Abstract
Objective
Determine the effectiveness of a COVID‐19 remote monitoring and management program in reducing preventable hospital utilization.
Design
A retrospective cohort study utilizing data from electronic health records.
Sample
Two hundred ninety‐three patients who tested positive for COVID‐19 at a drive‐through testing site in Michigan. [Correction added on 11 April 2022, after first online publication: In the preceding sentence, “Two hundred and ninety‐third” has been corrected to “Two hundred ninety‐three” in this version.] The intervention group, consisting of 139 patients, was compared to a control group of 154 patients.
Measurements
The primary outcome was the 30‐day probability of hospital utilization. The covariates included in the analysis were age, gender, tobacco use, body mass index (BMI), race, and ethnicity.
Intervention
A nurse‐led, telephone‐based active management protocol for COVID‐19 patients who were isolating at home.
Results
The intervention group had a non‐statistically significant 42% reduction in risk of hospital utilization within 30 days of a positive COVID‐19 test when compared to the control group (HR = 0.578, p‐value .111, HR 95% CI [0.29, 1.13]).
Conclusions
A nurse‐led remote monitoring and management program for COVID‐19 reduced the probability of 30‐day hospital utilization. Although the findings were not statistically significant, the program yielded practical significance by reducing hospital utilization, in‐person interaction, and the risk of infection for healthcare workers.
Keywords: COVID‐19, hospital resources, hospitalization, nursing, SARS‐CoV‐2, telemedicine
1. BACKGROUND
Coronavirus disease of 2019 (COVID‐19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which first appeared in Wuhan, China in December 2019 (Centers for Disease Control and Prevention, 2020a). After spreading to 114 countries, the World Health Organization (WHO) declared COVID‐19 as a pandemic on March 11, 2020 (World Health Organization, 2020). With the initial surge in COVID‐19 cases in the United States, the availability of hospital resources became a concern. The supply of available hospital beds, ventilators, and personal protective equipment (PPE) rapidly decreased in health systems across the United States.
COVID‐related hospitalizations place a financial burden on patients, families, employers, health insurance companies, health systems, and the federal government. Using data from the largest repository of private health insurance claims from January through May 2020, median dollar amounts charged by hospitals in the US for a COVID‐19 hospitalization ranged from $34,662 for patients ages 23–30 years to $45,683 for ages 51–60 years (FAIR Health, 2020). The median allowed amounts for reimbursement ranged from $17,216 to $24,012 (FAIR Health, 2020). According to data from the Centers for Medicare and Medicaid Services from January through September 2020, the average Medicare payment for a COVID‐19 hospitalization was $24,659 (The Centers for Medicare and Medicaid Services, 2020). Furthermore, an estimated $5.1 billion was spent on Medicare fee‐for‐service COVID‐19 hospitalizations over this same period (The Centers for Medicare and Medicaid Services, 2020).
Many patients with a mild clinical presentation can be managed from home (Centers for Disease Control and Prevention, 2020b). Based on a cohort of more than 44,000 patients with COVID‐19 in China, approximately 81% had a mild disease severity, which by the authors’ definition excluded hypoxia (Wu & McGoogan, 2020). However, there is still a risk of progression to severe disease requiring hospitalization following the first week of symptom onset (Centers for Disease Control and Prevention, 2020a). Through efforts to control the spread of COVID‐19 by social distancing, the number of visits to outpatient practices declined by nearly 60% in mid‐March 2020 (Rae et al., 2020). Due to reductions in outpatient clinical visits, alternative methods such as telemedicine needed to be utilized to maintain appropriate disease follow‐up.
The Infectious Diseases Society of America (IDSA) supports the use of telehealth and telemedicine practices to “provide evidence‐based, cost‐effective, subspecialty care to resource‐limited populations…and implement infection prevention and control (IPC) measures” (Young et al., 2019). Frequent follow‐up is especially important for COVID‐19 patients managing their illness at home. Remote monitoring and assessment of symptoms to risk stratify patients may help direct individuals to the appropriate level of care and reduce preventable hospital utilization. To help reduce hospital utilization and improve patient outcomes in Jackson, MI, Henry Ford Allegiance Health, a 300‐bed community hospital in the Henry Ford Health System, implemented a nurse‐led, telephone‐based active management protocol for patients that tested positive for SARS‐CoV‐2 at their drive‐through testing site and who were isolating at home.
This retrospective cohort study assessed the effectiveness of a nurse‐led, telephone‐based active management protocol for COVID‐19 patients who were isolating at home. The intervention group included patients who tested positive after the implementation of the protocol, and the control group included the patients who tested positive for COVID‐19 prior to the implementation of the protocol. The primary objective of this study was to compare the 30‐day probability of hospital utilization (Emergency Department visit and/or Inpatient Admission) between the control and intervention groups. Our secondary objectives included the separate 30‐day probabilities of an emergency department visit and inpatient admission. The information obtained from this study may help health systems in their response to not only the current COVID‐19 pandemic, but also other pandemics that may occur in the future.
2. METHODS
2.1. Active management protocol intervention
The intervention for this study was a nurse‐led, telephone‐based active management protocol for COVID‐19 patients who were isolating at home. This protocol was developed by a group of physicians and nurses and was initiated on April 19, 2020. Once a positive SARS‐CoV‐2 test result was reported, a registered nurse would telephone a patient to explain the role of the intervention and obtain an initial symptom assessment. A point system was used to assess for the overall symptom severity (Appendix A). There were four levels of illness severity that were used: Better (0 points), Mild (1–3 points), Moderate (4–6 points), and Severe (7 or more points). Each level of severity had specific instructions for the nurse to follow, with an escalation of management as severity increased (Appendix A). Patients with a “MODERATE” or “SEVERE” score were also scheduled an appointment (in‐person or video visit) with the respiratory clinic or their primary care provider. A visit from a community paramedic and the recommendation to seek further care at the Emergency Department were also recommendations for those with “SEVERE” illness scores. The frequency of the phone calls ranged from “every other day” to “twice daily,” depending on the severity of symptoms. During the phone calls, the patients were also reminded about appropriate isolation procedures and provided confirmation of their PCR re‐test date (which was recommended at that time during the pandemic). The nurses that administered the protocol participated in a formal training session led by one of the physicians. There were additional monthly meetings to discuss cases and ensure consistency in disease severity scoring and management, and a physician was available to the team of nurses during operating hours by phone and through secure messaging.
2.2. Design
This study is a retrospective cohort study utilizing data from the electronic health record. The subjects were drawn from a cohort of COVID‐19 patients who had SARS‐CoV‐2 PCR testing performed at the Henry Ford Allegiance Health drive‐through testing site in Jackson, MI from March 23, 2020 through May 31, 2020. Henry Ford Allegiance Health is the only hospital in a county with an estimated population of 158,510 people in 2019 (U.S. Census Bureau, 2021). At the time of the study, only county residents, individuals working in the county, and existing health system patients were eligible for testing at this location due to the unpredictable supply chain for testing components such as swabs and reagent. The state was also under executive order of the governor to “stay home, stay safe.” These orders significantly restricted the movement and gathering of residents who were not essential workers until being modified on June 1, 2020.
2.3. Sample
The data extraction was performed by the analytics department of the affiliated health system. The study sample included 293 patients who tested positive for COVID‐19 at the drive through testing site. Inclusion criteria included presence of COVID‐19 detected by SARS‐CoV‐2 PCR test, testing performed at the specific drive through testing site, and age of 18 years or older. Patients were excluded from the sample if the following criteria were met: currently incarcerated, under the age of 18 years, pregnant at the time of the test, and/or patients with testing performed at another site. The control group, consisting of 154 subjects, included the patients who tested positive for COVID‐19 prior to the implementation of the protocol (from March 23, 2020 through April 18, 2020). The intervention group consisted of the first 139 subjects who met the eligibility criteria and tested positive for COVID‐19 after the implementation of the active management protocol on April 19, 2020. To achieve nearly equal case counts and eligibility periods for the study groups, a cut‐off date of May 31, 2020, was used as the end of the eligibility period for the intervention group. Vaccinations and monoclonal antibody therapies were not yet available during the study period.
2.4. Measures
The following variables were collected for analysis: SARS‐CoV‐2 test date, emergency department visit, inpatient hospital admission, age, gender, tobacco use, body mass index (BMI), race, and ethnicity. Admitting diagnosis and chief complaint were also collected to help determine if the hospital utilization was related to COVID‐19.
Our primary outcome is the 30‐day probability of COVID‐19‐related hospital utilization, which is defined as an emergency department visit and/or inpatient hospital admission related to COVID‐19 within the first 30 days of diagnosis. We also calculated the separate 30‐day probabilities of an emergency department visit and inpatient hospital admission.
2.5. Analytic strategy
The basic descriptive statistics and tests to assess for demographic similarity between the study groups was performed using IBM's SPSS Software, Version 23. The time series analysis was performed using SAS Propriety Software, Version 9.4. The variables and outcomes were compared between the intervention and control groups. The basic demographic information was compared between the two groups to assess the need for adjustment in the outcome analysis. A p‐value of .05 or less was considered a statistically significant result. If patients had missing data for variables in a specific analysis, then those patients were excluded from that analysis. A comparison of means using t‐tests was performed for the numerical variables. A cross‐tabulation analysis was also performed for the categorical variables.
When analyzing hospital utilization, we excluded patients who presented to the hospital for reasons that were obviously unrelated to COVID‐19 (e.g., arm injury, motorcycle crash). After reviewing the chief complaints and admitting diagnoses for patients within 30 days of a positive COVID‐19 test, only one case needed to be excluded in the analysis. Survival analysis, using a Kaplan‐Meier estimator, was performed for the time‐to‐event outcomes. A log‐rank test was used to compare the two groups. A Cox proportional hazard regression was used to obtain hazard ratios. A Cox stepwise regression analysis was also used to allow for the inclusion of covariates and to determine the best model for predicting the 30‐day probability of hospital utilization. The stepwise inclusion requirement was p = .05 and the exclusion requirement was also p = .05. The variables considered for this model were: BMI, age, race, ethnicity, sex, and smoking status. The initial inclusion of BMI reduced the valid case count to 265 because of missing data. Once it was determined that BMI was not a significant factor in the model, by itself or in combination with other factors, BMI was dropped from all future analyses to ensure all 293 cases would be available. A less restrictive inclusion and exclusion cut‐off p‐value of .20 was also used to perform a stepwise regression analysis.
A sub‐group analysis was performed, comparing the hospital utilization in the intervention and control groups when stratified by the sociodemographic variables listed above. Fisher's Exact Test was used to compute the p‐values. Stata was used to perform a post‐hoc power analysis using the “power cox” command and utilizing the p‐value obtained for the primary outcome.
2.6. Ethical considerations
This study was approved by the Henry Ford Allegiance Health Institutional Review Board on August 17, 2020. The study was considered to have minimal to no risk to study participants. Informed consent was waived due to the retrospective nature of the study and the deidentification of patient information. Participants were not contacted for information; all data was obtained in a secure and confidential manner from the electronic health record. The data extraction was approved by the hospital's executive leadership team.
3. RESULTS
In the overall sample, the mean age was 46.03 years, and just over half 163 (55.6%) were female (Table 1). Most patients identified as White/Caucasian (79.5%), and 13.0% as Black/African American. The mean BMI was 31.96, which meets the classification for obesity. Regarding smoking status, 25 (8.5%) were current smokers, 86 (29.4%) were former smokers, 148 (50.5%) had never smoked, and 34 (11.6%) were unknown. For all these demographic factors there was no significant difference between the study groups.
TABLE 1.
Sociodemographic characteristics of the study sample
| Variable | Total sample (n = 293) | Range | Control group (n = 154) | Intervention group (n = 139) | p‐Value a |
|---|---|---|---|---|---|
| Age (years) | 46.03 ± 1.88 | 18–95 | 46.55 ± 2.31 | 45.45 ± 3.04 | .575 |
| Sex | .557 | ||||
| Female | 163 (55.6%) | 83 (53.9%) | 80 (57.6%) | ||
| Male | 130 (44.4%) | 71 (46.1%) | 59 (42.4%) | ||
| Race | .265 | ||||
| White | 233 (79.5%) | 128 (83.1%) | 105 (75.5%) | ||
| Black | 38 (13.0%) | 17 (11.0%) | 21 (15.1%) | ||
| Other/Refused | 22 (7.5%) | 9 (5.8%) | 13 (9.4%) | ||
| Ethnicity | .417 | ||||
| Not Hispanic | 272 (92.8%) | 144 (93.5%) | 128 (92.1%) | ||
| Hispanic | 7 (2.4%) | 2 (1.3%) | 5 (3.6%) | ||
| Unknown/Refused | 14 (4.8%) | 8 (5.2%) | 6 (4.3%) | ||
| Body mass index a | 31.96 ± 0.90 | 18.54–55.98 | 32.27 ± 1.31 | 31.61 ± 1.21 | .468 |
| Smoking status | .825 | ||||
| Current smoker | 25 (8.5%) | 12 (7.8%) | 13 (9.4%) | ||
| Former smoker | 86 (29.4%) | 45 (29.2%) | 41 29.5%) | ||
| Never smoked | 148 (50.5%) | 81 (52.6%) | 67 (48.2%) | ||
| Unknown | 34 (11.6%) | 16 (10.4%) | 18 (12.9%) |
Due to missing data, the sample sizes for BMI are the following: total sample n = 265, control n = 141, intervention n = 124.
A Kaplan‐Meier analysis for the 30‐day probability of hospital utilization showed no statistically significant difference between the intervention and control groups (p‐value = .105, Log‐Rank Test). Despite the lack of statistical significance, the Kaplan‐Meier curve (Figure 1) demonstrated that 30‐day hospital utilization was reduced in the intervention group.
FIGURE 1.
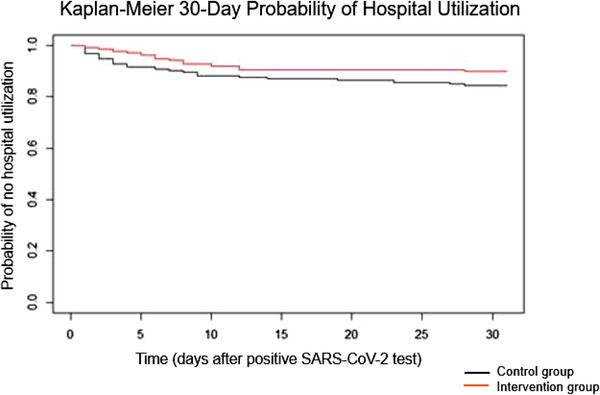
The effect of an active management protocol on 30‐day probability of hospital utilization in COVID‐19 patients [Colour figure can be viewed at wileyonlinelibrary.com]
A Cox proportional hazards regression with only the intervention variable included in the model was used to determine the impact of the protocol on the 30‐day probability of hospital utilization. This analysis showed that the intervention group had a 42% reduction in risk of hospital utilization within 30 days of a positive COVID‐19 test when compared to the control group (HR = 0.578, p‐value .111, HR 95% CI [0.29, 1.13]), however, this difference did not meet statistical significance. A post‐hoc power analysis revealed a power of 53% for the primary outcome.
When a Cox stepwise regression analysis with an inclusion and exclusion cutoff of p = .05 was performed, only patient age (p = .009) met the cutoff for statistical significance to be in the final model. This “age‐only” model had a hazard ratio of 1.027 (p = .005). Additionally, no statistically significant interaction effects were found.
A more liberal p‐value of .20 was also used for the stepwise inclusion and exclusion cutoff. The final model using this approach included age, smoking status, and the intervention variable. The model showed that there is a 42% reduction in risk of requiring hospital services in the intervention group compared to the control group (HR = 0.580, p‐value = .115). These results were similar to those we found for the “intervention only” model previously mentioned.
Hospital utilization was also stratified and analyzed by emergency department visit and hospital admission. There was no statistically significant difference between the study groups for 30‐day probability of an emergency department visit (p = .105) or 30‐day probability of a hospital admission (p = .406). A comparison between the intervention and control groups regarding emergency department visit and hospital admission, when stratified by patient demographics, did reveal two statistically significant differences (Table 2). In patients who were less than 30 years old, there were fewer patients in the intervention group that had an emergency department visit within 30 days of a COVID‐19 diagnosis (p = .040). In patients between the ages of 60 and 69 years, there were fewer patients in the intervention group that were admitted to the hospital within 30 days of a COVID‐19 diagnosis (p = .032).
TABLE 2.
Hospital utilization by the control and intervention groups when stratified by sociodemographic variables
| Emergency department visit | Hospital admission | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | Intervention group | Control group | Intervention group | ||||||||
| Variable b | N | % a | N | % a | N | p‐Value | N | % a | N | % a | p‐Value |
| Age (years) | <30 | 4 | 16.0% | 0 | 0.0% | .040 | 0 | 0.0% | 0 | 0.0% | NA |
| 30–39 | 0 | 0.0% | 2 | 6.5% | .514 | 0 | 0.0% | 1 | 3.2% | 1.000 | |
| 40–49 | 9 | 20.5% | 4 | 12.1% | .376 | 2 | 4.5% | 1 | 3.0% | 1.000 | |
| 50–59 | 3 | 9.1% | 2 | 14.3% | .627 | 1 | 3.0% | 1 | 7.1% | .512 | |
| 60–69 | 6 | 23.1% | 1 | 5.3% | .211 | 6 | 23.1% | 0 | 0.0% | .032 | |
| 70–79 | 2 | 40.0% | 1 | 25.0% | 1.000 | 1 | 20.0% | 1 | 25.0% | 1.000 | |
| ≥80 | 0 | 0.0% | 3 | 33.3% | 1.000 | 0 | 0.0% | 2 | 22.2% | 1.000 | |
| Gender | Male | 11 | 15.5% | 3 | 5.1% | .086 | 5 | 7.0% | 1 | 1.7% | .220 |
| Female | 13 | 15.7% | 10 | 12.5% | .655 | 5 | 6.0% | 5 | 6.3% | 1.000 | |
| Smoking | Yes | 1 | 8.3% | 0 | 0.0% | .480 | 0 | 0.0% | 0 | 0.0% | NA |
| No | 22 | 17.5% | 13 | 12.0% | .274 | 10 | 7.9% | 6 | 5.6% | .606 | |
| Race | White | 21 | 16.4% | 12 | 11.4% | .346 | 8 | 6.3% | 6 | 5.7% | 1.000 |
| Black | 1 | 5.9% | 1 | 4.8% | 1.000 | 1 | 5.9% | 0 | 0.0% | .447 | |
| Other | 0 | 0.0% | 0 | 0.0% | NA | 0 | 0.0% | 0 | 0.0% | NA | |
| Ethnicity | Hispanic | 0 | 0.0% | 0 | 0.0% | NA | 0 | 0.0% | 0 | 0.0% | NA |
| Non‐Hispanic | 24 | 15.6% | 13 | 9.4% | .276 | 9 | 6.3% | 6 | 4.7% | .607 | |
| BMI | Underweight (<18.5) | 0 | 0.0% | 0 | 0.0% | NA | 0 | 0.0% | 0 | 0.0% | NA |
| Normal (18.5–24.9) | 3 | 10.0% | 4 | 19.0% | .427 | 0 | 0.0% | 2 | 9.5% | .165 | |
| Overweight (25–29.9) | 7 | 25.9% | 4 | 10.0% | .103 | 4 | 14.8% | 2 | 5.0% | .211 | |
| Obese (≥30) | 14 | 16.7% | 5 | 7.9% | .141 | 6 | 7.1% | 2 | 3.2% | .467 | |
| Total | 24 | 15.6% | 13 | 10.1% | .117 | 10 | 6.5% | 6 | 4.3% | .452 | |
Percentages for Emergency Department Visit and Hospital Admission are the percent in each subgroup out of all patients in that subgroup.
Smoking, Race, BMI, and Ethnicity each had missing cases.
4. DISCUSSION
4.1. Contribution to current literature
Since the initiation of this study, numerous articles have been published about the use of telemedicine across different specialties and populations during the COVID‐19 pandemic. Telemedicine may decrease emergency department visits, preserve healthcare resources, and reduce the spread of COVID‐19 (Bokolo, 2020). A comprehensive review of patient satisfaction and experience with telemedicine found that patient satisfaction with telemedicine appears high, with commonly noted benefits including less travel time, accessibility, convenience, and cost‐efficiency (Nanda & Sharma, 2021). Additionally, when looking at outcome measures, telemedicine was found to be both useful and reliable (Nanda & Sharma, 2021).
At the time of writing, a review of the current literature revealed a few studies examining an outpatient approach to managing COVID‐19. Colleagues at the Cleveland Clinic were the first in the United States to implement a COVID‐19 home‐based intervention utilizing a self‐monitoring app (Medina et al., 2020). They found that approximately half (52%) of the enrolled patients actively used the app and only 1% required a hospital admission (Medina et al., 2020).
Similarly, a healthcare system in Minnesota adapted a previously established remote patient monitoring and educational application‐based platform for the use of COVID‐19 patients (Annis et al., 2020). Overall, they found that patient satisfaction was high in those who responded to the questionnaire (300 total), with 74% “extremely likely to recommend their doctor” (Annis et al., 2020). The found an overall activation rate of 61.2% for the patients offered to participate in the program (Annis et al., 2020). Out of the 1496 patients that activated the program, 91 utilized the emergency department and 13 were hospitalized (Annis et al., 2020). Another virtual care program involving weekly virtual assessments was trialed at Sunnybrook Health Sciences Center in Toronto, Ontario (Lam et al., 2020). This was a small study involving only 50 patients, with six of the patients requiring hospital care (Lam et al., 2020).
Overall, prior studies suggest that telemedicine can provide satisfactory, useful, and reliable care to patients during the COVID‐19 pandemic. Several healthcare systems have implemented remote care programs to manage COVID‐19 patients; however, the studies describing these programs lacked control groups. Without a control group, it is difficult to determine the effectiveness of these programs. This study contributes to the current literature by not only providing a protocol that can be adapted for future use by other healthcare systems, but it also includes a comparison group to allow for an evaluation of the effectiveness of the intervention.
4.2. Significance of results
Although there was not a statistically significant difference in 30‐day probability of hospital utilization between groups, there was a decrease in hospital utilization seen in the intervention group. Strict adherence to the traditional definition of statistical significance, a p‐value of .05, is a subject of debate because it potentially fails to identify clinically significant findings (Wasserstein et al., 2019). There is practical significance in a 42% reduction in risk of hospitalization during a global pandemic. Preventing unnecessary hospital visits relieves burden on patients, families, and strained health system resources. We believe the results of this study should be interpreted in context of the available sample of cases and practical impact on those affected. Additionally, the sub‐group analysis did reveal a statistically significant difference in hospital utilization in two age groups (less than 30 years and 60–69 years), directionally supporting the need for analysis in a larger cohort.
When we performed the Cox stepwise regression analysis, we did find that the “age‐only” model met statistical significance. In other words, we saw that hospital utilization increased with increasing age. This trend was not surprising to us and was consistent with the data released by CDC (Centers for Disease Control and Prevention, 2022). Older adults are more susceptible to developing severe COVID‐19 and have a higher risk of requiring hospitalization.
The protocol also allowed ongoing assessment of COVID‐19 patients with limited in‐person interactions, thereby reducing the risk of infection for healthcare workers. With the increased use of telemedicine, this protocol could be used as a guide for other health systems looking to improve access to care for COVID‐19 patients without increasing hospital utilization. Moreover, patient satisfaction should also be considered when determining the effectiveness of the protocol. Having COVID‐19 can be a stressful for some people, and the added support provided by the active management protocol may ease their concerns and provide psychological comfort. Unfortunately, an evaluation of patient satisfaction was not obtained during the implementation of this protocol. A follow‐up study would need to be performed to specifically assess patient satisfaction with the protocol.
4.3. Considerations in the application of this intervention
There are some additional factors to consider when determining the suitability of this intervention for a specific community or hospital system. At the time of this study, the health care system involved in the study managed the largest testing site in the surrounding community and had sufficient testing capacity. If COVID‐19 cases cannot be quickly identified and contacted, this protocol is unlikely to be effective in reducing hospital utilization. A health system utilizing this approach needs to have testing capacity or data exchange capability with other local testing sites to identify cases.
Community partnership played an important role in the success of this program. Nursing resources from local health departments and other health systems should collaborate on a common approach to cases. The COVID‐19 pandemic exposed a disconnect between local public health and hospitals, which were more likely to operate in parallel than in collaboration. In the community where this study occurred, strain on the local health department was significant. The hospital and health department have a strong working relationship that helped provide consistent communication when contacting patients, balance the workload of nurses, and prevented confusion around continuously evolving COVID‐19 guidelines.
The ongoing shortage of nurses may adversely affect the ability to implement this program. Staffing shortages have posed a problem throughout the COVID‐19 pandemic. In our institution, we did not find it difficult to attract nurses. The work is lower intensity in nature than what many were experiencing in the acute environment and helped keep several individuals near retirement in the workforce. The more significant challenge is flexing the staffing model with demand, as pandemic surges could easily overwhelm the resources available to contact patients. In the absence of additional staff resources, modification to the protocol may be necessary to prioritize patients at highest risk of a poor outcome.
4.4. Limitations
Our study does have several limitations to consider. First is the inability to fully differentiate hospital utilization related to COVID‐19 and those that were unrelated. We chose to exclude obvious unrelated diagnoses; however, there were certain diagnoses (e.g., melena, transient cerebral ischemic attack) for which we could not definitively determine the etiology without more information. We decided to be conservative with our case exclusions, with the assumption that each group would have a similar number of ambiguous hospital admission diagnoses and patient chief complaints.
Second, the post‐hoc power analysis, which demonstrated a power of 53%, indicates that the sample size may not have been sufficient to identify a significant difference in hospital utilization between groups. The COVID‐19 pandemic was a time of rapid change, and we elected to limit the duration of the study to minimize the impact of new knowledge and treatment options on the management of COVID‐19. This approach resulted in a slightly smaller intervention group.
Third, we also assumed that patients tested at this specific location only utilized the affiliated hospital for acute care needs. There is only one hospital in the county, it is centrally located, and at this early stage in the pandemic testing was limited to county residents, those who worked in the county, and established health system patients. The testing restrictions were necessary due to the unpredictable supply of PCR testing components. Additionally, effective March 24, 2020, the state was under executive order of the governor to “stay home, stay safe,” which limited gatherings and reduced the movement of residents who were not essential workers. The most significant reduction in restrictions did not occur until June 1, 2020. The study timeline is confined to this period of most restricted travel. Finally, while there is a possibility of acute care utilization outside the sole hospital in the county despite the geographic, testing and travel barriers, it is unlikely to disproportionally affect one study group over the other.
A final limitation to consider is the generalizability of the study. The county involved in the study has a relatively small population, so this intervention may not be as effective in a large county, a densely populated city, or an area with high COVID‐19 activity. Additionally, underserved populations with insufficient testing capacity may not be equipped to effectively implement the intervention.
5. CONCLUSION
The COVID‐19 pandemic altered our lives in many ways, and the United States health care system adapted quickly. New approaches to delivering health care were necessary to ensure access to care and to protect the healthcare workforce. Hospitals became overwhelmed during the early months of the pandemic and hospital resources became scarce. Although, this study took place in the initial COVID‐19 surge in the United States, the findings remain relevant. Over time, we have seen the evolution of the SARS‐CoV‐2 virus with subsequent more easily transmissible variants. Even with public health measures in place (e.g., masking, social distancing, hand hygiene), hospitals have continued to be strained during surges. Hospitals could use this protocol as a more proactive approach to help alleviate demand on the acute care environment during a COVID‐19 surge.
Overall, this retrospective study demonstrated that a nurse‐led, telephone‐based active management protocol for COVID‐19 patients is a viable option for health systems looking to reduce in‐person interactions with COVID‐19 patients while maintaining access to quality care. A similar protocol may also help reduce hospital utilization. This protocol is applicable to the current COVID‐19 pandemic or could be adapted for future pandemics.
APPENDIX A. SARS‐CoV‐2 ACTIVE MANAGEMENT PROTOCOL FOR COVID‐19 POSITIVE PATIENTS
A.1.
SARS‐CoV‐2 (COVID‐19) symptom assessment
“Can you please describe your current symptoms?”
| Criteria | Points | Definition |
|---|---|---|
| Shortness of breath | 4 | Breathless at rest, or not improved at rest. |
| Fever | 3 | Severe chills, drenching sweats or measured temperature over 100.0F. |
| Cough | 1 | |
| Unable to eat/drink | 1 | |
Other
|
1 | Any number of symptoms in this category receive one (1) point total. More symptoms DOES NOT result in more points. |
Sum the points and follow the protocol according to the severity of illness.
| Severity of Illness | Point Total |
|---|---|
| Better | 0 points |
| Mild illness | 1–3 points |
| Moderate illness | 4–6 points |
| Severe illness | 7 or more points |
Instructions for patients that are BETTER: 0 points
Can occur via phone or video visit.
Schedule follow‐up visits at random times.
Schedule calls for EVERY OTHER DAY.
Review isolation procedure.
Confirm retest date.
Instructions for patients that have MILD illness: 1–3 points
Can occur via phone or video visit.
Schedule follow‐up visits at random times.
Schedule calls for DAILY.
Review isolation procedure.
Confirm retest date.
Review that patients often worsen at 10–14 days.
Schedule appointment with respiratory clinic at day 7 of symptoms OR recommend call PCP if independent.
Instructions for patients that have MODERATE illness: 4–6 points
Can occur via phone or video visit.
Schedule follow‐up visits at random times.
Schedule calls for TWICE DAILY.
Review isolation procedure.
Confirm retest date.
Review that patients often worsen at 10–14 days.
Schedule appointment with respiratory clinic for a consult OR recommend call PCP if independent. Patient with independent PCP has option to call their PCP or schedule a visit with the Respiratory Clinic. Respiratory Clinic visits may be in person or video.
Consider Community Paramedic.
Instructions for patients that have SEVERE illness: 7 or more points
Can occur via phone or video visit.
Schedule follow‐up visits at random times.
Schedule calls for TWICE DAILY.
Contact Physician/Provider ON CALL at Respiratory Clinic for consultation (517‐205‐8991). Provider may choose any of following options:
Schedule phone/video visit with Respiratory Clinic.
Schedule in person visit with Respiratory Clinic.
Schedule Community Paramedic.
Advise patient to go to the emergency room.
Korycinski, S. , Metcalf, D. , & Keteyian, C. (2022). Effectiveness of a telephone‐based nursing intervention to reduce hospital utilization by COVID‐19 patients. Public Health Nursing, 1–9. 10.1111/phn.13074
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the ICPSR COVID‐19 Data Repository at https://doi.org/10.3886/E145581V1.
REFERENCES
- Annis, T. , Pleasants, S. , Hultman, G. , Lindemann, E. , Thompson, J. A. , Billecke, S. , Badlani, S. , & Melton, G. B. (2020). Rapid implementation of a COVID‐19 remote patient monitoring program. Journal of the American Medical Informatics Association : JAMIA, 27(8), 1326–1330. 10.1093/jamia/ocaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokolo Anthony Jnr (2020). Use of telemedicine and virtual care for remote treatment in response to COVID‐19 pandemic. Journal of Medical Systems, 44(7), 132. 10.1007/s10916-020-01596-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2020a). COVID‐19 and your health . Retrieved 11 May 2020, from https://www.cdc.gov/coronavirus/2019‐ncov/faq.html#Coronavirus‐Disease‐2019‐Basics
- Centers for Disease Control and Prevention . (2020b). Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID‐19) . Retrieved 11 May 2020, from https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐guidance‐management‐patients.html.#clinical‐management‐treatment≤
- Centers for Disease Control and Prevention . (2022). Cases, data, and surveillance. Retrieved 16 February 2022, from http://www.cdc.gov/coronavirus/2019‐ncov/covid‐data/investigations‐discovery/hospitalization‐death‐by‐age.html
- FAIR Health . (2020). Key characteristics of COVID‐19 patients‐profiles based on analysis of private healthcare claims. A FAIR Health Brief. FAIR Health. Retrieved from https://www.fairhealth.org/publications/briefs
- Lam, P. , Sehgal, P. , Andany, N. , Mubareka, S. , Simor, A. , Ozaldin, O. , Leis, J. A. , Daneman, N. , & Chan, A. K. (2020). A virtual care program for outpatients diagnosed with COVID‐19: A feasibility study. CMAJ Open, 8(2), E407–E413. 10.9778/cmajo.20200069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina, M. , Babiuch, C. , Card, M. , Gavrilescu, R. , Zafirau, W. , Boose, E. , Giuliano, K. , Kim, A. , Jones, R. , & Boissy, A. (2020). Home monitoring for COVID‐19. Cleveland Clinic Journal of Medicine. 10.3949/ccjm.87a.ccc028 [DOI] [PubMed] [Google Scholar]
- Nanda, M. , & Sharma, R. (2021). A review of patient satisfaction and experience with telemedicine: A virtual solution during and beyond COVID‐19 pandemic. Telemedicine Journal and e‐health : the official Journal of the American Telemedicine Association, 27(12), 1325–1331. 10.1089/tmj.2020.0570 [DOI] [PubMed] [Google Scholar]
- Rae, M. , Claxton, G. , Kurani, N. , McDermott, D. , & Cox, C. (2020). Potential costs of COVID‐19 treatment for people with employer coverage. Peterson‐KFF Health System Tracker. Peterson‐KFF Health System Tracker. Retrieved 11 May 2020, from https://www.healthsystemtracker.org/brief/potential‐costs‐of‐coronavirus‐treatment‐for‐people‐with‐employer‐coverage/ [Google Scholar]
- The Centers for Medicare and Medicaid Services (2020). Preliminary medicare COVID‐19 data snapshot medicare claims and encounter data: Services January 1 to September 12, 2020, Received By October 9, 2020. Retrieved 9 December 2020, from https://www.cms.gov/research‐statistics‐data‐systems/preliminary‐medicare‐covid‐19‐data‐snapshot
- U.S. Census Bureau QuickFacts: Jackson County, Michigan. Census Bureau QuickFacts . (2021). Retrieved 17 February 2021, from http://www.census.gov/quickfacts/fact/table/jacksoncountymichigan/PST045219
- Wasserstein, R. , Schirm, A. , & Lazar, N. (2019). Moving to a World Beyond “p < 0.05”. The American Statistician, 73(sup1), 1–19. 10.1080/00031305.2019.1583913 [DOI] [Google Scholar]
- Wu, Z. , & McGoogan, J. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China. JAMA, 323(13), 1239. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2020). WHO Director‐General's opening remarks at the media briefing on COVID‐19 ‐ 11 March 2020. Retrieved 11 May 2020, from https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19—11‐march‐2020
- Young, J. D. , Abdel‐Massih, R. , Herchline, T. , McCurdy, L. , Moyer, K. J. , Scott, J. D. , Wood, B. R. , & Siddiqui, J. (2019). Infectious diseases society of America position statement on telehealth and telemedicine as applied to the practice of infectious diseases. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 68(9), 1437–1443. 10.1093/cid/ciy907 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in the ICPSR COVID‐19 Data Repository at https://doi.org/10.3886/E145581V1.


