP and PT) performed the search and screened the titles and abstracts of identified potential studies to remove any irrelevant publications as per the inclusion and exclusion criteria. Additional publications were considered and registered in this study by screening review articles or relevant articles after searching for “myopia progression and COVID‐19” in Google Scholar.
Data extraction and quality assessment
Two authors (TP and AW) independently extracted the data from selected studies. The extracted data included the following: first author name, study design, study region, study date and time, the total number of subjects, subjects' age, change in mean SER before COVID‐19 and during COVID‐19, standard deviation (SD) of the change in mean before COVID‐19 and during COVID‐19 and refraction methods. Discrepancies between the authors were discussed to resolve issues, and a third author (PT) was included to achieve the final consensus. The Newcastle–Ottawa Scale (NOS) 14 , 15 was used to evaluate the quality of the included studies, which was assessed by two authors (AW and PT) independently, and the third author (TP) resolved any disagreement. NOS was divided into three components: selection, comparability and outcome. A higher score was considered an indicator of good quality.
Data analysis
The studies without change in mean SER and SD were calculated as shown below. 16
Number 1 indicated baseline, number 2 indicated the follow‐up before the COVID‐19 pandemic, and number 3 indicated the follow‐up during the COVID‐19 pandemic. If the study provided the mean difference with 95% confidence intervals (CIs), the SD was calculated as: . The correlation coefficient (Corr) was calculated from studies by Aslen et al. 17 and Ma et al., 18 according to the Cochrane Handbook for Systematic Reviews of Interventions: 16 . The average Corr was 0.82. Differences in SER before and during the COVID‐19 pandemic were transformed into mean difference in annualised progression, which may be meaningful on a practical level. 19
The heterogeneity of the included studies was evaluated using the I 2 value with Cochran's Q test. We used a fixed‐effect model to derive estimates due to the lack of evidence on heterogeneity (I 2 lower than 50%, p‐value of Cochran's Q test lower than 0.05). I 2 above 50%, the random effected model with generic inverse variance method recommended by the Cochrane Handbook for Systematic Reviews, was used to analyse the difference in mean as a primary outcome. 16 Subgroup analysis was classified into noncycloplegic and cycloplegic to explore the possible cause of heterogeneity. The radial plot was used to assess outlier influence when heterogeneity was detected. The radial plot showed the inverse of the standard errors on the x‐axis and outcomes standardised by their corresponding standard errors on the y‐axis. A regression line ran centrally across the plot, and a single dot represented the individual studies. Publication bias was identified by funnel plot with pseudo‐95% CIs and Egger's statistics. Lastly, sensitivity analysis was performed by subsequently omitting the studies to confirm the results. The meta‐analysis was analysed using Review Manager (RevMan) version 5.4.1 (Cochrane Library, cochrane.org), 20 and Egger's test and radial plot were calculated by MetaHUN. 21 The significant value was p < 0.05.
RESULTS
Our initial search revealed 58 articles from three international databases. After removing duplicates, the titles were screened to select potential eligible articles. In total, 17 articles and full‐texts were reviewed, and eligible articles were selected according to the inclusion criteria (Figure 1). Subsequently, three related articles eligible for inclusion were identified after a search in Google Scholar. Accordingly, the information extracted from eight studies 17 , 18 , 22 , 23 , 24 , 25 , 26 , 27 was included in this meta‐analysis.
FIGURE 1.
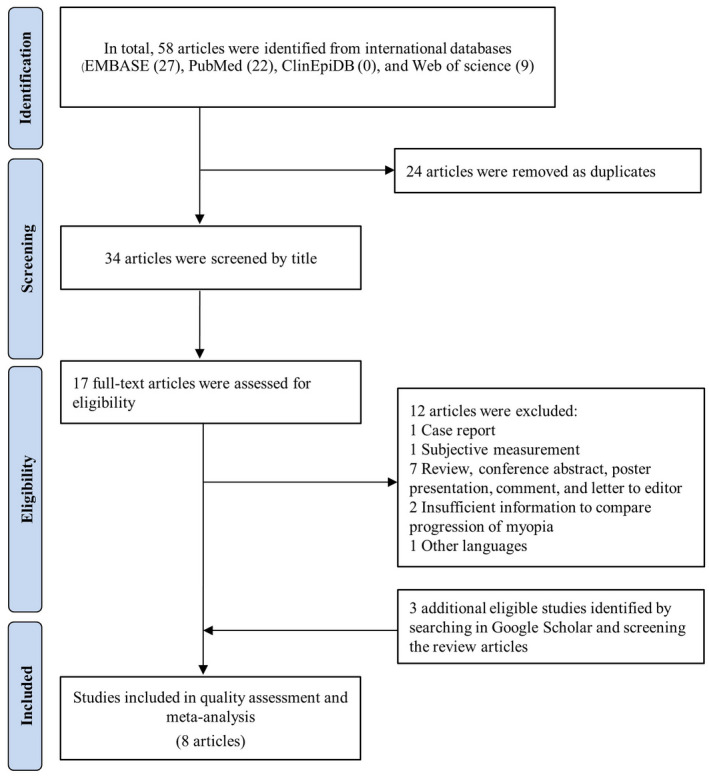
The selection process diagram to identify eligible studies following Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA)
Study demography and quality assessment
The characteristics of the included eight studies are presented in Table 1. Participants' age ranged from 5 to 18 years, with more than 773, 797 individuals. Three studies were conducted with relatively large sample sizes. Six studies were conducted in China, one in Turkey and one in Argentina. Three studies performed the refractive error measurement using noncycloplegic refraction. There were two cross‐sectional studies, one case–control study and five cohort studies. Based on NOS, included studies represented a median of good quality (Table 2). Due to differing durations of refractive error measurement, the data were converted into annual myopia progression (Table 3).
TABLE 1.
General information on the included studies
| Study | Location | Time points | Age (years) | N | Pre‐COVID‐19 | During‐COVID‐19 | Measurement method | ||
| Baseline | Before‐COVID‐19 | During‐COVID‐19 | ΔMean SER in D (SD) | ΔMean SER in D (SD) | |||||
| Aslan et al. 17 | Turkey | N/A ‡ 2018 | N/A ‡ 2019 | Aug‐20 | 8–17 | 115 | 0.54 (0.43) | 0.71 (0.46) | CA |
| Chang et al. 22 | Zhejiang, China | Mar‐19 | Oct‐19 | May‐20 | 6–15 | 29 719 | 0.20 (0.99) † | 0.50 (1.02) † | NCA |
| Hu et al. 23 | Guangdong, China | Nov‐18 | Nov‐19 | Nov‐20 | 6–7 | 1060 § | 0.31 (0.46) | 0.67 (0.56) | CA |
| Ma et al. 24 | Shanghai, China | Apr‐19 | Oct‐19 | May‐20 | 7–12 | 201 | 0.39 (0.58) | 0.98 (0.52) | CA |
| Ma et al. 18 | Hebei, China | Jul‐19 | Jan‐20 | Aug‐20 | 8–10 | 208 | 0.33 (0.47) | 0.93 (0.65) | CA |
| Picotti et al. 25 | Argentina | N/A ‡ 2018 | N/A ‡ 2019 | Sep‐20 | 5–18 | 115 | 0.44 (0.52) | 0.58 (0.53) | CA |
| Wang et al. 26 | Shandong, China | Sep‐18 | Sep‐19 | Jun‐20 | 6–13 | 194 904 | 0.02 (1.55) † | 0.17 (1.54) † | NCP |
| Xu et al. 27 | Zhejiang, China | Jun‐19 | Dec‐19 | Jun‐20 | 7–18 | 547 475 ¶ | 0.26 (0.37) † | 0.39 (0.38) † | NCA |
Abbreviations: CA, cycloplegic autorefraction; D, dioptre; N/A, not available; NCA, noncycloplegic autorefraction; NCP, noncycloplegic photoscreener; SD, standard deviation; SER, spherical equivalent refraction.
The durations have to be at least or assumed to be 8 months between each of the three different examinations.
Calculated difference in mean SER or SD.
The sample during COVID‐19 was 575,597.
This was the pre‐COVID‐19 sample; the sample during COVID‐19 was 1054.
TABLE 2.
Quality assessment using Newcastle‐Ottawa Scale of cross‐sectional, case–control and cohort study
| Study | Cross‐sectional study 14 | Case–control study 15 | Cohort study 15 | Total (10) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | Selection | Comparability | Outcome | Selection | Comparability | Outcome | ||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Aslan et al. 17 | ★ | ★★ | ★ | ★★ | ★ | 7 | ||||||||||||||||||
| Wang et al. 26 | ★ | ★ | ★ | ★ | ★ | ★★ | ★ | 8 | ||||||||||||||||
| Hu et al. 23 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | |||||||||||||||
| Chang et al. 22 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 8 | |||||||||||||||
| Ma et al. 24 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | ||||||||||||||||
| Ma et al. 18 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | 9 | |||||||||||||||
| Picotti et al. 25 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | ||||||||||||||||
| Xu et al. 27 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | 7 | ||||||||||||||||
Cross‐sectional study: 1, representative of sample; 2, justified sample size; 3 non‐respondents; 4, ascertainment of exposure; 5, the subjects in different outcome groups are comparable, based on study design or analysis; 6, assessment of outcome; 7, statistical test.
Case–control study: 1, is the case definition adequate; 2, representativeness of the cases; 3, selection of controls; 4, definition of controls; 5, comparability of cases and controls on the basis of the design or analysis; 6, ascertainment of exposure; 7, same method of ascertainment for cases and controls; 8, non‐response rate.
Cohort study: 1, representativeness of the exposed cohort; 2, selection of the non‐exposed cohort; 3, ascertainment of exposure; 4, demonstration that outcome of Interest was not present at start of study; 5, comparability of cohorts on the basis of the design or analysis; 6, assessment of outcome; 7, was follow‐up long enough for outcomes to occur; 8, adequacy of follow up of cohorts.
TABLE 3.
Duration of refractive measurement and annual myopia progression
| Study | Duration of refractive measurement (months) | Annualised progression in D (SD) | ||
|---|---|---|---|---|
| Pre‐COVID‐19 | During‐COVID‐19 | Pre‐COVID‐19 | During‐COVID‐19 | |
| Aslan et al. 17 | 8 † | 8 † | 0.81 (0.65) | 1.07 (0.69) |
| Chang et al. 22 | 8 | 8 | 0.30 (1.48) | 0.75 (1.53) |
| Hu et al. 23 | 12 | 12 | 0.31 (0.46) | 0.67 (0.56) |
| Ma et al. 24 | 7 | 7 | 0.67 (0.99) | 1.68 (0.89) |
| Ma et al. 18 | 6 | 6 | 0.66 (0.94) | 1.86 (1.30) |
| Picotti et al. 25 | 8 † | 8 † | 0.66 (0.78) | 0.87 (0.80) |
| Wang et al. 26 | 12 | 9 | 0.02 (1.55) | 0.22 (2.05) |
| Xu et al. 27 | 6 | 6 | 0.52 (0.74) | 0.78 (0.76) |
Abbreviations: D, dioptre; SD, standard deviation.
Duration of refractive measurement was estimated to be at least 8 months between each of the three different examinations.
Meta‐analysis of myopia progression, sensitivity analysis and publication bias
According to the heterogeneity analysis (I 2 > 50%, p‐value < 0.05), the random effects model was applied to merge the results of the eight studies. This meta‐analysis illustrated that the COVID‐19 pandemic accelerated myopia progression among children and adolescents (ages 5–18 years). The mean difference of myopia progression changes before and during COVID‐19 was 0.20–1.20 dioptres (D), with a pooled mean difference of 0.41 D. (95% CI, 0.35–0.48, p < 0.01) (Figure 2a). Sensitivity analysis showed that both results were consistent after 1‐layer omitting study, confirming the stability and reliability of this meta‐analysis (Table 4). The radial plot showed no significant outliers in the meta‐analysis (Figure 2c). Egger's test (p = 0.10) indicated no publication bias in the studies. However, the funnel plot analysis was likely to be asymmetric, showing the possible presence of publication bias or a small study effect (Figure 2d).
FIGURE 2.
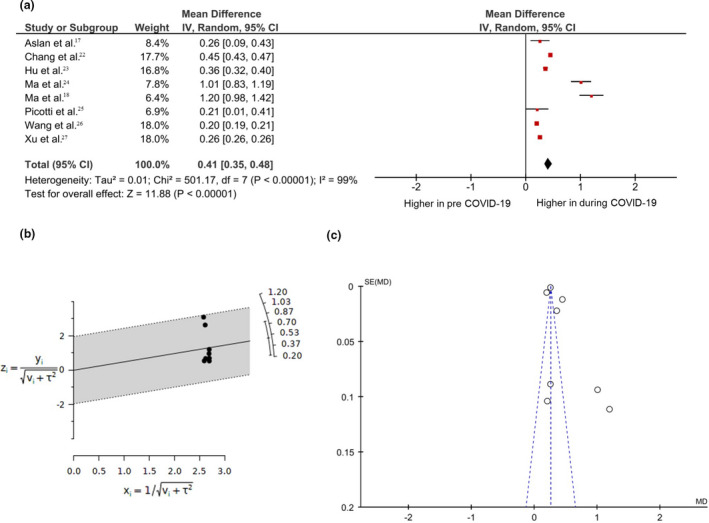
Meta‐analysis of the myopic shift in 773 797 individuals from eight studies. (a) Forest plot of mean difference in annual myopia progression showing the comparison before and during the COVID‐19 pandemic. The square's area was proportionate to study weight. The horizontal line indicates 95% CIs. The overall impact is shown as diamonds, with CIs at the lateral points. (b) Radial plot (c) Funnel plot with pseudo‐95% CIs. CI, confidence interval; MD, difference in mean; SE, standard error
TABLE 4.
Sensitivity analysis of included studies
| Omitted study | Mean difference in D (95% CI) | p‐Value of mean difference |
|---|---|---|
| Aslan et al. 17 | 0.43 [0.36–0.50] | <0.01 |
| Chang et al. 22 | 0.38 [0.32–0.44] | <0.01 |
| Hu et al. 23 | 0.43 [0.35–0.50] | <0.01 |
| Ma et al. 24 | 0.36 [0.29–0.43] | <0.01 |
| Ma et al. 18 | 0.36 [0.29–0.42] | <0.01 |
| Picotti et al. 25 | 0.43 [0.36–0.50] | <0.01 |
| Wang et al. 26 | 0.50 [0.38–0.62] | <0.01 |
| Xu et al. 27 | 0.50 [0.36–0.65] | <0.01 |
Abbreviations: CI, confidence interval; D, dioptre; MD, mean difference.
Subgroup analysis
Because heterogeneity and publication bias were observed, subgroup analysis was introduced to resolve issues. The included studies were classified into noncycloplegic and cycloplegic refraction. The COVID‐19 pandemic showed significant annual myopia progression in both noncycloplegic and cycloplegic refraction, with the pooled mean difference of 0.30 D (95% CI, 0.22–0.38; p < 0.01) (Figure 3a) and 0.60 D (95% CI, 0.27–0.93; p < 0.01) (Figure 3b), respectively.
FIGURE 3.
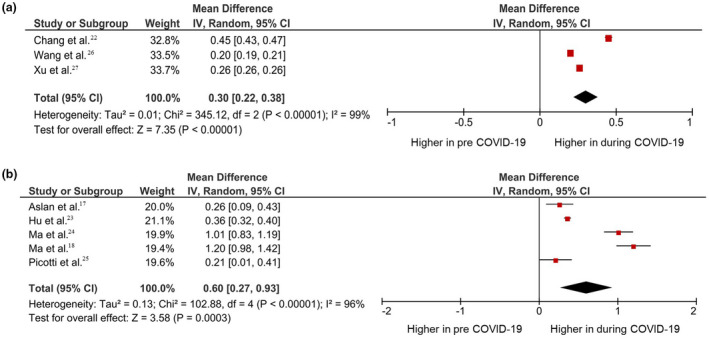
Subgroup analysis using (a) non‐cycloplegic and (b) cycloplegic measurement. Forest plot comparing myopia progression before and during the COVID‐19 pandemic. The square area was proportionate to study weight. The horizontal line indicates 95% CIs. The overall impact is shown as diamonds, with CIs at the lateral points. CI, confidence interval
DISCUSSION
To the best of our knowledge, this is the first meta‐analysis comparing myopia progression before and during the COVID‐19 outbreak. Our results showed that the COVID‐19 outbreak significantly increased myopia progression, which was approximately 0.41 D higher than before the pandemic.
During the COVID‐19 outbreak, lifestyle and behaviours changed. Traditional schooling was shut down for children and adolescents and replaced with various technologies utilising visual display terminals. UNESCO estimated that the COVID‐19 crisis affected over 365 million students worldwide, and in Asia, the schools were closed for approximately 40 weeks. 28 The students learned from home and communicated using social networking platforms, which resulted in increased use of digital screens and decreased outdoor activity. 29 , 30 Zhao's study in China showed that most children spent more than 3 h daily using digital screens and less than 2 h per day in outdoor activities. 31 Francisco et al. demonstrated that the pattern of digital screen use and daily physical activity significantly differed before and during the COVID‐19 pandemic. 32 Therefore, we speculate that the pandemic rapidly promoted myopia progression because of increasing near work and decreasing outdoor activity, which are both well‐known risk factors of myopia. 10 , 33
Subgroup analysis using refractive measurements could not reduce heterogeneity. A possible explanation of high heterogeneity is baseline refraction. The degree of myopia at the beginning of the follow‐up, for which enrolment studies of this meta‐analysis included the children who displayed true progression (≤−0.5 D) and progression in the non‐myopic eye (SER > −0.5 D) at baseline measurement, was associated with myopia progression. 34 Additionally, the progression of myopia may be higher in younger compared with older children, 35 , 36 for which our meta‐analysis combined the results from ages 5–18. Myopia progression in children aged ≤15 years is greater depending on the severity, ranging from high to normal in patients/children with myopia. 37 , 38 Thus, greater myopic baseline, younger age at baseline and age of myopic onset are important factors in the progression of myopia. Although the causes for heterogeneity were unknown, one‐layer sensitivity analysis yielded reliable results.
The study encountered limitations that should not be neglected. First, this analysis lacks sufficient data on other ethnic groups, which have been suggested to be at greater risk of myopic shift. 39 , 40 Although the study combined large sample sizes, the majority represented Asian and Chinese populations, and only one study was conducted in South America. Therefore, such conditions may not appropriately represent populations in North America and Europe. Thus, data relevant to other ethnicities should be carefully considered. Second, differences in refractive measurement durations before and during the COVID‐19 pandemic in each study might have affected the progression rate. However, different mean SER during and before COVID‐19, represented in this study, showed increased myopic progression. Third, our study identified three relevant articles after manual searching, which was a protocol deviation. Finally, the funnel plot likely showed publication bias across the included studies. Therefore, caution should be undertaken while interpreting the results. A future study in which the inclusion and exclusion criteria are set based on younger age at baseline and age at myopic onset with respect to different ethnicities is required to validate the effect of public health measures on the progression of myopia.
CONCLUSION
Meta‐analysis is a useful method to summarise studies that provide valid evidence on myopia progression amongst children and adolescents, which increased during the pandemic, creating a long‐lasting effect on children. Eyecare professionals, policymakers, educators and parents must work together to minimise childhood myopia, which might become a public health emergency due to COVID‐19. Governments should take responsibility for placing a high priority on preventing and controlling myopia amongst children and adolescents.
CONFLICT OF INTEREST
There is no conflict of interest relevant to this paper. The authors received no financial support for the study.
AUTHOR CONTRIBUTIONS
Akarapon Watcharapalakorn: Conceptualization (equal); data curation (lead); methodology (equal); writing – original draft (equal). Patarakorn Tawonkasiwattanakun: Conceptualization (equal); data curation (equal); supervision (lead); writing – review and editing (lead).
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
Akarapon Watcharapalakorn, Teera Poyomtip, Patarakorn Tawonkasiwattanakun are grateful for the support provided by the Faculty of Optometry, Ramkhamhaeng University. The authors would like to thank Paninee Mongkolsuk who provided the EMBASE search result for our study. Finally, the authors would like to thank Enago (www.enago.com) for the English language review.
Watcharapalakorn AP, Poyomtip T, Tawonkasiwattanakun P. Coronavirus disease 2019 outbreak and associated public health measures increase the progression of myopia among children and adolescents: Evidence synthesis. Ophthalmic Physiol Opt. 2022;42:744–752. 10.1111/opo.12976
Contributor Information
Teera Poyomtip, Email: teera.p@rumail.ru.ac.th.
Patarakorn Tawonkasiwattanakun, Email: patarakorn.t@rumail.ru.ac.th.
REFERENCES
- 1. Haarman AEG, Enthoven CA, Tideman JWL, Tedja MS, Verhoeven VJM, Klaver CCW. The complications of myopia: a review and meta‐analysis. Invest Ophthalmol Vis Sci 2020;61:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, et al. Causes of vision loss worldwide, 1990‐2010: a systematic analysis. Lancet Glob Health. 2013;1:e339–49. [DOI] [PubMed] [Google Scholar]
- 3. Xiang ZY, Zou HD. Recent epidemiology study data of myopia. J Ophthalmol. 2020;2020:4395278. 10.1155/2020/4395278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang Y, Li R, Ting D, Wu X, Huang J, Zhu Y, et al. The associations of high academic performance with childhood ametropia prevalence and myopia development in China. Ann Transl Med. 2021;9:745. 10.21037/atm-20-8069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Takashima T, Yokoyama T, Futagami S, Ohno‐Matsui K, Tanaka H, Tokoro T, et al. The quality of life in patients with pathologic myopia. Jpn J Ophthalmol. 2001;45:84–92. [DOI] [PubMed] [Google Scholar]
- 6. Parmet WE, Sinha MS. Covid‐19 – the law and limits of quarantine. N Engl J Med. 2020;382:e28. 10.1056/NEJMp2004211 [DOI] [PubMed] [Google Scholar]
- 7. Sumitha M, Sanjay S, Kemmanu V, Bhanumathi MR, Shetty R. Will COVID‐19 pandemic‐associated lockdown increase myopia in Indian children? Indian J Ophthalmol. 2020;68:1496–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wen L, Cao Y, Cheng Q, Li X, Pan L, Li L, et al. Objectively measured near work, outdoor exposure and myopia in children. Br J Ophthalmol. 2020;104:1542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lingham G, Mackey DA, Lucas R, Yazar S. How does spending time outdoors protect against myopia? A review. Br J Ophthalmol. 2020;104:593–9. [DOI] [PubMed] [Google Scholar]
- 10. Huang HM, Chang DS, Wu PC. The association between near work activities and myopia in children‐a systematic review and meta‐analysis. PloS One. 2015;10:e0140419. 10.1371/journal.pone.0140419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu J, Li B, Chen Q, Dang J. Student health implications of school closures during the COVID‐19 pandemic: new evidence on the association of e‐learning, outdoor exercise, and myopia. Healthcare (Basel). 2021;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu J, Li B, Sun Y, Chen Q, Dang J. Adolescent vision health during the outbreak of COVID‐19: association between digital screen use and myopia progression. Front Pediatr. 2021;9:662984. 10.3389/fped.2021.662984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Modesti PA, Reboldi G, Cappuccio FP, Agyemang C, Remuzzi G, Rapi S, et al. Panethnic differences in blood pressure in Europe: a systematic review and meta‐analysis. PloS One. 2016;11:e0147601. 10.1371/journal.pone.0147601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses Ottawa Hospital Research Institute. 2000. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 13 March, 2022.
- 16. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from: www.training.cochrane.org/handbook/current. Accessed 13 March, 2022.
- 17. Aslan F, Sahinoglu‐Keskek N. The effect of home education on myopia progression in children during the COVID‐19 pandemic. Eye (London, England). 2021:1–6. 10.1038/s41433-021-01655-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ma M, Xiong S, Zhao S, Zheng Z, Sun T, Li C. COVID‐19 home quarantine accelerated the progression of myopia in children aged 7 to 12 years in China. Invest Ophthalmol Vis Sci 2021;62:37. 10.1167/iovs.62.10.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilkinson L. Statistical methods in psychology journals: guidelines and explanations. Am Psychol. 1999;54:594–604. [Google Scholar]
- 20. Review Manager (RevMan) Version 5.4 . Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration 2020.
- 21. Umaroglu M., Ozdemir P. editors. metaHUN: a web tool for meta‐analysis. 3rd International and 20th National Biostatistics Congress; 2018; Gaziantep, Turkey
- 22. Chang P, Zhang B, Lin L, Chen R, Chen S, Zhao Y, et al. Comparison of Myopic Progression before, during, and after COVID‐19 lockdown. Ophthalmology. 2021;128:1655–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu Y, Zhao F, Ding X, Zhang S, Li Z, Guo Y, et al. Rates of myopia development in young chinese schoolchildren during the outbreak of COVID‐19. JAMA Ophthalmol. 2021;139:1115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma D, Wei S, Li SM, Yang X, Cao K, Hu J, et al. Progression of myopia in a natural cohort of Chinese children during COVID‐19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2021;259:2813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Picotti C, Sanchez V, Fernandez Irigaray L, Morgan IG, Iribarren R. Myopia progression in children during COVID‐19 home confinement in Argentina (preprint). Lancet. 10.2139/ssrn.3781660 [DOI]
- 26. Wang J, Li Y, Musch DC, Wei N, Qi X, Ding G, et al. Progression of myopia in school‐aged children after COVID‐19 home confinement. JAMA Ophthalmol. 2021;139:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu L, Ma Y, Yuan J, Zhang Y, Wang H, Zhang G, et al. COVID‐19 quarantine reveals that behavioral changes have an effect on Myopia progression. Ophthalmology. 2021;128:1652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. UNESCO . Education: From disruption to recovery 2020 [internet]. France: UNESCO. Available from: https://en.unesco.org/covid19/educationresponse. Accessed 13 March, 2022.
- 29. Alvarez‐Peregrina C, Martinez‐Perez C, Villa‐Collar C, Andreu‐Vazquez C, Ruiz‐Pomeda A, Sanchez‐Tena MA. Impact of COVID‐19 home confinement in children's refractive errors. Int J Environ Res Public Health. 2021;18:5347. 10.3390/ijerph18105347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mirhajianmoghadam H, Piña A, Ostrin LA. Objective and subjective behavioral measures in myopic and non‐myopic children during the COVID‐19 pandemic. Transl Vis Sci Technol 2021;10:4. 10.1167/tvst.10.11.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao Y, Guo Y, Xiao Y, Zhu R, Sun W, Huang W, et al. The effects of online homeschooling on children, parents, and teachers of grades 1‐9 during the COVID‐19 pandemic. Med Sci Monit. 2020;26:e925591. 10.12659/MSM.925591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Francisco R, Pedro M, Delvecchio E, Espada JP, Morales A, Mazzeschi C, et al. Psychological symptoms and behavioral changes in children and adolescents during the early phase of COVID‐19 quarantine in three European countries. Front Psych. 2020;11:570164. 10.3389/fpsyt.2020.570164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xiong S, Sankaridurg P, Naduvilath T, Zang J, Zou H, Zhu J, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta‐analysis and systematic review. Acta Ophthalmol. 2017;95:551–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parssinen O, Lyyra AL. Myopia and myopic progression among schoolchildren: a three‐year follow‐up study. Invest Ophthalmol Vis Sci. 1993;34:2794–802. [PubMed] [Google Scholar]
- 35. French AN, Morgan IG, Burlutsky G, Mitchell P, Rose KA. Prevalence and 5‐ to 6‐year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology. 2013;120:1482–91. [DOI] [PubMed] [Google Scholar]
- 36. Saw SM, Tong L, Chua WH, Chia KS, Koh D, Tan DT, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46:51–7. [DOI] [PubMed] [Google Scholar]
- 37. Verkicharla PK, Kammari P, Das AV. Myopia progression varies with age and severity of myopia. PloS one. 2020;15:e0241759. 10.1371/journal.pone.0241759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wong K, Dahlmann‐Noor A. Myopia and its progression in children in London, UK: a retrospective evaluation. J Optom. 2020;13:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jones‐Jordan LA, Sinnott LT, Chu RH, Cotter SA, Kleinstein RN, Manny RE, et al. for the CLEERE Study Group . Myopia progression as a function of sex, age, and ethnicity. Invest Ophthalmol Vis Sci 2021;62:36. 10.1167/iovs.62.10.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Donovan L, Sankaridurg P, Ho A, Naduvilath T, Smith EL 3rd, Holden BA. Myopia progression rates in urban children wearing single‐vision spectacles. Optom Vis Sci. 2012;89:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


